电子垃圾拆解地翠鸟对多氯联苯的累积及风险评估
莫凌,吴江平,张云,邢巧,林彰文,罗孝俊,麦碧娴
1. 海南省环境科学研究院,海口 570100 2. 中国科学院广州地球化学研究所 有机地球化学国家重点实验室,广州 510640 3. 华南师范大学 生命科学学院,广州 510631
电子垃圾拆解地翠鸟对多氯联苯的累积及风险评估
莫凌1,吴江平2,,张云3,邢巧1,林彰文1,罗孝俊2,麦碧娴2
1. 海南省环境科学研究院,海口 570100 2. 中国科学院广州地球化学研究所 有机地球化学国家重点实验室,广州 510640 3. 华南师范大学 生命科学学院,广州 510631
粗犷的电子垃圾拆解活动已造成当地野生生物多氯联苯(PCBs)严重污染,但PCBs在野生鸟类中的生物累积特征及潜在的毒害作用研究较少。本研究采集了广东省某电子垃圾拆解地翠鸟(Alcedo atthis)及其食物(各种小型鱼类)样品,研究翠鸟对PCBs的累积特征、生物放大效应及毒性风险。翠鸟肌肉中PCBs中值含量为220 μg·g-1脂重,比其他报道值高1~3个数量级。计算的生物放大因子(BMF)显示,大部分PCB单体的BMF值都大于1,表明翠鸟对PCBs具有生物放大效应。计算的共面PCBs毒性当量(TEQs)范围为39~23 600 pg·g-1湿重,已经达到或超过了影响某些鸟类生殖或发育障碍的报道值。上述结果表明,电子垃圾拆卸活动已经造成了当地翠鸟PCBs严重污染,PCBs污染物对电子垃圾拆解地翠鸟及其他野生生物的毒性效应尚需进一步研究。
多氯联苯;鸟类;生物积累;生物放大;电子垃圾
Received 14 November 2015 accepted 22 December 2015
多氯联苯(polychlorinated biphenyls,PCBs)是一类人工合成的多氯芳香烃类化合物。由于PCBs具有物理化学性质稳定、耐热性、电绝缘性等特点,常用作热载体、绝缘材料、溶剂等广泛添加于电子电器等产品中[1]。这些产品在使用过程中或废弃后PCBs很容易进入到环境。研究证实,PCBs在环境中具有持久性、可生物累积性和较高的生物毒性[2-3]。2001年5月,PCBs被列入《斯德哥尔摩公约》持久性有机污染物(POPs)首批受控名单,在全球禁用[4]。
随着PCBs的禁用,全球PCBs主要生产和使用地区(主要为北美和欧洲)环境中PCBs含量显著下降[5]。然而,作为全球废弃电子电器产品(电子垃圾)主要集中地亚洲和非洲一些国家环境中PCBs含量却有上升的趋势[5-6]。PCBs在电子垃圾集中地的环境行为及其生态风险评估已引起了广泛关注。我们的初步研究发现,广东省某电子垃圾拆解地野生鱼类体内的PCBs含量很高[7],这些污染物是否传递到高营养级生物(如食鱼鸟)及其对这些生物可能的毒害作用尚不清楚。本研究在广东省某电子垃圾拆解地同时采集了翠鸟(Alcedo atthis)及其食物(3种小型鱼类)样品,通过测定这些样品中PCBs的含量,研究了翠鸟对PCBs的生物放大效应,并评估了这些污染物对翠鸟潜在的毒性效应。
1 材料与方法 (Materials and methods)
1.1 样品采集与前处理
翠鸟是一种食鱼性鸟类,主要是以其栖息地附近水体中各种小型鱼类为食[8]。翠鸟(A. atthis)样品(n = 22)于2010年5月到2010年7月在广东省某电子垃圾拆解地池塘边用网捕法采集,鸟类采集经广东省林业局批准。同时在池塘中采集了中国斗鱼(Macropodus opercularis,n = 9)、食蚊鱼(Gambusia affinis,n = 11)和马口鱼(Opsariichthys bidens,n = 9) 3种小型鱼类。鸟类样品采集后用N2进行安乐死。所有样品低温运输至实验室后,-20 ℃冷冻保存待分析。
翠鸟样品解剖后,取肌肉组织。每种鱼类样品(整鱼样)随机混合成3个混合样。取约4 g翠鸟肌肉组织和8 g鱼类样品,冷冻干燥并混匀后,加入回收率指示物CB 30、CB 65和CB 204后,用正己烷/丙酮混合溶剂(1/1,V/V)索氏抽提48 h。抽提液一部分用于测定脂肪含量(重量法)。余下抽提液浓缩至1 mL左右,经凝胶渗透色谱柱(GPC)去除脂肪和其他干扰物。洗脱液浓缩至1~2 mL后,过复合硅胶柱(酸性硅胶:中性硅胶=8:8)净化,最后浓缩定容至200 μL,加入内标(CB 24、CB 82和CB 198)。样品的抽提及净化具体方法参见文献[7]。
1.2 仪器测定
75种PCBs单体用安捷伦气相色谱质谱联用仪(Agilent 6890 GC-5975B Series MS)测定。采用EI源、选择性离子扫描模式(SIM),使用色谱柱DB-5MS (60 m × 0.25 mm i.d.,0.25 μm film thickness;J&W Scientific, Folsom,CA)进行分离。选择无分流进样,进样量为1 μL。载气为高纯氦气,柱流速为1.50 mL·min-1。升温程序:起始温度120 ℃,6 ℃·min-1升温至180 ℃,1 ℃·min-1升温至240 ℃,然后6 ℃·min-1升温至290 ℃并保留17 min。进样口、离子源温度和界面温度分别为290 ℃、250 ℃和290 ℃。目标化合物采用内标法(6点校正曲线)定量。
1.3 质量保证与质量控制(QA/QC)
QA/QC体系主要包括回收率指示物添加、程序空白、加标空白、基质加标、样品重复样测定等。程序空白样中有少量PCB 118和PCB 138检出,实际样品进行了相应扣除。样品中PCB 30、PCB 65和PCB 204的回收率分别为83% ± 16%、94% ±17%和94% ± 15%,定量结果未经回收率校正。空白加标和基质加标中PCBs单体(20种PCBs)的回收率范围分别为75%~107%和75%~104%。样品平行样中所有目标化合物的相对标准偏差均小于15%。PCBs的最低检测限(LOQs)按方法空白中各单体的含量平均值加3倍标准偏差计算。对于空白中没有检测出的目标化合物,按5倍信噪比(S/N)计算。样品中PCBs的检测限为0.01~0.20 μg·g-1脂重。
1.4 生物放大因子(BMF)计算
普通翠鸟体内中各PCB单体的生物放大因子(BMF)按照下式计算:
BMF=C翠鸟/C食物
式中C翠鸟为化合物在翠鸟中的浓度,单位为μg·g-1脂重,C食物为翠鸟食物(3种小鱼)中对应化合物的浓度,单位为μg·g-1脂重。
1.5 毒性当量(TEQ)计算
利用联合国卫生组织提出的鸟类二噁英类化合物毒性当量因子(TEFs)[7],计算了几种主要的共面PCB单体(包括PCB 77、PCB 81、PCB 105、PCB 114、PCB 118、PCB 123、PCB 126、PCB 156、PCB 167和PCB 169)的毒性当量(TEQ)。其计算公式如下:
TEQ= ∑(PCBi×TEFi)
式中PCBi和TEFi分别为某种共面PCB单体的浓度(pg·g-1湿重)和其TEF。
2 结果与讨论(Results and discussion)
2.1 PCBs的含量与组成
翠鸟及3种小型鱼类体内7种指示性PCBs及75种PCB单体总含量(ΣPCBs)见表1。翠鸟肌肉中ΣPCBs的浓度范围为4.0~3 300 μg·g-1脂重(中值浓度为220 μg·g-1脂重)。目前,仅有一篇文献报道了华南某自然保护区(中值1 800 ng·g-1脂重)和农村区域(中值410 ng·g-1脂重)普通翠鸟中PCBs的含量[7]。但已有不少研究报道了其他食鱼性鸟类肌肉中PCBs的含量。Luo等[9]研究的电子垃圾区池鹭肌肉中的PCBs的含量达到了120 000 ng·g-1脂重。比利时的牛背鹭肌肉中的浓度达到90 000 ng·g-1脂重[10]。Tanabe等[11]报道了采集于南印度湿地和沿海区域的白胸翡翠(翠鸟)肌肉中PCBs残留浓度为400 ng·g-1脂重。Kunisne等[12]报道的日本北海道黑尾鸥肌肉浓度为2 700~11 000 ng·g-1脂重。采自日本Lake Biwa湖区和罗马尼亚Danube Delta区域的普通鸬鹚肌肉中PCBs的浓度分别为2 900~7 700 000和700 ng·g-1脂重[12-14]。Braune等[15]研究了加拿大西部秋沙鸭和普通潜鸟胸部肌肉中PCBs的残留情况,这2种鸟类中PCBs的残留浓度为1.1~1 000 ng·g-1脂重。Elliott等[16]测定了英国、哥伦比亚和加拿大鹊鸭和棕胁秋沙鸭肌肉中PCBs含量,其浓度分别为1 600 ng·g-1脂重和78 ng·g-1脂重。相比于前人报道的食鱼性鸟类肌肉中PCBs的残留浓度,本研究翠鸟PCBs含量与日本Lake Biwa湖区的鸬鹚和中国电子垃圾拆解地池鹭相当(范围为4.0~3 300 μg·g-1脂重),但高于其他报道值1~3个数量级。翠鸟肌肉中PCBs含量也高于采集于同一电子垃圾拆卸区的其他7种水生鸟类(1 800~18 000 ng·g-1脂重)和1种陆生鸟类(7 300 ng·g-1脂重)以及华南某保护区4种陆生鸟类(45~1 770 ng·g-1脂重)肌肉中PCBs含量[9, 17-19]。虽然监测的PCBs单体总数量的不同,会影响本文翠鸟中PCBs的残留浓度与其他研究鸟类中浓度的对比,但是这些结果也初步表明,电子垃圾拆解地翠鸟已经受到PCBs严重污染。此外,诸多因素如食性和摄食量、迁移性和生态位(营养级)等会影响不同鸟类对PCBs的累积。

表1 电子垃圾拆卸区翠鸟及3种小型鱼类中PCBs含量(单位:μg·g-1脂重)
注:9 (3)表示样品的个数,括号里面的数字表示混合后样品的数量;3.5 (2.2~5.9)表示中值和范围;∑PCBs表示75个PCBs单体的总浓度。
Note: 9 (3) is the number of individual samples collected; figures in brackets indicate analyses number of pooled samples when individual were pooled; 3.5 (2.2~5.9) means median and range; ∑PCBs is the sum concentrations of the 75 PCB congeners examined.

图1 电子垃圾拆卸区翠鸟和其食物中PCBs的同系物分布模式Fig. 1 Congener profiles of PCBs in the kingfishers and their prey fish species collected from an e-waste recycling site
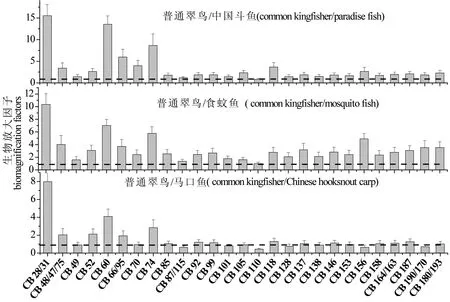
图2 翠鸟/鱼类的3种捕食关系中PCBs的BMFs值Fig. 2 Biomagnification factors (BMFs) for PCB congeners derived from the three predator/prey pairs
翠鸟及其食物(3种小鱼)中PCBs同系物组成模式如图1所示。翠鸟肌肉中CB 118是最主要的同系物,占ΣPCBs的16% ± 1.5%。CB 28/31、CB 153、CB 101和CB 138也是主要的同系物,共占ΣPCBs的47% ± 1.0%。翠鸟的食物与翠鸟有着相似的PBDEs同系物分布模式,其中CB 118、CB 153、CB 101和CB 138是最主要的同系物。本研究普通翠鸟肌肉中的PCB同系物组成特征与之前报道的华南某自然保护区和农村区域普通翠鸟中PCBs的同系物相同[7],都以CB 118、CB 28/31和CB 153为最主要同系物。本研究翠鸟体内PCBs同系物组成与电子垃圾拆卸区其他水生鸟类PCBs同系物组成也基本相同,都是以5~6氯等低氯代的PCBs单体为主[9],但与陆生鸟类PCB的同系物组成特征(主要是以5~8氯为主PCBs单体为主)具有较大的差异[17, 20]。水生鸟类和陆生鸟类生活环境和食性的不同,可能是导致这种差异的主要原因。相对于高氯代的PCB单体,低氯代的PCBs具有较高的水溶性,更容易在水生生物体内富集,造成翠鸟体内较高含量的低氯代PCB单体。
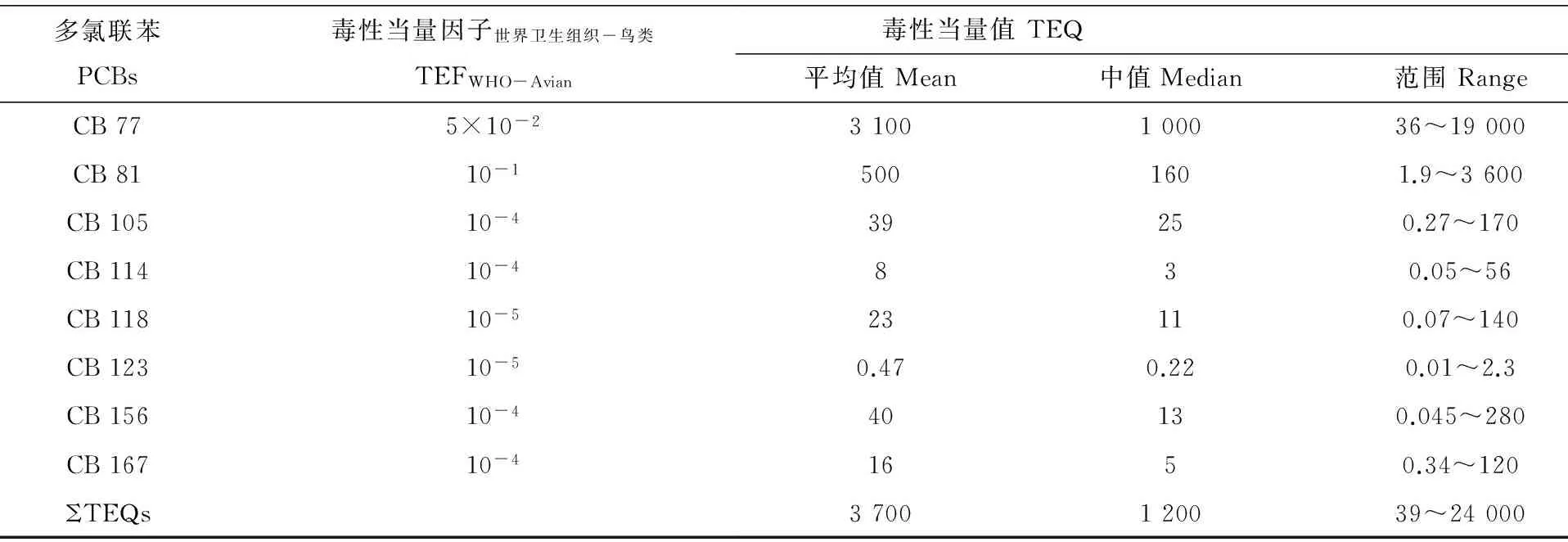
表2 电子垃圾拆卸区普通翠鸟中共面PCBs的毒性当量值(单位:pg·g-1湿重)
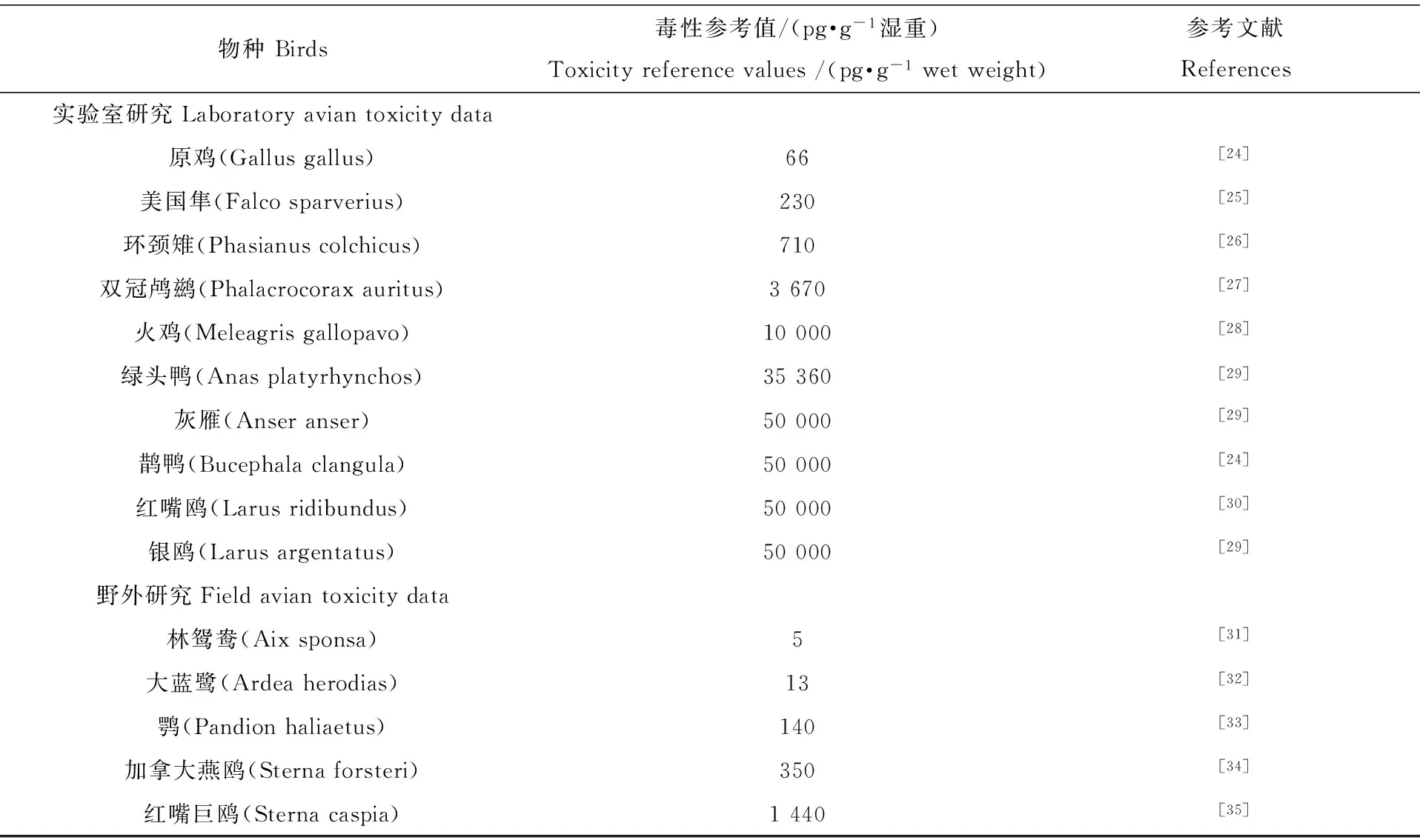
表3 文献报道的PCBs的TEQs对鸟类的毒性参考值
2.2 PCBs的生物放大
为了调查PCBs单体在普通翠鸟食物链中可能的生物放大效应,我们计算了这些化合物的生物放大因子(BMFs)(图2)。大部分的PCB单体的BMF值都大于1(ΣPCBs的平均BMF值为1.1~2.5),表明翠鸟对这些PCB单体产生了生物放大效应。通过对比PCB单体在3种捕食关系中的BMFs发现,翠鸟/中国斗鱼和翠鸟/食蚊鱼之间BMF值都大于1,而PCB少数单体(CB 49、CB87/115、CB110、CB156、CB190/170)在翠鸟/马口鱼之间BMF值小于1,通过查询文献,普通翠鸟其食物中99%都是以2.3 cm左右小型淡水鱼类(最大也能达到12.5 cm)[8],但是本次样品中马口鱼的平均长度为7.0[21],较其他2种鱼类长。因此,马口鱼可能不是翠鸟的主要食物之一,不同鱼类在翠鸟食物中的比例可能是造成这种差异的原因。本研究计算的PCBs的BMF值与之前报道的华南某自然保护区普通翠鸟对这些污染物的BMF值基本相似(ΣPCBs的平均BMF值为1.1~1.4)[7]。Drouillard等[22]采用肠道累积放大的方法预测了环鸽(Streptopelia risoria)对PCBs的生物放大效果,预测的BMF范围为18.5~33.8,高于我们当前的研究值。野外实验条件和实验室预测条件的差别以及物种间对于PCBs不同的累积特性,可能是导致不同研究BMF值不尽相同的主要原因。
2.3 TEQ值
目前还未见PCBs对于翠鸟的毒性风险评价数据。我们计算了几种主要的共面PCB单体(包括CB 77、CB 81、CB 105、CB 114、CB 118、CB 123、CB 156和CB 167)的毒性当量(TEQ),并通过对比TEQs对其他水生鸟类的毒性参考值(TRVs)或风险评估的阈值,评估PCBs对普通翠鸟潜在的毒害作用。计算的TEQs范围为39~24 000 pg·g-1湿重,平均值为3 700 pg·g-1湿重(表2)。较多研究报道了TEQs对鸟类的TRVs(表3),超过这些参考值将对鸟类产生胚胎死亡和发育障碍等方面的影响[23]。与这些结果比较,电子垃圾拆卸区翠鸟体内的TEQs含量超过了大部分水生鸟类的TRV值。这一结果预示着电子垃圾拆卸区翠鸟体内的PCBs可能对其生殖和发育方面带来潜在的毒害作用。
[1] Safe S, Hutzinger O. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): Biochemistry, toxicology, and mechanism of action [J]. Critical Reviews in Toxicology, 1984, 13(4): 319-395
[2] Fischer L J, Seegal R F, Ganey P E, et al. Symposium overview: Toxicity of non-coplanar PCBs [J]. Toxicological Sciences, 1998, 41(1): 49-61
[3] Jones K C, de Voogt P. Persistent organic pollutants (POPs): State of the science [J]. Environmental Pollution, 1999, 100(3): 209-221
[4] United Nations Environment Programme. The Stockholm Convention on Persistent Organic Pollutants (POPs) [R/OL]. Switzerland, 2001. http://chm.pops.int/Convention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx
[5] Breivik K, Gioia R, Chakraborty P, et al. Are reductions in industrial organic contaminants emissions in rich countries achieved partly by export of toxic wastes? [J]. Environmental Science & Technology, 2011, 45(21): 9154-9160
[6] Zhang K, Schnoor J L, Zeng E Y. E-waste recycling:Where does it go from here? [J]. Environmental Science & Technology, 2012, 46(20): 10861-10867
[7] Mo L, Wu J P, Luo X J, et al. Using the kingfisher (Alcedo atthis) as a bioindicator of PCBs and PBDEs in the Dinghushan Biosphere Reserve, China [J]. Environmental Toxicology and Chemistry, 2013, 32(7): 1655-1662
[8] Kasahara S, Katoh K. Food-niche differentiation in sympatric species of kingfishers, the common kingfisher Alcedo atthis and the greater pied kingfisher Ceryle lugubris [J]. Ornithological Science, 2008, 7(2): 123-134
[9] Luo X J, Zhang X L, Liu J, et al. Persistent halogenated compounds in waterbirds from an e-waste recycling region in South China [J]. Environmental Science & Technology, 2009, 43(2): 306-311
[10] Jaspers V L B, Covaci A, Voorspoels S, et al. Brominated flame retardants and organochlorine pollutants in aquatic and terrestrial predatory birds of Belgium:Levels, patterns, tissue distribution and condition factors [J]. Environmental Pollution, 2006, 139(2): 340-352
[11] Tanabe S, Senthilkumar K, Kannan K, et al. Accumulation features of polychlorinated biphenyls and organochlorine pesticides in resident and migratory birds from South India [J]. Archives of Environmental Contamination and Toxicology, 1998, 34(4): 387-397
[12] Kunisue T, Higaki Y, Isobe T, et al. Spatial trends of polybrominated diphenyl ethers in avian species: Utilization of stored samples in the environmental specimen bank of ehime university (es-bank) [J]. Environmental Pollution, 2008, 154(2): 272-282
[13] Covaci A, Gheorghe A, Hulea O, et al. Levels and distribution of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in sediments and biota from the Danube Delta, Romania [J]. Environmental Pollution, 2006, 140(1): 136-149
[14] Kubota A, Iwata H, Tanabe S, et al. Levels and toxicokinetic behaviors of PCDD, PCDF, and coplanar PCB congeners in common cormorants from Lake Biwa, Japan [J]. Environmental Science & Technology, 2004, 38(14): 3853-3859
[15] Braune B M, Malone B J, Burgess N M, et al. Chemical residues in waterfowl and gamebirds harvsted in Canada, 1987-95 [J]. Canadian Wildlife Service Technical Report, 1999, 326(1): 392-432
[16] Elliott J E, Martin P A. Chlorinated hydrocarbon contaminants in grebes and seaducks wintering on the coast of British Columbia, Canada: 1988-1993 [J]. Environmental Monitoring and Assessment, 1998, 53(2): 337-362
[17] Sun Y X, Hao Q, Zheng X B, et al. PCBs and DDTs in light-vented bulbuls from Guangdong Province, South China: Levels, geographical pattern and risk assessment [J]. Science of the Total Environment, 2014, 490(15): 815-821
[18] Zhang X L, Luo X J, Liu H Y, et al. Bioaccumulation of several brominated flame retardants and dechlorane plus in waterbirds from an e-waste recycling region in South China: Associated with trophic level and diet sources [J]. Environmental Science & Technology, 2010, 45(2): 400-405
[19] Peng Y, Wu J P, Tao L, et al. Contaminants of legacy and emerging concern in terrestrial passerines from a nature reserve in South China: Residue levels and inter-species differences in the accumulation [J]. Environmental Pollution, 2015, 203(1): 7-14
[20] Chen D, Zhang X L, Mai B X, et al. Polychlorinated biphenyls and organochlorine pesticides in various bird species from northern China [J]. Environmental Pollution, 2009, 157(7): 2023-2029
[21] Mo L, Wu J P, Luo X J, et al. Bioaccumulation of polybrominated diphenyl ethers, decabromodiphenyl ethane, and 1,2-bis(2,4,6-tribromophenoxy) ethane flame retardants in kingfishers (Alcedo atthis) from an electronic waste-recycling site in South China [J]. Environmental Toxicology and Chemistry, 2012, 31(9): 2153-2158
[22] Drouillard K G, Paterson G, Liu J, et al. Calibration of the gastrointestinal magnification model to predict maximum biomagnification potentials of polychlorinated biphenyls in a bird and fish [J]. Environmental Science & Technology, 2012, 46(18): 10279-10286
[23] United States Environmental Protection Agency (US EPA). Analyses of Laboratory and Field Studies of Reproductive Toxicity in Birds Exposed to Dioxin-like Compounds for Use in Ecological Risk Assessment [R]. Washington DC: National Center for Environmental Assessment Office of Research and Development, 2003
[24] United States Environmental Protection Agency (US EPA). Analyses of Laboratory and Field Studies of Reproductive Toxicity in Birds Exposed to Dioxin-like Compounds for Use in Ecological Risk Assessment [R]. Washington DC: US EPA, 2003
[25] Hoffman D J, Melancon M J, Klein P N, et al. Comparative developmental toxicity of planar polychlorinated biphenyl congeners in chickens, American kestrels, and common terns [J]. Environmental Toxicology and Chemistry, 1998, 17(4): 747-757
[26] Nosek J, Craven S, Sullivan J, et al. Toxicity and reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in ring-necked pheasant hens [J]. Journal of Toxicology and Environmental Health, 1992, 35(3): 187-198
[27] Powell D C, Aulerich R J, Meadows J C, et al. Effects of 3,3',4,4',5-pentachlorobiphenyl (PCB 126), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), or an extract derived from field-collected cormorant eggs injected into double-crested cormorant (Phalacrocorax auritus) eggs [J]. Environmental Toxicology and Chemistry, 1997, 16(7): 1450-1455
[28] Brunström B, Andersson L. Toxicity and 7-ethoxyresorufin-O-deethylase-inducing potency of coplanar polychlorinated biphenyls (PCBs) in chick embryos [J]. Archives of Toxicology, 1988, 62(4): 263-266
[29] Brunström B. Sensitivity of embryos from duck, goose, herring gull, and various chicken breeds to 3,3',4,4'-tetrachlorobiphenyl [J]. Poultry Science, 1988, 67(1): 52-56
[30] Brunström B, Reutergårdh L. Differences in sensitivity of some avian species to the embryotoxicity of a PCB, 3,3',4,4'-tetrachlorobiphenyl, injected into the eggs [J]. Environmental Pollution, 1986, 42(1): 37-45
[31] White D H, Seginak J T . Dioxins and furans linked to reproductive impairment in wood ducks at Bayou Meto, Arkansas [J]. Journal of Wildlife Management, 1994, 25(4): 100-106
[32] Henshel D S, Martin J W, Norstrom R, et al. Morphometric abnormalities in brains of great blue heron hatchlings exposed in the wild to PCDDs [J]. Environmental Health Perspectives, 1995, 103(4): 61-66
[33] Woodford J E, Karasov W H, Meyer M W, et al. Impact of 2,3,7,8-TCDD exposure on survival, growth, and behavior of ospreys breeding in Wisconsin, USA [J]. Environmental Toxicology and Chemistry, 1998, 17(7): 1323-1331
[34] Kubiak T J, Harris H J, Smith L M, et al. Microcontaminants and reproductive impairment of the Forster's tern on Green Bay, Lake Michigan-1983 [J]. Archives of Environmental Contamination and Toxicology, 1989, 18(5): 706-727
[35] Ewins P J, Weseloh D V, Norstrom R J, et al. Caspian terns on the Great Lakes: Organochlorine contamination, reproduction,diet, and population changes 1972-91 [J]. Canadian Wildlife Service, 1994, 85(5): 1-33
◆
Bioaccumulation and Risk Assessment of Polychlorinated Biphenyls in the Common Kingfisher (Alcedoatthis) from an Electronic Waste Recycling Site in South China
Mo Ling1, Wu Jiangping2,*, Zhang Yun3, Xing Qiao1, Lin Zhangwen1, Luo Xiaojun2, Mai Bixian2
1. Hainan Research Academy of Environmental Sciences, Haikou 570100, China 2. State Key Laboratory of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China 3. School of Life Science, South China Normal University, Guangzhou 510631, China
The wildlife from electronic waste (e-waste) sites have been heavily polluted by polychlorinated biphenyls (PCBs), due to the primitive e-waste recycling activities. However, information on the bioaccumulation and the toxic effects of PCBs in wild avian species from e-waste sites is limited. In the present study, we investigated the levels and congener profiles of PCBs in the common kingfisher (Alcedo atthis) from an e-waste recycling site in Guangdong Province, South China. Additionally, PCBs in the diet items including three fish species collected from the same sampling site were also examined, to evaluate the potential biomagnification of PCBs in the common kingfisher. Finally, we assessed the potential toxic effects of PCBs to these birds by estimating the toxic equivalent quantity (TEQ) of the co-planar PCBs. Elevated PCB residues (median = 220 μg·g-1lipid weight for total PCBs) were detected in the kingfishers, which were one to three orders of magnitude higher than the values previously reported in the species from other sampling sites. The calculated predator/prey biomagnification factors (BMFs) were greater than unity for most of the PCB congeners examined, suggesting biomagnification of these chemicals in the common kingfisher. The TEQ concentrations estimated in the common kingfisher ranged from 39 to 23 600 pg·g-1wet weight, with some of these values reaching or exceeding the levels known to impair bird reproduction and survival. Our results revealed that the common kingfisher from the e-waste recycling site has been heavily contaminated by PCBs. The need for further examination is warranted to determine the potential adverse effects resulting from the PCBs exposure, in the common kingfishers and other wildlife that are habitants of e-waste sites.
polychlorinated biphenyls; bird; bioaccumulation; biomagnification; electronic waste
10.7524/AJE.1673-5897.20151114001
海南省自然科学基金(20154176);国家自然科学基金(41230639,41173109)
莫凌(1984-),男,博士,助理研究员,高级工程师,研究方向为持久性有毒污染物的环境行为及毒害作用,E-mail: morning.ml@163.com
*通讯作者(Corresponding author), E-mail: jpwu@gig.ac.cn
2015-11-14 录用日期:2015-12-22
1673-5897(2016)2-155-08
X171.5
A
简介:吴江平(1976-),男,博士,副研究员,主要研究方向为持久性有毒污染物的生物累积、沿食物链传递及其毒害作用,近5年在国内外环境领域主流刊物发表文章50余篇,被SCI期刊引用共1200余次。
莫凌, 吴江平, 张云, 等. 电子垃圾拆解地翠鸟对多氯联苯的累积及风险评估[J]. 生态毒理学报,2016, 11(2): 155-162
Mo L, Wu J P, Zhang Y, et al. Bioaccumulation and risk assessment of polychlorinated biphenyls in the common kingfisher (Alcedo atthis) from an electronic waste recycling site in south China [J]. Asian Journal of Ecotoxicology, 2016, 11(2): 155-162 (in Chinese)

