Aqueous reduction of Au3+ by natural organic matter under light-limited irradiation*
LIU Zilu, MA Jiahai
(School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, China)(Received 8 March 2016; Revised 20 April 2016)
Abstract In this study, we find that one standard natural organic matter (NOM) called Suwannee River humic acid (SRHA) reduces Au3+ to Au nanoparticles under short-time simulated sunlight irradiation or in full-course darkness. The results suggest that the reduction of metal ions by the active NOM occurs widely, even in special aquatic environments that lack sunlight exposure, such as underground and cavern water. Moreover, analogous experiments covering other two kinds of NOMs, Pony Lake fulvic acid (PLFA) and Aldrich humic acid (AHA) , demonstrate that NOMs behave differently in reduction of Au3+ with limited irradiation.
Key words natural organic matter; Au3+; Au nanoparticles
With the rapid development of nanotechnology in recent decades, industrial scale engineered nanop-articles (ENPs) are produced every year, including non-metals, carbon, polymers, lipids, metal oxides and metals[1]. ENPs are used in various fields such as energy, medical treatment, food products, electronics, and communications. Among them, gold nanoparticles (AuNPs) is one of the most common noble metal-containing nanoparticles (NPs)[2], whose applications have covered cellular imaging, bio-chemical sensing, drug, and medical therapeutics[3]. The expanding applications and production of AuNPs inevitably lead to its accumulating release and exposure in surface environment, which has raised wide concern about environmental toxicity of AuNPs. In the aquatic ecosystem, AuNPs can be taken up by organisms, which then causes toxicity and adhesion[4-6], as well as cycling through the food chain.
NOM is a complex mixture of poorly understood organic molecules. NOM exsits in abundance in natural waters, and the concentration can be up to 15 mg/L[7]. Containing carbonyl-, carboxyl-, phenol-, nitrogen-, and sulfur-containing groups, NOM has high affinities for metal ions[8]. Recent reports has suggested that natural organic matter (NOM) reduces Au(III) to form AuNPs in the aquatic environment under continuous irradiation[9-10]. Additionally, NOM influences fate of NPs and transport NPs by stabili-zing and dispersing NPs as a coating agent[11-12].
Gold concentration can be up to about 80 μg·L-1in ground water[13-16]. Recently, the stable existence of Au(III)-complexes in aqueous environment has also been reported[17]. So it is necessary to further expand our knowledge of the aqueous production of AuNPs from Au(III) complexes by NOM in aquatic systems. Existing studies[18-21]have focused on the transformation of ionic metals under continuous irradiation in systems with NOM. However, to our best knowledge, research on the photoreduction of Au3+by different NOMs with limited irradiation exposure has not been reported. In this work, the specific behaviors of Au3+with different NOMs in aqueous environment undergoing full-course darkness or simulated sunlight of short durations are examined.
1 Experimental section
1.1 Materials
Gold(III) chloride hydrate (>99.9% purity) was from J&K. Suwannee River humic acid (SRHA) and Pony Lake fulvic acid (PLFA) were from the International Humic Substances Society (IHSS). Aldrich humic acid (AHA) was obtained from Aldrich. Sodium borohydride was obtained from Alfa Aesar. Water was obtained from a Milli-Q purification system. Total organic carbon (TOC) of the NOM solutions was analyzed by a Tekmar-Dohrmann Apollo 9000 TOC analyzer.
1.2 Reaction procedures
All the experiments were performed in quartz beakers, and the total volume of each reaction solution was 20 mL. Solution conditions were 0.2 mmol/L HAuCl4and 10 mg C/L (carbon content per litre) NOM (SRHA, PLFA or AHA). The control samples in the presence of 0.2 mmol/L HAuCl4and 10 mg C/L NOM were exposed to simulated sunlight for 5-30 min and then kept in dark. Simulated sunlight was provided by a 250 W Xe lamp.
1.3 Analysis
AuNPs were completely removed from the solutions by centrifuging the samples at 16 000 rpm for 15 min. Then, 1 mL supernatant was taken out and diluted five times with water for measuring the remaining Au3+concentration. The Au3+concentration determination was performed via the flame method using an atomic absorption spectrometer (PinAAcle 900T, PerkinElmer, USA). Temporal reduction ratios of Au3+were calculated according to the fomula, [AuNP]t/[Au3+]0=([Au3+]0-[Au3+]t)/[Au3+]0. [Au3+]0is the Au3+concentration at timet=0; [Au3+]tis the Au3+concentration at timet=t; and [AuNP]tis the AuNPs concentration at timet=t.
UV-Vis spectra were recorded from 200 to 800 nm by a TU-1900 spectrophotometer (Beijing Purkinje General Instrument Co. Ltd.). Transmission electron microscopy (TEM) was performed using a Tecnai G2 F30 instrument (FEI, USA).
2 Results and discussion
2.1 Reduction of Au3+ by SRHA after short-term simulated solar irradiation
The formation of AuNPs in the presence of 10 mg C/L SRHA and 0.2 mmol/L HAuCl4in darkness is shown in TEM picture (Fig.1). The shape of AuNPs are mainly sphere, flake, and rod-like structures.

Solution conditions: 0.2 mmol/L HAuCl4 and 10 mg C/L SRHA.Fig.1 TEM images of gold nanoparticles formed in darkness
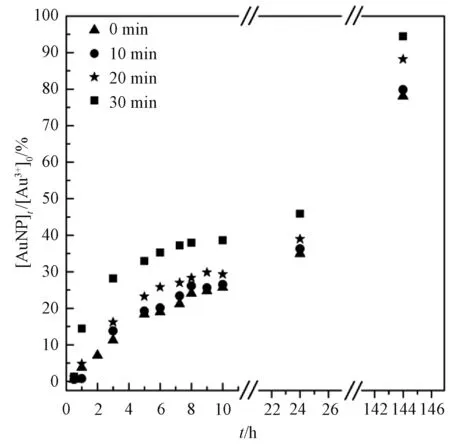
Solution conditions: 0.2 mmo/L HAuCl4 and 10 mg C/L SRHA.Fig.2 Temporal yields of AuNPs in the dark after different durations of irradiation

Besides, temporal reduction ratios of Au3+by SRHA under continuous irradiation reached 86.8% in 1 h and almost 100% in 2 h (Table 1). These results illustrate that both short-term irradiation and dark condition led to significant decrease of AuNPs formation rate, compared with continuous irradiation process.

Table 1 Temporal reduction ratios of Au3+ under continuous simulated sunlight %
Note:Solution conditions: 0.2 mmol/L HAuCl4and 10 mg C/L NOM (SRHA, PLFA or AHA).
For the full-course darkness experiments, it should be taken into consideration that the absolute darkness was unreached in the lab. Once the superoxide free radical produced before the samples were placed into darkness, it may partly cause the reduction of Au3+. Gold atoms nucleate within minutes or even seconds of irradiation, and once the concentration of AuNPs increases, the likelihood of AuNPs to fuse, grow, or aggregate increases.
Under some circumstances, free ionic Au3+and other metal ions in the aquatic environment are exposed to sunlight for a short period and then remain in the dark or shadow. For example, in a substantial part of the Earth surface such as deepwater areas, there is only short-term exposure or even no exposure to sunlight. In addition, the complexity of the ground-surface conditions should be taken into consideration, for example, underground streams and cavern water. Hence, the above results imply that, even in aquatic environments with low sunlight exposure, reduction of Au3+by active NOM like SRHA can still occur.
2.2 Analogous experiments using AHA and PLFA
For PLFA and AHA, the processes of short-time irradiation and full-course darkness were studied (Fig.3). In full-course darkness or after short irradiation, the reduction ratios of Au3+were much lower than under the lasting simulated sunlight irradiation (Table 1) for both PLFA and AHA, which is consistent with the results of SRHA. For both PLFA and AHA, we found small difference in the results in full-course darkness or after 10-to 30 min irradiation. It is further demonstrated that these samples are obviously less active than SRHA.

Solution conditions: 0.2 mmol/L HAuCl4 and 10 mg C/L PLFA (a) or AHA (b).Fig.3 Temporal yields of AuNPs in the dark after different durations of irradiation by NOMs
As shown in Fig.4, in electron paramagnetic resonance (EPR) spectra of PLFA there was no significant signal of superoxide species in darkness (0 min). However, after simulated sunlight irradiation, the superoxide species content increased evidently with time. This clearly proves the effect of O2in the Au3+reduction by NOMs and explains why NOM samples under lasting exposure reduce Au3+to gold nanoparticles fast.

Initial concentrations: 100 mg C/L PLFA; 40 mmol/L DMPO.Fig.4 Corresponding EPR spectra of PLFA after different durations of simulated sunlight irradiation
These results suggest that not all NOMs can operate non-photochemical processes. The active NOM like SRHA could reduce metal ions in dim environment. But NOMs like PLFA, whose aromatic and carbonyl contents are much lower, cannot reduce Au3+in the dark unless with long term photoexcitation. The specific reasons may be complex and related to some physical and chemical properties of different samples, which needs further studies in the future.
3 Conclusion
In brief, this study covers the Au3+photoreduction by three kinds of NOMs under different irradiation conditions, including full-course darkness and short-term and continuous simulated sunlight irradiation. It provides valuable information about different reaction process in the complex surface water environment. The almost complete reduction of Au3+to AuNPs by SRHA in the dark after short-term irradiation suggests that the reduction of metal ions by active NOM may occur widely, even in special aquatic environments that lack sunlight exposure such as underground and cavern water.

