35岁及以下年轻乳腺癌患者的临床资料分析
崔芳,刘红△,陆苏,于辰华,宋佳,方志沂
35岁及以下年轻乳腺癌患者的临床资料分析
崔芳1,刘红1△,陆苏1,于辰华1,宋佳2,方志沂1
目的分析35岁及以下年轻乳腺癌患者的临床病理特征、生存情况及影响预后的因素。方法回顾性分析天津医科大学肿瘤医院2008年1月—12月收治的105例治疗规范的年龄≤35岁年轻乳腺癌患者(年轻组)的临床及病理资料,并与同年收治的103例治疗规范的65~70岁老年乳腺癌患者(老年组)进行比较,并对患者的预后情况进行随访。结果年轻组随访期间有25例(23.8%)出现局部复发或转移,17例(16.2%)死亡;老年组有8例(7.8%)出现局部复发或转移,9例(8.7%)死亡。与老年组相比,年轻组初诊时原发肿瘤直径大,组织学分级高,P53突变率较高(均P<0.05)。年轻组5年无病生存率低于老年组(76.2%vs.92.2%,Log-rank χ2=9.799,P=0.002),5年总生存率与老年组差异无统计学意义(83.8%vs.91.3%,Log-rank χ2=2.758,P=0.097)。Cox多因素分析结果显示,肿瘤直径、是否有腋窝淋巴结转移是影响年轻组乳腺癌患者5年无病生存率的独立预后因素,而P53阳性与否和是否有腋窝淋巴结转移是影响年轻组乳腺癌5年总生存率的独立预后因素。结论年轻乳腺癌患者的生物学行为恶性程度更高,预后更差。因此,对乳腺癌高风险年轻女性需采用针对性的预防筛查策略,制定个体化治疗方案。
乳腺肿瘤;预后;临床病理;年轻患者
乳腺癌是女性最常见的恶性肿瘤,在女性中其死亡率仅次于肺癌[1]。随着饮食结构的改变、现代女性社会压力的增大,乳腺癌的发病率呈逐年上升趋势。国内乳腺癌发病集中于50~55岁,较美国提早约10年[2-3]。虽然乳腺癌多发生在绝经后,但仍有不少年轻女性遭受乳腺癌的折磨。相关资料显示年轻乳腺癌患者占乳腺癌患病人群的3%~20%[4-6],目前研究普遍认为年轻乳腺癌分化低、病期晚、雌激素受体(estrogen receptor,ER)阴性率高及人类表皮生长因子受体2(human epidermal growth factor receptor-2,HER-2)阳性率高[7-10],但对于年轻乳腺癌与老年乳腺癌的预后差异及年龄是否为影响乳腺癌预后的因素仍有不同观点[5-6,11],且对年轻的定义国际上尚有争议,有大型流行病学研究报道35岁为合理的界限[12]。本研究就本院2008年全年进行乳腺癌手术且治疗规范的35岁及以下女性乳腺癌患者的临床病理特征及生存状态进行分析。
1 资料与方法
1.1 病例资料天津医科大学肿瘤医院2008年1月—12月收治原发性乳腺癌患者共2 178例,其中≤35岁的患者133例(6.1%),排除治疗不规范(治疗不规范是指术后辅助化疗、内分泌治疗及必要的放疗未做或未完成)、临床病理资料和随访资料不完整者28例,最终年轻组入组105例,年龄23~35岁,平均(31.5±3.3)岁;另择同期收治的老年乳腺癌患者103例为老年组,年龄60~75岁,平均(66.7±5.1)岁。所有病例均经病理学确诊为原发性乳腺癌,治疗方式包括手术、化疗、放疗和内分泌治疗。
1.2 指标收集根据病例记载收集患者年龄、初产年龄、初潮年龄、体质指数(BMI)、肿瘤家族史、病理类型、肿瘤大小、组织学分级、淋巴结转移情况及分子分型等资料。分子分型的判断标准采取2011年圣加伦会议的专家共识:ER和孕激素受体(progestogen receptor,PR)阳性的定义为免疫组织化学结果中有≥1%的细胞核染色阳性,P53的阳性定义为免疫组织化学结果中有≥10%的细胞核染色阳性。血管内皮生长因子(VEGF)阳性定义为≥30%的细胞胞浆棕黄色颗粒着色。
1.3 随访通过电话、信件、门诊等方式随访,截至2013年12月。患者的死亡定义为乳腺癌相关性死亡,出现的复发、转移均需经过病理学的确诊。无病生存期(disease free survival,DFS)定义为患者手术日至出现复发转移的时间;总生存期(overall survival,OS)定义为患者初次确诊乳腺癌至死亡或随访截止的时间。
1.4 统计学方法采用SPSS 19.0软件进行统计学分析。计量资料以均数±标准差表示,2组间比较采用t检验;计数资料组间比较采用χ2检验。采用Kaplan-Meier法进行生存分析,并采用Cox比例风险模型进行预后的多因素分析。P<0.05为差异有统计学意义。
2 结果
2.1 2组临床病理特征比较年轻组初潮年龄较老年组早,BMI≥24 kg/m2者比例较老年组低;年轻组原发肿瘤直径>5 cm、组织学分级Ⅲ级、HER-2免疫组化(++/+++)和P53阳性患者比例较老年组高;2组其余指标差异均无统计学意义,见表1。年轻组乳腺癌患者中,接受乳腺癌改良根治术80例,保乳手术20例,其他术式(乳腺癌根治术合并再造术或假体植入术)5例;老年组接受改良根治术97例,保乳术(乳腺肿瘤切除加腋窝淋巴结清扫)3例,其他术式(乳腺局部扩大切除术或乳房全切术)3例。

Tab.1Comparison of clinicopathologic characteristics between two groups of patients with breast cancer表1 年轻乳腺癌与老年乳腺癌临床病理特征的比较
2.2 2组生存情况比较年轻组患者的随访时间为21~72个月,中位随访时间64个月,随访期间有25例(23.8%)出现局部复发或转移,17例(16.2%)死亡;老年组患者的随访时间为18~72个月,中位随访时间66个月,随访期间有8例(7.8%)患者出现局部复发或转移,9例(8.7%)死亡。年轻组5年无病生存率低于老年组(76.2%vs.92.2%,Log-rank χ2= 9.799,P=0.002),5年总生存率与老年组差异无统计学意义(83.8%vs.91.3%,Log-rank χ2=2.758,P= 0.097),见图1、2。

Fig.1Kaplan-Meier analysis of disease-free survival between two groups of patients图1 年轻组与老年组DFS比较

Fig.2Kaplan-Meier analysis of overall survival between two groups of patients图2 年轻组与老年组OS比较
2.3 年轻乳腺癌患者不同年龄组临床病理特征比较将105例年轻乳腺癌患者分成≤25岁、26~30岁、31~35岁组进行比较,3组间不同临床病理特征差异无统计学意义,且3组间5年无病生存率(分别是66.7%、66.7%和81.8%)与5年总生存率(分别是83.3%、75.8%和87.9%)差异均无统计学意义(Logrank χ2分别为3.362、2.233,P>0.05),见表2,图3、4。

Fig.3Kaplan-Meier analysis of disease-free survival between different age groups in young patients图3 年轻乳腺癌不同年龄组的DFS
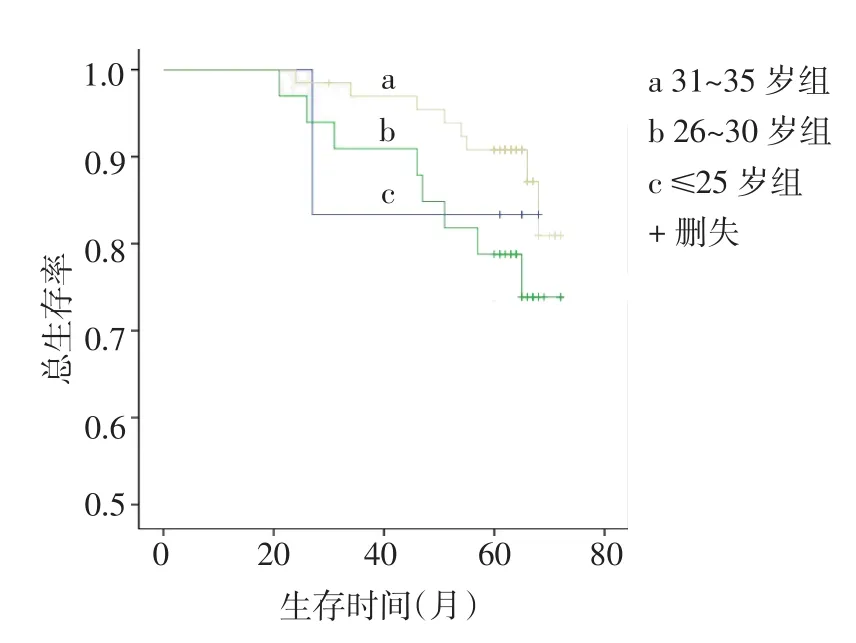
Fig.4Kaplan-Meier analysis of overall survival between different age groups of young patients图4 年轻乳腺癌不同年龄组的OS
2.4 影响年轻乳腺癌预后的因素分析Kaplan-Meier单因素分析结果显示,P53阳性与否和是否有腋窝淋巴结转移是影响年轻组乳腺癌患者5年无病生存率和总生存率的预后因素;肿瘤直径是影响患者5年无病生存率的预后因素,但不是影响OS的预后因素,见表3。对单因素分析中有统计学意义的变量采用前进法进行Cox回归分析,结果显示肿瘤直径、是否有腋窝淋巴结转移是影响年轻组乳腺癌患者5年无病生存率的独立预后因素,而P53阳性与否和是否有腋窝淋巴结转移是影响年轻组乳腺癌患者5年总生存率的独立预后因素,见表4。

Tab.2Comparison of clinical and pathological features between different age groups of young patients with breast cancer表2 年轻乳腺癌不同年龄组间临床病理特征的比较例(%)

Tab.3Univariate analysis of prognostic factors affecting 5-year survival rate in the young group表3 年轻乳腺癌5年生存率的单因素分析

Tab.4Multivariate analysis of prognostic factors affecting 5-year survival of the young group表4 年轻乳腺癌5年生存率多因素分析
3 讨论
国内外乳腺癌多发病于绝经后,年轻乳腺癌(≤35岁)并不多见,据相关文献统计约占3%~20%[4-6],本研究中年轻乳腺癌占6.1%,与文献报道基本符合。虽然多数研究显示年轻患者生存期短,但年龄是否为乳腺癌的独立预后因素目前仍存在争议,本研究中年轻组5年无病生存率低于老年组,5年总生存率与老年组无明显差异,此结果与施晓通等[11]的研究结果一致;而导致年轻患者预后较差的原因很可能与其不利的临床病理特征和分子分型有关。国内外多数文献报道年轻患者肿瘤直径大,组织学分化低,淋巴结转移率高,ER、PR阳性率低,HER-2阳性率高[7-10]。本研究中,年轻组HER-2阳性率高于老年组,但2组ER、PR阳性率未见明显差异;另外,年轻组P53阳性表达率较老年组高且为年轻乳腺癌生存的独立预后因素,虽然国内文献对此报道较少,但国外却有年轻乳腺癌P53的突变率更高的相关报道[13-15],但本研究样本量较小,相关结论仍需大样本研究证实。本研究年轻乳腺癌患者不同年龄组的临床病理特征和生存率差异均无统计学意义,这与Collins等[16]的研究结果一致。
目前值得关注的是,随着生育年龄的推迟和国家政策的变化,年轻乳腺癌患者确诊时还面临着未生育或仍有生育计划的问题。针对乳腺癌的各项治疗如手术、化疗、放疗及内分泌治疗均会对年轻患者的生育带来不同程度的影响,且生育对患者的预后也会有所影响。因此保护患者卵巢功能,恢复患者生育能力是首要解决的问题,目前的方法有辅助生殖技术、治疗过程中保护卵巢功能和卵巢组织冷冻保存;各项治疗中化疗对于卵巢功能的影响最大,因此在化疗过程中加用保护卵巢功能的药物[如促性腺激素释放激素(GnRH)激动剂],不仅可以使ER/ PR阳性乳腺癌患者生存获益,而且治疗结束后可以恢复患者月经和生育能力。因此对于年轻、肿瘤分期较早,估计治疗结束后丧失生育能力可能性不大的患者,在化疗过程中可选择使用GnRH来保护卵巢;而年龄相对较大,治疗后卵巢功能丧失可能性较大的患者,可选择人工辅助生殖技术[17-18]。而治疗后妊娠的时机是接下来面临的问题,相关研究显示术后妊娠对乳腺癌预后并无消极影响,并建议依据复发风险来决定妊娠时机,低危组患者建议2年后妊娠,高危组患者建议5年后妊娠[19-20]。且乳腺癌患者的新生儿与正常人群新生儿相比,不良事件发生率并没有明显增加,但相关研究同时指出新生儿分娩时的并发症、剖宫产率、早产儿、低体质量儿及畸形率均较正常人群新生儿有所增加[21]。乳腺癌患者保乳术后患侧乳汁分泌会减少,但乳汁安全性并没有问题,且术后哺乳并不会影响预后,甚至与更好的预后有一定关系[22-24]。
另外,随着人类基因组计划的实现及生物信息的数据化,精准医学应运而生,携带乳腺癌相关突变基因的女性乳腺癌的发病率会提高、发病时间提前。除了熟知的BRCA1/BRCA2突变外,还有PALB2、ATM和CHEK2等基因的突变与乳腺癌的发生发展有关[25]。最新的中国抗癌协会乳腺癌筛查指南推荐从40岁开始对女性进行常规筛查,但是对于乳腺癌高危人群有必要将年龄提前,因此除了有乳腺癌家族史、乳腺导管或小叶中重度不典型增生和胸部放疗史者,对于采用基因检测技术筛查出有乳腺癌相关突变基因的女性亦有必要提前预防。
年轻乳腺癌复发窗口期较长,因此需更多的治疗手段来控制复发转移,目前对乳腺癌的治疗主要还取决于肿瘤的分期及分子分型,年龄相关的其他指标的改变或许可以成为个体化治疗努力的方向。最新文献报道了年轻组与老年组女性乳腺癌基因组方面的改变,随着年龄的增长,体细胞的突变及染色体拷贝数的改变也增加,在老年女性乳腺癌患者中KMT2D及FOXA1突变率较高[26];而在年轻女性乳腺癌中GATA3的突变率较高,且该基因与ER复合体有关联,其突变可能会影响ER的转录活性[27-28],调节乳腺癌细胞雌激素调节通路,GATA3与luminal型的乳腺癌内分泌治疗有关[29]。因此对年轻乳腺癌特殊突变的位点进行调控不失为一种有效手段。
综上所述,年轻组乳腺癌患者较老年组患者具有更多的不良预后因素,如肿瘤直径大,淋巴结转移率高及组织分化差等;但本研究中年轻组5年总生存率与老年组无明显差异,而对于年轻乳腺癌患者的生育问题及综合治疗手段仍需更深入的研究。
[1]Siegel RL,Miller KD,Jemal A.Cancer statistics,2015[J].CA Cancer J Clin,2015,65(1):5-29.
[2]Yang HJ,Yu XF,He XM,et al.Age interactions in breast cancer:an analysis of a 10-year multicentre study in China[J].J Int Med Res,2012,40(3):1130-1140.
[3]Zeng H,Zheng R,Zhang S,et al.Female breast cancer statistics of 2010 in China:estimates based on data from 145 population-based cancer registries[J].J Thorac Dis,2014,6(5):466-470.
[4]Cancello G,Maisonneuve P,Rotmensz N,et al.Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women(<35 years)with operable breast cancer[J].Ann Oncol,2010,21(10):1974-1981.
[5]Rapiti E,Fioretta G,Verkooijen HM,et al.Survival of young and older breast cancer patients in Geneva from 1990 to 2001[J].Eur J Cancer,2005,41(10):1446-1452.
[6]Yang H,Wang SY,Qu W,et al.Clinical characteristics and prognosis of very young patients with breast cancer in the southern of China[J].Chinese Journal of Cancer,2009,28(12):1310-1316.[杨桦,王思愚,区伟,等.华南地区年轻乳腺癌患者的临床特征及预后因素分析[J].癌症,2009,28(12):1310-1316].
[7]Cuan Martínez JR,Mainero Ratchelous FE,Aguilar Gallegos IU,et al.Comparative study of clinical and pathological features of breast cancer in women with 40 years old and younger vs 70 years old and older[J].Ginecol Obstet Mex,2008,76(6):299-306.
[8]Goksu SS,Tastekin D,Arslan D,et al.Clinicopathologic features and molecular subtypes of breast cancer in young women(age</= 35)[J].Asian Pac J Cancer Prev,2014,15(16):6665-6668.
[9]Kwong A,Cheung P,Chan S,et al.Breast cancer in Chinese women youngerthanage40:aretheydifferentfromtheirolder counterparts?[J].World J Surg,2008,32(12):2554-2561.
[10]Zhang Q,Ma B,Kang M.A retrospective comparative study of clinicopathological features between young and elderly women with breast cancer[J].Int J Clin Exp Med,2015,8(4):5869-5875.
[11]Shi XT,Shen JG,Wang LB.The clinical features of breast cancer in young patients[J].Tumor,2014,34(12):1138-1143.[施晓通,沈建国,王林波.年轻乳腺癌患者临床特征的研究[J].肿瘤,2014,34(12):1138-1143].
[12]Han W,Kang SY.Relationship between age at diagnosis and outcome of premenopausal breast cancer:age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer[J]. Breast Cancer Res Treat,2010,119(1):193-200.
[13]Sabiani L,Houvenaeghel G,Heinemann M,et al.Breast cancer in young women:Pathologic features and molecular phenotype[J]. Breast,2016,29:109-116.doi:10.1016/j.breast.2016.07.007.
[14]Lin CH,Lu YS,Huang CS,et al.Prognostic molecular markers in women aged 35 years or younger with breast cancer:is there a difference from the older patients?[J].J Clin Pathol,2011,64(9):781-787.
[15]Sidoni A,Cavaliere A,Bellezza G,et al.Breast cancer in young women:clinicopathological features and biological specificity[J]. Breast,2003,12(4):247-250.
[16]Collins LC,Marotti JD,Gelber S,et al.Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer[J].Breast Cancer Res Treat,2012,131(3):1061-1066.
[17]Sun RH,Su XL,Wu KN.Fertility problems of young patients with breast cancer[J].Chin J Clin Onco,2013,40(23):1468-1472.[孙荣华,苏新良,吴凯南.年轻乳腺癌患者的生育问题[J].中国肿瘤临床,2013,40(23):1468-1472].doi:10.3969/j.issn.1000-8179.20130815.
[18]Zheng DP,Dong X,Wang XJ.Fertility plan,knowledge and decision-makingpreferences in young breast cancer women[J]. Journal of Nursing,2015,30(22):31-34.[郑丹萍,董鑫,王晓晶.年轻乳腺癌患者生育计划及知识与治疗决策的研究[J].护理学杂志,2015,30(22):31-34].doi:10.3870/j.issn.1001-4152. 2015.22.031.
[19]Chabbert-Buffet N,Uzan C,Gligorov J,et al.Pregnancy after breast cancer:a need for global patient care,starting before adjuvant therapy[J].Surg Oncol,2010,19(1):e47-55.
[20]Largillier R,Savignoni A,Gqligorov J,et al.Prognostic role of pregnancy occurring before or after treatment of early breast cancer patients aged<35 years:a GET(N)A Working Group analysis[J]. Cancer,2009,115(22):5155-5165.
[21]Dalberg K,Eriksson J,Holmberg L.Birth outcome in women with previously treated breast cancer——a population-based cohortstudy from Sweden[J].PLoS Med,2006,3(9):e336.
[22]Azim HA Jr,Bellettini G,Gelber S,et al.Breast-feeding after breast cancer:if you wish,madam[J].Breast Cancer Res Treat,2009,114(1):7-12.doi:10.1007/s10549-008-9983-7.
[23]Goetz O,Burgy C,Langer C,et al.Breastfeeding after breast cancer:survey to hospital health professionals in Alsace[J]. Gynecol Obstet Fertil,2014,42(4):234-239.
[24]Higgins S,Haffty BG.Pregnancy and lactation after breastconserving therapy for early stage breast cancer[J].Cancer,1994,73(8):2175-2180.
[25]Couch FJ,Nathanson KL,Offit K.Two decades after BRCA:setting paradigms in personalized cancer care and prevention[J].Science,2014,343(6178):1466-1470.
[26]Azim HA Jr,Nguyen B,Brohée S,et al.Genomic aberrations in young and elderly breast cancer patients[J].BMC Med,2015,13:266.
[27]Chou J,Provot S,Werb Z.GATA3 in development and cancer differentiation:cells GATA have it![J].J Cell Physiol,2010,222(1):42-49.
[28]Liu Z,Merkurjev D,Yang F,et al.Enhancer activation requires trans-recruitment of a mega transcription factor complex[J].Cell,2014,159(2):358-373.
[29]Adomas AB,Grimm SA,Malone C,et al.Breast tumor specific mutation in GATA3 affects physiological mechanisms regulating transcription factor turnover[J].BMC Cancer,2014,14:278.doi: 10.1186/1471-2407-14-278.
(2016-04-07收稿2016-07-05修回)
(本文编辑陈丽洁)
Analysis on the clinical data of breast cancer in women aged 35 years and younger
CUI Fang1,LIU Hong1△,LU Su1,YU Chenhua1,SONG Jia2,FANG Zhiyi1
1 The Second Department of Breast Cancer,Tianjin Medical University Cancer Institute and Hospital,National Clinical Research Center for Cancer;Tianjin Key Laboratory of Cancer Prevention and Therapy;Key Laboratory of Breast Cancer Prevention and Therapy,Tianjin Medical University,Ministry of Education,Tianjin 300060,China;2 Department of Breast Cancer,the Affiliated Cancer Hospital of Hebei University△
ObjectiveTo analyze the clinical and pathological characteristics and prognosis of breast cancer in women aged 35 years and younger.MethodsThe clinical data of 105 breast cancer patients(≤35 years)who received comprehensive standardized treatment in our hospital in 2008 were retrospectively analyzed.And the clinical data of 103 breast cancer patients aged 65-70 years treated in our hospital during the same year were chosen as control group.Results During the follow-up period,local recurrence and metastasis were found in 25 patients(23.8%),and dead in 17 cases (16.2%)in young group.In elderly group,local recurrence or metastasis were found in 8 patients(16.2%)and dead in 9 cases(8.7%).Compared with elderly group,the primary tumor diameter was large,the histological grade was high and the mutation rate of P53 was higher in the younger group(P<0.05).The 5-year disease-free survival rate was lower in the young group than that of the elderly group(76.2%vs.92.2%,Log-rank χ2=9.799,P=0.002).There was no significant difference in the 5-year overall survival rate between the two groups(83.8%vs.91.3%,Log-rank χ2=2.758,P=0.097).Cox multivariate analysis showed that tumor diameter and axillary lymph node metastasis were independent prognostic factors influencing 5-year disease-free survival rate in young group.The positive expression of P53 and axillary lymph node metastasis were independent prognostic factors influencing 5-year overall survival rate in young group.ConclusionOur study indicates that the histological grade of breast cancer is higher and the prognosis is even worse in young patients. Therefore,young women with the high risk of breast cancer need to be targeted for prevention and screening strategies to develop individualized treatment programs.
breast neoplasms;prognosis;clinical and pathological features;young patient
R737.9
A
10.11958/20160229
1天津医科大学肿瘤医院乳腺二科,国家肿瘤临床医学研究中心,天津市肿瘤防治重点实验室,乳腺癌防治教育部重点实验室(邮编300060);2河北大学附属医院乳腺外科
崔芳(1990),女,硕士在读,主要从事乳腺癌个体化治疗研究
△通讯作者E-mail:lh713@163.com

