Histopathological changes associated with oxidative stress induced by electromagnetic waves in rats’ ovarian and uterine tissues
Ali S. H. Alchalabi, Hasliza Rahim, Erkihun Aklilu, Imad I. Al-Sultan, Abd Rahman Aziz, Mohd F. Malek, Suzanna. H. Ronald, Mohd Azam Khan
1Faculty of Veterinary Medicine, UMK City Campus, Pengkalan Chepa, Locked Bag36, 16100 Kota Bharu, Kelantan, Malaysia
2Veterinary Medicine College, Mosul University, Mosul, Iraq
3School of Electrical System Engineering, University Malaysia Perlis (UniMAP), Pauh Putra, 02600 Arau, Perlis, Malaysia
Histopathological changes associated with oxidative stress induced by electromagnetic waves in rats’ ovarian and uterine tissues
Ali S. H. Alchalabi1,2*, Hasliza Rahim3, Erkihun Aklilu1, Imad I. Al-Sultan1, Abd Rahman Aziz1, Mohd F. Malek3, Suzanna. H. Ronald3, Mohd Azam Khan1
1Faculty of Veterinary Medicine, UMK City Campus, Pengkalan Chepa, Locked Bag36, 16100 Kota Bharu, Kelantan, Malaysia
2Veterinary Medicine College, Mosul University, Mosul, Iraq
3School of Electrical System Engineering, University Malaysia Perlis (UniMAP), Pauh Putra, 02600 Arau, Perlis, Malaysia
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Histopathological changes Female sex organs Oxidative stress
Objective: To evaluate the histopathological changes in ovarian and uterine tissues associated with oxidative stress induced by electromagnetic waves. Methods: Thirty female Sprague Dawley of 180 g body weight and 3 months old were used in the experimental work. Animals were divided into control and two experimental groups. Experimental groups were exposed to 1 800 MHz Global System for Mobile Communication radiofrequency radiation emitted by a signal generator for 2 h per day for 30 and 60 d respectively. Following exposure, serum, ovaries and uteri were collected for biochemical and histopathological investigations. Results: Biochemical analysis showed alterations in oxidative stress parameters in both ovaries and uteri tissues in comparison to control group. The histopathological changes were more prominent in experimental groups, in the ovary were included vacuolation in interstitial, granulosa, luteal cells and ooplasm. Other histopathological changes are disorientation of corona radiata, disruption and thinning of the zona pellucida. Cellular nucleus changes similar to fragmentation of the nucleus indicate the start of a degeneration process at Graffian follicles as well as micronuclei formation in oocyte nucleus and in some luteal cells. Histopathological changes in uterine tissue confined to increase height of luminal epithelium cells, sever apoptosis of glandular and luminal epithelium cells, and sever eosinophils, polymorphonucleocyte lymphocytes and macrophage’s infiltration in myometrium and endometrium layers. Vascular congestion points out for the existence of inflammatory response changes in the endometrium. Conclusion: Results executed that the potential alteration of antioxidant capacity may contribute to endometrial oxidative damage that could be related to pathogenesis and progression of endometritis.
or indirect role in females’ fertility, has contradictory outcomes in most cases and possibly due to variation in the experimental design in terms of source of EMWs, frequency, exposure duration and the specific absorption rate (SAR) used. For instance, Sprague-Dawley rats exposed to low frequency 50 Hz for 18 weeks (24 h/ d) exhibited a harmful effect on rats’ fertility and reproduction [11]. In the experiment carried out by researchers on the impact of electromagnetic waves on young rats aged 21 d of mothers exposed during pregnancy to mobile phone for 11 h. and 45 min. in the standby and 15 min. in speech mode to evaluate the ovarian follicle’s activity, number of follicles and growth were significantly affected due to apoptosis among follicles, hyperplasia in ovarian stroma and elongation cell mitosis time was observed [12]. Another study on Wister rats which exposed to mobile phone 12 times for 10 min. (calling mode) for 2 weeks and 1 month, the study suggests that mobile phone wave can increase ovarian and atresia follicles as well as changing in sex hormone’s levels [13]. Devrim and his term found that exposure to mobile phone radiation “four times a day for 10 min. in “call position,” electromagnetic radiation (EMR) emitted by mobile phones causes oxidative stress and lipid peroxidation in kidney and erythrocytes, and vitamin C can give some protection against the oxidant stress [14]. On other sides, some studies reported no adverse effects on reproduction activity and fertility in rats and mice exposed to EMWs (GSM /Wi-Fi signals) [15-18]. The correlations between mobile phone use and histopathological changes were evaluated in different organs and different electromagnetic field (EMF) setup based on the aims of the studies [19-21]. In 2007, a Turkish study concluded that female rats exposed to mobile phone frequency of 900 MHz exhibited oxidative endometrial damage which is responsible for endometrial impairment and vitamin E & C supplement reduces this damage at a tissue level [22]. An investigation of EMF (50 Hz, 0.5 mT) on epididymis and deferens duct in mice exposed for 2 months revealed low reproductive efficiency in mice due to a decrease in diameter of reproductive ducts, the height of epithelial cells and weight of the testes [23]. In a study to clarify the effect of low-frequency electromagnetic field (ELFEMF) on fertility and heights of epithelial cells in pre-implantation stage of endometrium and fallopian tube in mice, daily 4 h for 2-week exposure to 50 Hz 0.5 mT EMF showed that ELF-EMF has the detrimental effect on a female genital tract via increasing the fallopian tube epithelial cell’s height and reduction in flushed blastocyst’s number [24]. Female rats whole body exposure by EMF at 50 Hz for 40 d revealed a detrimental effect on ovarian tissue via increasing fibrosis and venous congestion, and these bad effects were minor in other exposed groups received Ocimum basilicum at a dose 1.5 g/kg B.W. as antioxidant therapy [25]. Iranian researchers group found that in-utero exposure by ELF-EMF at 50 Hz 3 mT, 4 h/d for 21 d in rats led to induce oxidative stress and granulosa cells interspaced from the base membrane with narrow and irregular zona pellucida, vacuolization in ooplasm were observed [26]. Our study, it was aimed to evaluate the correlation between chronic exposure to 1 800 MHz GSM signals “heavy use of mobile phone” induces oxidative stress and histopathological changes in ovarian and uterine tissues in adult female rats.
2. Material and methods
2.1. Animal and EMF setup
The study was approved by the scientific committee of faculty veterinary medicine of University Malaysia Kelantan (UMK) and was conducted in accordance with the UMK guidelines for animal experiments (FPV-PGSC-2014). Thirty female Sprague Dawley type rats at an average body weight 180 g and 3 months old were used throughout the study. Oestrus synchronization was done before starting the experiment, and the animals in pro-oestrus phase were used throughout the experiment. The animals were distributed over the three groups (control group and the two exposure groups as whole-body exposure for 2 h/d, 7 d/week for 30 and 60 continuous days). The animals were obtained from the laboratory animal research unit of faculty of veterinary medicine (UMK). Animals were kept in plastic cages at room temperature (25±1) C and humidity (60±10) % (relative humidity) with light / dark cycle 12-12 h, and tap water and standard rat pellet were provided ad libitum. Especial designed exposure Plexiglas box (60 cm x 40 cm x 20 cm) was used during the RF-EMR exposure time.
Whole-body exposure with 1 800 MHz GSM-like frequency of mobile phone at SAR level value 0.974 W/kg was calculated using this equation:
SAR = (δ/ ρ) E²
Where (E) is the magnitude of electric field 28.156, (δ) is the conductivity 1.34 s/m and (ρ) is the mass density of the tissueequivalent media 1 090 kg/m3. The exposure setup described by previous publication [27].
2.2. Biochemical analysis
At the end of experiment, rats were anesthetized by intraperitoneal (IP) injection of Ketamine and Xylazine combination at a dose 0.1 mL/100 g b.w. (80 mg/kg b.w. ketamine and 5 mg/kg b.w. Xylazine) and then sacrificed. Ovaries and uterus were surgically removed for biochemical and histopathological investigations. Kits were purchased from Cusabio Biotech CO., LTD. and Abcam for biochemical analysis. Malondialdehyde (MDA) was assessed as lipid peroxidation biomarker by using of lipid peroxidation (MDA)
assay kit (ab119870, Abcam, U.K.) according to the manufacturer’s instructions [28]. Glutathione peroxidase (GSH-PX) activity and melatonin (MT) concentration in serum were estimated by using of rat Glutathione Peroxidase ELISA kit and rat Melatonin ELISA kit (CUSABIO Rat GSH-PX ELISA Kit, CSB-E12146r, CUSABIO Melatonin ELISA Kit, CSB-E13433r, Wuhan University Science, Wuhan, Hubei province 430223 P.R. China) respectively. According to the manufacturer’s instructions, estimation was performed.
2.3. Histopathological sample preparation
All rats were sacrificed in the oestrus cycle. Removed ovaries and uterus were cleaned from fatty tissues and washed by normal saline. Tissue paper was used to remove the excess fluids and weighed using a digital scale with precision of 0. 001 g. Uterus and ovary samples were fixed in 10% neutral buffer formalin for 48 h before starting the process of preparation for histological slides. Specimens were embedded in paraffin, and sections at 5 μm was prepared and stained with H&E. Ovarian and uterine tissue slides were examined under the light microscope for histopathological study and measurement of uterine horn layer’s thickness (five slices from each sample were randomly selected) at a magnification of 4x and 40x for luminal epithelium layer height. Tissue samples were assessed at a magnification of 20x and 40x and photographed by Olympus microscope (model: BX43F Japan). The evaluation of ovarian and uterine pathological changes was confirmed by histologist and pathologist in veterinary medicine college of UMK. Uterine layer’s thickness was measured by using Cellsens Dimension software.
2.4. Statistical analysis
Data were presented as mean ± standard deviation (mean ± S.D.). SPSS programs V. 22 software (SPSS In. Chicago, IL., USA) was used to test significant differences among groups. One-way ANOVA and LSD test were used to evaluate the significance between groups. P values of less than 0.05 were considered as significant.
3. Results
3.1. Body, ovarian and uterine weight
Table 1 shows the effect of 1 800 MHz GSM electromagnetic field on body and sex organ’s weight of rats after 30 and 60-day irradiation by GSM signals. As observed no significant difference between the body and uterine weight of females of all groups. However, ovarian weight was significantly decreased in 60-day EMF exposure when compared with 30-day EMF exposure and control groups (P = 0.024).
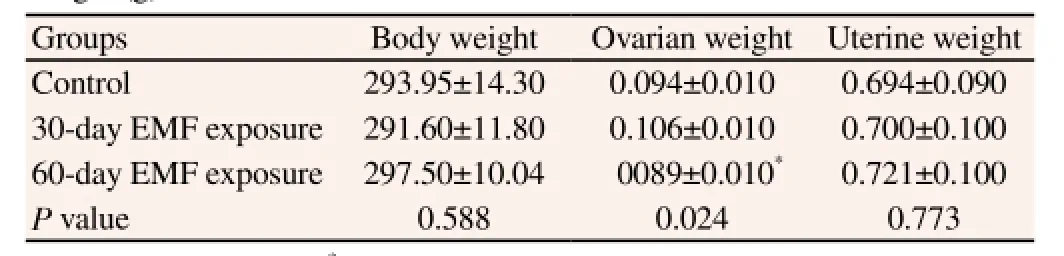
Table 1 Effect of 1 800 MHz GSM electromagnetic field on body, ovarian and uterine weight (g).
Table 2 Effect of 1 800 MHz GSM electromagnetic field on oxidative stress parameters.
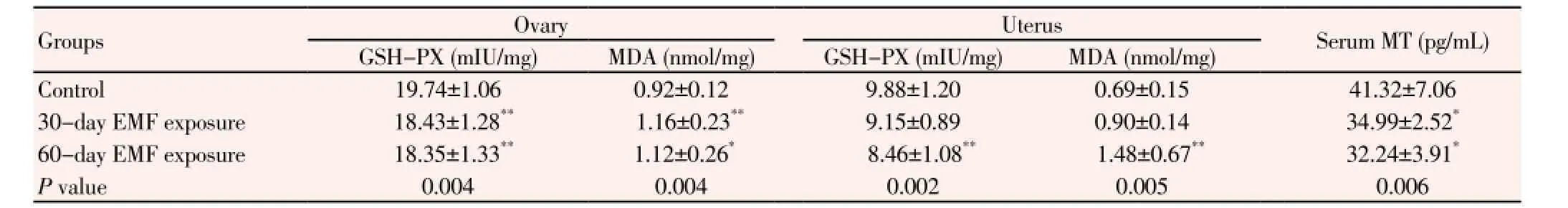
Values are Mean±S.D.**P < 0.01;*P < 0.05 statistically significant difference vs control group.
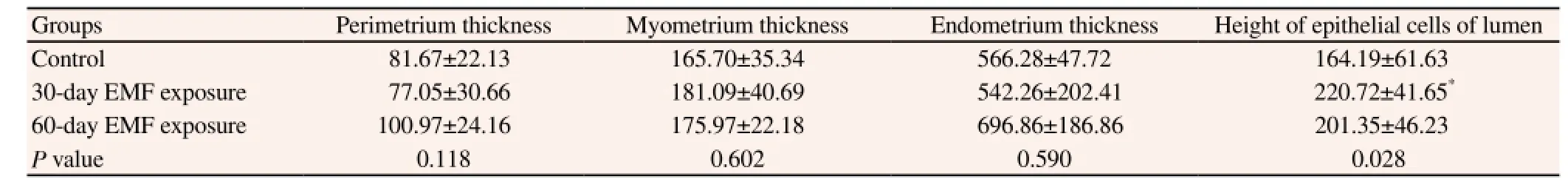
Table 3 Effect of 1 800 MHz GSM electromagnetic field on thickness of the uterine horns’ layers and height of epithelial cells of lumen (μm).
3.2. Biochemical analysis
Biochemical analysis results in Table 2 showed a significant decrease in GSH-PX activity and an increase in MDA level in ovarian tissue in both EMF exposure groups compares to control group. Uterine tissue GSH-PX activity in the 60-day EMF exposure group was significantly lower than 30-day exposure and control groups. Moreover, endometrial MDA level in a 60-day EMF exposure group for was significantly higher than 30-day EMF
exposure and control groups. Serum melatonin level was reduced significantly in both EMF exposure groups compare to control animals.
3.3. Measurement of thickness of the uterine layers
The statistical analysis in Table 3 did not show a significant difference in the thickness of the uterine horns’ layers in terms of micrometers among the exposure and control groups. However, the height of epithelial cell’s layer was significantly increased in 30-day EMF exposure group compared to epithelial cells in control group (P < 0.028).
3.4. Histopathological changes
3.4.1. Ovarian tissue changes
Different histologic sections of the ovaries from the female control group indicate well development of ovarian follicles, normal blood vessels and normal stroma cells. The pre-ovulatory follicle showed typical morphology of oocyte and euchromatin nucleus, which was surrounded by typical zona pellucida and several layers of granulosa cells are resting on the basement membrane. Theca interna and theca externa are clearly developed. The primary follicles with the normal oocyte surrounded by single layer of cubical granulosa cells are showed to rest on the basement membrane. Hypertrophy of corpus luteum and proliferation and differentiation of steroidogenic cells with extensive angiogenesis are evident in Figure 1.
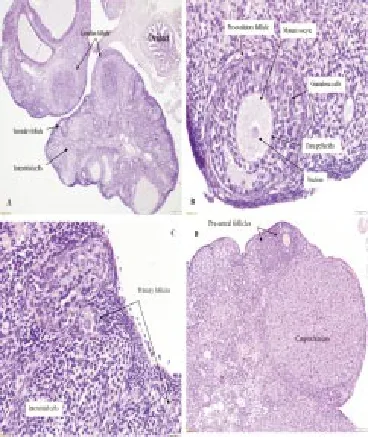
Figure 1. Ovarian sections from the female control group.(A) Normal ovary showing well follicular development 4x. (B) Pre-ovulatory follicle with mature oocyte surrounded by granulosa cells with normal zona pellucida and theca interna and externa 40x. (C) Well developed primary follicles indicated by arrows 20x. (D) 1. Preantral follicles, 2. Corpus luteum showing normal luteal cells 10x. H&E staining.
The most evident histopathological changes in the ovaries of females EMF exposure group 30 d post exposures are congestion and reduction in the number of ovarian follicles. Other changes include vacuolation in luteal cells, ooplasm and in granulosa cells of preovulatory follicles. Moreover, the histologic changes seen indicate a thin and irregular zona pellucida. Granulosa cells perform a strong contact with the oocyte in the complementary process of autophagy leading to cell death. Micronuclei formation and persistence of preovulatory follicle oocyte and in luteal cells are an indication of DNA damage (Figure 2).
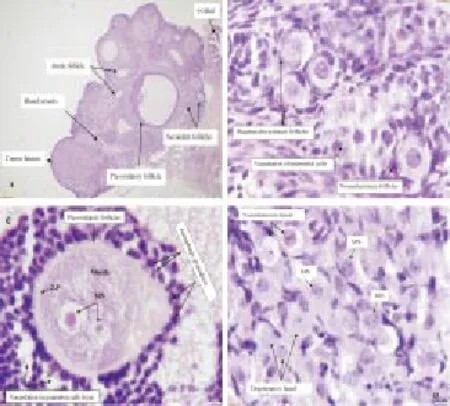
Figure 2. Ovarian sections from 30-day EMF exposure group.(A)Ovary showing reduction in ovarian follicle’s numbers with congestion of blood vessels and degeneration of interstitial cells and some follicles. (G. F) Pre-ovulatory follicle, (A. F) Atretic follicle, (C. L) Corpus Luteum, (b.v) blood vessel, (o.d) oviduct. 4x. (B) Primordial follicles 1. Degenerative oocytes with vacuolization in ooplasm. 2. Normal primordial follicle with a single layer of flattened pre-granulosa cells surrounding the oocyte 40x. (C) Pre-ovulatory follicles shows oocyte with micronucleus formation indicated by (MN) enclosed by the thin, irregular zona pellucida indicated by (Z.P). Granulosa cells were separated from each other and vacuolization in ooplasm and granulosa cell’s layer. Autophagy granulosa cell is strong contact with the oocyte indicated by arrows 100x. (D) Corpus luteum showing degenerative luteal cells with vacuolation and micronucleus formation (MN) in some luteal cells indicated by arrows 100x. H&E staining.
Microscopic examination of the ovarian tissue sections of females EMF exposure group 60 d post exposure showed a reduction in ovarian follicles, congestion of blood vessels, and degeneration of pre-ovulatory follicle cells and infiltration of macrophages. Vacuolation of interstitial cells and granulosa cell’s layers is activated. Micronuclei formation was found in pre-ovulatory follicle’s oocytes. Further changes disclose on the disorientation of corona radiata, disruption and thinning of the zona pellucida. Cellular nucleus changes similar to fragmentation of the nucleus indicate the start of the degeneration process at pre-ovulatory follicles (Figure 3).
3.4.2. Uterine tissue changes
Tissue sections from the uterus of females control group shows common uterine histology with regular columnar epithelial cells lining the uterine lumen and glands. The mitotic figures were observed clearly in both epithelial and glandular cells along with normal histology of blood vascularity. Fibrosis, necrosis, apoptosis or any other adaptive cellular changes did not observe in the control uterine tissue sections (Figure 4).
Electromagnetic irradiation for 30 d had the significant effect on female uterine tissue. Histopathological alterations were apoptosis demonstrated in the luminal epithelial cells in a form of individual cell debris that form a halo within the luminal epithelium. Changes in glandular epithelium range from desquamation of epithelial cells to degenerative process. Infiltration of the endometrium with an abundant number of lymphocytes and polymorph nuclear cells along with the vascular congestion point out for the existence of inflammatory response in the endometrium (Figure 5).
Endometritis of acute onset was observed in of females exposed to EMF for 60 d. The cellular inflammatory reaction was composed of infiltrative neutrophils, fewer numbers of lymphocytes as well as severe diffuse eosinophil’s infiltration in endometrium and perimetrium layers with apoptosis and desquamation of luminal and glandular epithelium (Figure 6).
4. Discussion
The interaction between electromagnetic waves emitted from cellular phone devices and vital organ’s activity, especially the female reproductive system performance in terms of the biological role of these waves in the development of oxidative stress and pathological changes became controversial about the reality of the impact of these waves on public health.
This study proved that chronic exposure to mobile phone have no effect on body weight, but the exposure for long periods led to significantly reduced ovarian weight compared to the control group as well as induced oxidative stress represented by elevation of MDA (a lipid peroxidation biomarker) and decline in the activity of antioxidant enzyme GSH-PX in both exposure periods 30 and 60 d at the tissue level of the ovary. Sixty-day exposure group encompassed the reduction of GSH-PX enzyme activity and increased MDA level at the uterine tissue level with reduction in melatonin level. Results are consistent with previous study [22]. One-month old infant rabbit showed an increased hepatic lipid peroxidation as a result of intrauterine exposure of their mothers to 1 800 MHz for 15 min/d for 7 d [29]. Other experimental studies on pregnant and non-pregnant rabbits indicated that exposure to electromagnetic radiation resulted in oxidative damage to DNA and lipid molecules [30]. Pregnant Wister rats exposed to the extremely low-frequency electromagnetic field (ELF-EMF) showed an increase in MDA serum level and also caused an adverse effect in F1 generation ovarian follicle’s development, which may affect fertility [26].
The present study showed chronic exposure to EMF 1 800 MHz causes pathological changes in ovarian tissues ranging from minor to powerful changes (congestion, decreased ovarian follicle number and development, vacuolation, autophagy, apoptosis and micronuclei formation). These results are similar to previous studies [26]. The Wister rats exposed to the ELF-EMF showed that the ovarian tissue revealed separation of granulosa cells from the basement membrane and thinning, irregular zona pellucida, ooplasm vacuolation. Another study found that mice exposed to ELF-EMF for 4 h/d during pregnancy, microscopic examination revealed that the oocyte’s nests were irregularly arranged, and the primordial follicles were undeveloped as well as ooplasm vacuolation was observed. The oocyte changes and the separation of granulosa cells from neighboring cells create the main feature of atretic follicle and characteristic of zona pellucida apoptosis with theca interna vacuolation [31].
Our findings showed that EMF 1 800 MHz frequency increases the oocyte degenerative changes and autophagy apoptosis in granulosa cells, which is in consistence with other study [32]. In the exposed animals, oocytes’ nuclei had the irregular shape with micronuclei formation and dark chromatin condensation and had several autophagy granulocytes with different size and shape. This is in consistent with previous researches [33,34]. The production of ova results from organized sequence of events in the folliculogenesis involving oocyte, granulosa cells and theca interna and externa cells. During folliculogenesis, the follicles not eclectic for the process of ovulation get rid of physiologically by apoptosis and/or autophagy, which is considered as another type of programmed cell death [35]. GSH-Px is an antioxidant enzyme that participates by using reduced glutathione as a hydrogen donor in the process of excluding H2O2and lipid hydroperoxides and reduces peroxides to reduce the oxidative damage. The reduction in GSH-Px activity due to over
consumption of reduced glutathione and reflected on the increase in MDA level that mean tissue oxidation was occurred.
Reduction of melatonin, a potent free radical’s scavenger, decreased GSH-Px activity and development of oxidative damage may contribute to increase lipid peroxidation of ovarian tissue [36,37].
The mechanisms of incidence of DNA damage and follicular atresia are not well known, which can be proposed by free radical’s production and leads to activation of the apoptotic process [38]. Theca interstitial cells are sensitive to free radicals’ level and reduction in antioxidant triggers apoptosis and antioxidants with precisely different mechanisms of action activate a course of actions consistent with the apoptosis mechanism in ovarian mesenchyme [39]. Endometrial bio-activities may be involved oxidative stress, which induces macrophage’s activation leading to excessive production of reactive oxygen and nitrogen species. These free radicals interact with low-density lipoproteins and other proteins leading to produce MDA [40-42]. Our findings showed that MDA level in uterine tissue was elevated after 60 d of exposure to EMF, suggesting involvement of lipid peroxidation as end product of oxidative damage and accompanied by decrease antioxidant enzyme GSH-Px activity. This is in consistence with previous studies [43-45]. In our study, we found that mobile phone radiation increases the height of the uterine luminal epithelium cells, which is consistent with an experimental study on mice exposed to the extremely low-frequency field (50 Hz, 4 h/d, 6 d a week for 2 weeks). The experiment indicates an increase in height of luminal epithelium layer of Fallopian tubes and decrease in flushed blastocysts contributed to an imbalance in the function of the female reproductive system [24].
Previous studies found that electromagnetic fields cause a decrease in oestrogen level in rats due to low level of FSH after exposure to EMF, which reduce ovarian follicle capacity to reach the developmental stage that is essential for reproductive success and cause alteration in oestrogen secretion that exert many biological effects throughout the body [46,47]. But physiologically the increase in height of the luminal epithelium occurred as a result of oestrogen effect on uterine tissue causing an increase in cell proliferation and size and inducing uterine growth [48-50]. This indicates presence of an unknown mechanism resulting from involving other factors that effect on luminal epithelium proliferation and growth, finally resulting in increased height of luminal epithelium cells. Therefore, further investigations to clarify the correct mechanism that contributed to the increase luminal epithelial layer growth.
The present study revealed that EMF causes cellular inflammatory reaction composed of infiltrative neutrophils, lymphocytes, severe diffuse eosinophils in endometrium and perimetrium layers with apoptosis and desquamation of luminal and glandular epithelium. This in consistent with previous results [22], which proved that the EMF induced diffuse and severe apoptosis in glandular and luminal epithelium cells and diffused eosinophils, leucocytes and lymphocyte’s infiltration. Several factors were involved in endometritis, including red blood cell’s damage, apoptotic endometrial cells, cellular debris and some inflammatory factors. All these factors contribute in activation of polymorphonuclear cells and macrophage, which might be stimulated by immune response or free radicals [51]. Our data suggest that the potential alteration of antioxidant capacity associated with excessive production of free radicals may contribute to endometrial oxidative damage, which could be related to the pathogenesis and progression of endometritis. Further studies need to evaluate the correlation between other factors such as interleukins, vascular endothelial growth factor, tumor necrosis factor, granulosa cell’s apoptosis and leucocyte activity and pathological changes of uterine tissue under effect of radiofrequency electromagnetic radiation emitted from mobile phone.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The project was fully supported by Faculty of Veterinary Medicine of University Malaysia Kelantan. The authors are indebted to our histologist (prof. Dr. Zahirul Islam) and research assistants at the histopathology laboratory for their help.
[1] Burlaka A, Tsybulin O, Sidorik E, Lukin S, Polishuk V, Tsehmistrenko S, et al. Overproduction of free radical species in embryonal cells exposed to low intensity radiofrequency radiation. Exp Oncol 2013; 35(3): 219-225.
[2] Haghdoost S. Biomarkers of oxidative stress and their application for assessment of individual radiosensitivity. Stockholm University , Stockholm, Sweden; 2005.
[3] Markkanen ARI. Effects of electromagnetic fields on cellular responses to agents causing oxidative stress and DNA damage. University of Kuopio, Kuopio, Finland; 2009.
[4] Mattsson MO, Simkó M. Grouping of experimental conditions as an approach to evaluate effects of extremely low-frequency magnetic fields on oxidative response in in vitro studies. Frontiers in public health. 2014;2.
[5] Yakymenko I, Tsybulin O, Sidorik E, Henshel D, Kyrylenko O, Kyrylenko S. Oxidative mechanisms of biological activity of low-
intensity radiofrequency radiation. Electromagnetic biology and medicine. 2015; Jul (9):1-7.
[6] Tök L, Nazıro lu M, Do an S, Kahya MC, Tök O. Effects of melatonin on Wi-Fi-induced oxidative stress in lens of rats. Indian J Ophthalmol 2014; 62(1): 12-15.
[7] Ozgur E, Kismali G, Guler G, Akcay A, Ozkurt G, Sel T, et al. Effects of prenatal and postnatal exposure to GSM-like radiofrequency on blood chemistry and oxidative stress in infant rabbits, an experimental study. Cell Biochem Biophys. 2013; 67(2): 743-751.
[8] Kismali G, Ozgur E, Guler G, Akcay A, Sel T, Seyhan N. The influence of 1800 MHz GSM-like signals on blood chemistry and oxidative stress in non-pregnant and pregnant rabbits. Int J Radiat Biol 2012; 88: 414-419.
[9] Kerman M, Senol N. Oxidative stress in hippocampus induced by 900 MHz electromagnetic field emitting mobile phone : Protection by melatonin. Biomed Res 2012; 23(1): 147-151.
[10] Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 2007; 35: 1147-1150.
[11] Al-Akhras MA. Influence of 50 Hz magnetic field on sex hormones and body, uterine, and ovarian weights of adult female rats. Electromagn Biol Med 2008; 27(2): 155-163.
[12] Gul A, Çelebi H, Uǧra S. The effects of microwave emitted by cellular phones on ovarian follicles in rats. Arch Gynecol Obstet 2009; 280(5): 729-733.
[13] Vahid HJ, Khatereh D, Esmaeal F, Maryam N, Mohammad F. The effects of mobile phone waves on the reproductive physiology in adult female rats. Adv Enviromental Biol 2012; 6(10): 2735-2741.
[14] Devrim E, Ergüder IB, Kılıço lu B, Yayka lı E, Cetin R, Durak I. Effects of electromagnetic radiation use on oxidant/antioxidant status and DNA turn-over enzyme activities in erythrocytes and heart, kidney, liver, and ovary tissues from rats: Possible protective role of vitamin c. Toxicol Mech Methods 2008; 18(9): 679-683.
[15] Akdag MZ, Dasdag S, Uzunlar AK, Ulukaya E, Oral AY, Çelik N, et al. Can safe and long-term exposure to extremely low frequency (50 Hz) magnetic fields affect apoptosis, reproduction, and oxidative stress? Int J Radiat Biol 2013; 89(12): 1053-1060.
[16] Bayat PD, Ghanbari A, Babaei S, Khazaei M, Ghorbani R, Ayubian M. Effect of exposure to extremely low electro-magnetic field during prenatal period on mice spleen. Indian J Exp Biol 2011; 49: 634-638.
[17] Gathiram P, Kistnasamy B, Lalloo U. Effects of a unique electromagnetic field system on the fertility of rats. Arch Environ Occup Health 2009; 64(2): 93-100.
[18] Poulletier de Gannes F, Billaudel B, Haro E, Taxile M, Le Montagner L, Hurtier A, et al. Rat fertility and embryo fetal development: influence of exposure to the Wi-Fi signal. Reprod Toxicol 2013; 36: 1-5.
[19] Aydogan F, Unlu I, Aydin E, Yumusak N, Devrim E, Samim EE, et al. The effect of 2100 MHz radiofrequency radiation of a 3G mobile phone on the parotid gland of rats. Am J Otolaryngol 2015; 36(1): 39-46.
[20] Aït-Aïssa S, Billaudel B, Poulletier de Gannes F, Ruffié G, Duleu S, Hurtier A, et al. In utero and early-life exposure of rats to a Wi-Fi signal: Screening of immune markers in sera and gestational outcome. Bioelectromagnetics 2012; 33(5): 410-420.
[21] Misa Agustiño MJ, Leiro JM, Jorge Mora MT, Rodríguez-González JA, Jorge Barreiro FJ, Ares-Pena FJ, et al. Electromagnetic fields at 2.45 GHz trigger changes in heat shock proteins 90 and 70 without altering apoptotic activity in rat thyroid gland. Biol Open 2012; 1(9): 831-838.
[22] Guney M, Ozguner F, Oral B, Karahan N, Mungan T. 900 MHz radiofrequency-induced histopathologic changes and oxidative stress in rat endometrium: protection by vitamins E and C. Toxicol Ind Health 2007; 23(7): 411-420.
[23] Rajaei F, Farokhi M, Ghasemi N, Pahlevan AA. Effects of extremely lowfrequency magnetic field on mouse epididymis and deferens ducts. Iran J Reprod Med 2009; 7(2): 85-89.
[24] Rajaei F, Borhani N, Sabbagh-Ziarani F, Mashayekhi F. Effects of extremely low-frequency electromagnetic field on fertility and heights of epithelial cells in pre-implantation stage endometrium and fallopian tube in mice. J Chin Integr Med 2010; 8: 56-60.
[25] Khaki A, Khaki AA, Ezzatzadeh A, A-Ashteani H. Effect of Ocimum basilicum on ovary tissue histopathology after exposure to electromagnetic fields (EMF) in rats. Afr J Pharm pharmacol 2013; 7(25): 1703-1706.
[26] Gharamaleki H, Parivar K, Rad JS, Roshangar L, Shariati M. Effects of low-frequency electromagnetic field exposure during the prenatal period on biomarkers of oxidative stress and pathology of ovarian tissue in F 1. Int J Cur Res Rev 2013; 5(21): 23-29.
[27] Alchalabi ASH, Aklilu E, Aziz AR, Malek F, Ronald SH, Khan MA. Different periods of intrauterine exposure to electromagnetic field: Influence on female rats’ fertility, prenatal and postnatal development. Asian Pacific J Reprod 2015; 5(1): 14-23.
[28] Botsoglou NA, Fletouris DJ, Papageorgiou GE, Vassilopoulos VN, Mantis AJ, Trakatellis AG. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J Agric Food Chem 1994; 42(9): 1931-1937.
[29] Güler G, Tomruk A, Ozgur E, Sahin D, Sepici A, Altan N, et al. The effect of radiofrequency radiation on DNA and lipid damage in nonpregnant and pregnant rabbits and their newborns. Int J Radiat Biol 2012; 88(4): 367-373.
[30] Guler G, Tomruk A, Ozgur E, Seyhan N. The effect of radiofrequency radiation on DNA and lipid damage in non-pregnant and pregnant rabbits and their newborns. Gen Physiol Biophys 2010; 29(1): 59-66.
[31] Roshangar L, Hamdi BA, Khaki AA, Rad JS, Soleimani-Rad S. Effect of low-frequency electromagnetic field exposure on oocyte differentiation and follicular development. Adv Biomed Res 2014; 3: 76.
[32] Cecconi S, Gualtieri G, Bartolomeo A Di, Giulia Troiani MGC, Canipari R. Evaluation of the effects of extremely low frequency electromagnetic fields on mammalian follicle development. Hum Reprod 2000; 15(11):
2319-2325.
[33] Kheradmand A, Roshangar L, Taati M, Sirotkin AV. Morphometrical and intracellular changes in rat ovaries following chronic administration of ghrelin. Tissue Cell 2009; 41(5): 311-317.
[34] Kowalczuk CI, Robbins L, Thomas JM, Butland BK, Saunders RD. Effects of prenatal exposure to 50 Hz magnetic fields on development in mice: I. Implantation rate and fetal development. Bioelectromagnetics 1994; 15(4): 349-361.
[35] M.L. Escobar, O. M. E. and G. H. V.-N. Autophagy - A Double-Edged Sword - Cell Survival or Death? (Y. Bailly, Ed.) 2013. InTech. http://doi. org/10.5772/50855
[36] Chernoff N, Rogers JM, Kavet R. A review of the literature on potential reproductive and developmental toxicity of electric and magnetic fields. Toxicology 1992; 74(2-3): 91-126.
[37] Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicular-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocriniolgy 1995; 136: 242-252.
[38] Hughes FM, Gorospe WC. Biochemical identification of apoptosis (programmed cell death) in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology 1991; 129(5): 2415-2422.
[39] Rzepczynska IJ, Foyouzi N, Piotrowski PC, Celik-Ozenci C, Cress A, Duleba AJ. Antioxidants induce apoptosis of rat ovarian theca-interstitial cells. Biol Reprod 2011; 84(1): 162-166.
[40] Szczepanska M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril 2003; 79(6): 1288-1293.
[41] Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online 2006; 13(1): 126-134.
[42] Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 21: 1-21.
[43] Tkalec M, Stambuk A, Srut M, Malari K, Klobu ar GI. Oxidative and genotoxic effects of 900 MHz electromagnetic fields in the earthworm Eisenia fetida. Ecotoxicol Environ Saf 2013; 90: 7-12.
[44] Ibrahim NK, Gharib OA. The protective effect of antioxidants on oxidative stress in rats exposed to the 950 MHz electromagnetic field. J Radiat Res Appl Sci 2010; 3(4): 1143-1155.
[45] Avci B, Akar A, Bilgici B, Tunçel ÖK. Oxidative stress induced by 1.8 GHz radio frequency electromagnetic radiation and effects of garlic extract in rats. Int J Radiat Biol 2012; 88(11): 799-805.
[46] Hajiuon B. Effects of cell phone radiation on estrogen and progesterone levels and ovarian changes in rats treated with garlic (Allium sativum L.) hydro-alcoholic extract. J Herb Drugs 2013; 4(2): 81-88.
[47] Al-Akhras MA, Darmani H, Elbetieha A. Influence of 50 Hz magnetic field on sex hormones and other fertility parameters of adult male rats. Bioelectromagnetics 2006; 27(2): 127-131.
[48] Davoudi M, Zavareh S, Ghorbanian MT, Hassanzadeh H. Effects of steroid hormones on uterine tissue remodeling of mouse menopause model. J Paramed Sci 2008; 6(1): 65-71.
[49] Peyghambari F, Salehnia M, Moghadam MF, Valujerdi MR, Ebrahim H. The changes in morphology and morphometrical indices of endometrium of ovariectomized mice in response to exogenous ovarian hormones. Iran J Reprod Med 2008; 6(3): 125-131.
[50] Pollard RM, Finn CA. The effect of ovariectomy at puberty on cell proliferation and differentiation in the endometrium of the aged mouse. Biol Reprod 1974; 10(1): 74-77.
[51] Barcelos, Ionara, and Paula Navarro. Endometriosis and Infertility: The Role of Oxidative Stress. INTECH Open Access Publisher, 2012: 399-416.
1. Introduction
ment heading
10.1016/j.apjr.2016.06.008
*Corresponding author: Ali S. H. Alchalabi, Faculty of Veterinary Medicine, UMK City Campus, Pengkalan Chepa, Locked Bag36, 16100 Kota Bharu, Kelantan, Malaysia.
Phone No: +60112947731
E-mail: alisaeedchalaby@yahoo.com
Electromagnetic radiation
Global System for Mobile Communication (GSM) is one of the operating systems used in cellular phone’s communications. Our cellular phones are emitting electromagnetic waves (EMW) which are non-ionizing radiation in its nature. This mean does not have an ability to ionize the water and macromolecules in human or animal tissues, in contrast to the ionizing radiation such as x-ray. Logically, this is right, but now the researchers’ data change this opinion due to of many studies were conducted about the non-thermal action of EMWs mediated by oxidative stress or other mediators on living tissues [1-5]. Some of the recent studies have performed to explain the role of oxidative stress as the harmful effect on living tissues induced by GSM signals [6-9]. Oxidative stress is a status result due to imbalance between oxidant agents and antioxidant system leading to production of free radicals who are responsible for oxidative damage to proteins, lipids and DNA in the body [10]. Impact of EMW on the effectiveness of the reproductive tract and its direct
 Asian Pacific Journal of Reproduction2016年4期
Asian Pacific Journal of Reproduction2016年4期
- Asian Pacific Journal of Reproduction的其它文章
- Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters
- Evaluation of the academic achievement of rural versus urban undergraduate medical students in pharmacology examinations
- The relationship between trace mineral concentrations of amniotic fluid with placenta traits in the pregnancy toxemia Ghezel ewes
- Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation
- Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle
- Spontaneous pregnancy after vaginoplasty in a patient presenting a congenital vaginal aplasia
