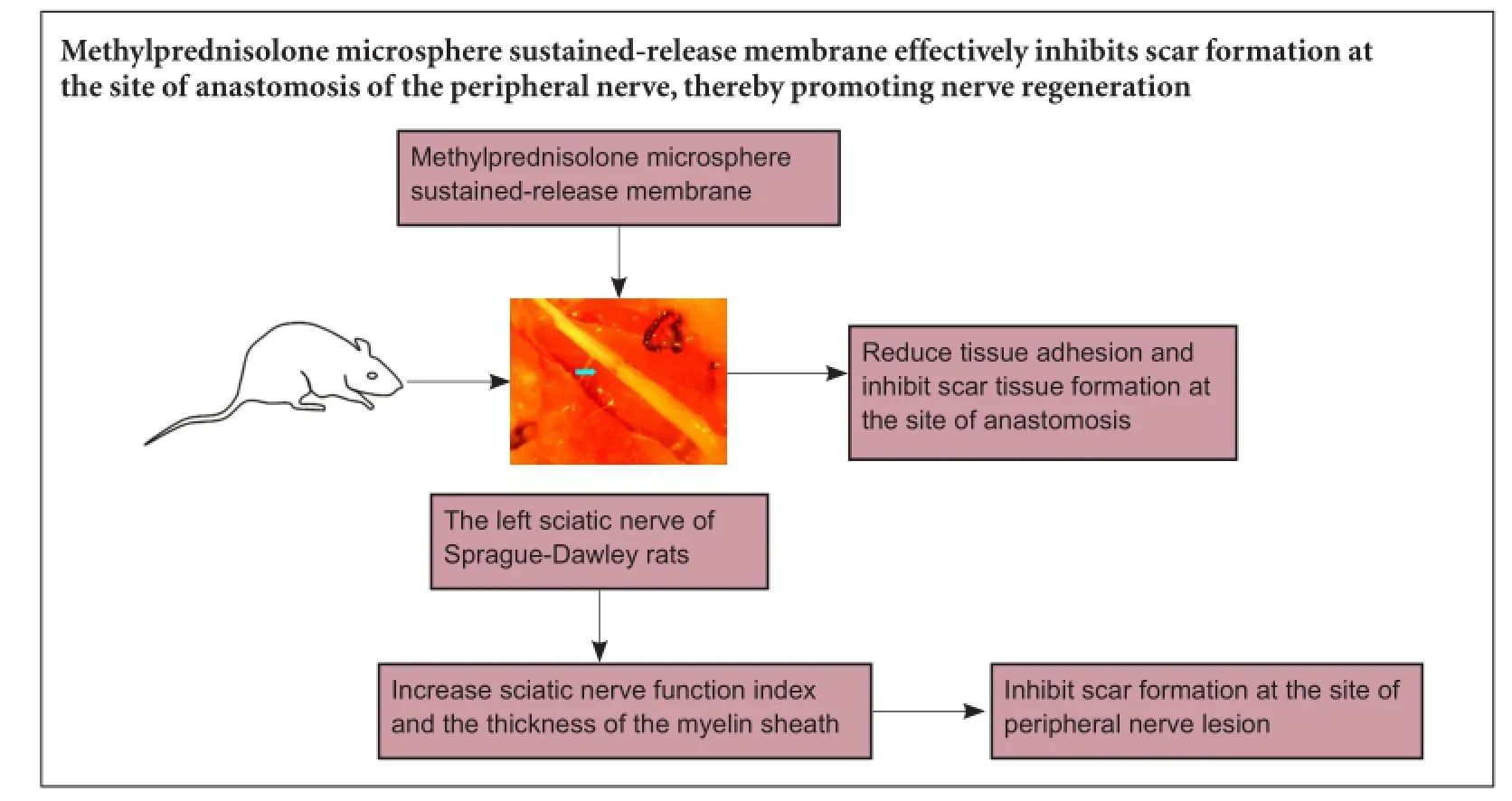
Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion
Qiang Li, Teng Li, Xiang-chang Cao, De-qing Luo, Ke-jian Lian
The Affiliated Dongnan Hospital of Xiamen University, Zhangzhou, Fujian Province, China
RESEARCH ARTICLE
Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion
Qiang Li#, Teng Li#, Xiang-chang Cao, De-qing Luo, Ke-jian Lian*
The Affiliated Dongnan Hospital of Xiamen University, Zhangzhou, Fujian Province, China
Graphical Abstract
#These authors contributed equally to this study.
orcid: 0000-0001-7030-8009 (Ke-jian Lian)
Corticosteroids are widely used for the treatment of acute central nervous system injury. However, their bioactivity is limited by their short half-life. Sustained release of glucocorticoids can prolong their efficacy and inhibit scar formation at the site of nerve injury. In the present study, we wrapped the anastomotic ends of the rat sciatic nerve with a methylprednisolone sustained-release membrane. Compared with methylprednisone alone or methylprednisone microspheres, the methylprednisolone microsphere sustained-release membrane reduced tissue adhesion and inhibited scar tissue formation at the site of anastomosis. It also increased sciatic nerve function index and the thickness of the myelin sheath. Our findings show that the methylprednisolone microsphere sustained-release membrane effectively inhibits scar formation at the site of anastomosis of the peripheral nerve, thereby promoting nerve regeneration.
nerve regeneration; peripheral nerve injury; nanometer; scar; methylprenisolone; sustained-release membrane; biological agents; peripheral nerve; neural regeneration
Introduction
Scar formation at the site of nerve repair is a major factor inhibiting recovery after peripheral nerve injury (Ngeow, 2010; Yin et al., 2010). Scar tissue prevents nerve regeneration primarily by increasing nerve adhesion, which provides a direct physical block to the nerve, and by inhibiting angiogenesis (Abrams and Widenfalk, 2004). The scar grows into the site of the lesion, and hinders extension of the regenerating nerve fibers (Park et al., 2011). Therefore, although various drugs and techniques have been used to promote nerve growth, regeneration remains limited.
Recently, the prevention of scar hyperplasia has become a hot topic in neural regeneration research. However, outcomes have not been satisfactory in both basic and clinical studies (Silver and Miller, 2004). There have been two major strategies for reducing the impact of the scar on nerve regeneration. The first approach has been to separate the nerve ends from the surrounding tissues to prevent tissueadhesion (Jeong and Gutowska, 2002). The second has been the use of steroidal hormones to inhibit the inflammatory response. These hormones selectively inhibit fibroblast growth and granulocyte migration and phagocytosis, but efficacy is limited because of rapid degradation and absorption. Furthermore, continuous systemic application of steroids produces severe side effects (Mukudai et al., 2014).
Biomembranes with controlled-release function have shown strong potential in anti-inflammatory and anti-neoplastic treatments, and have been used to improve nerve regeneration (Ciardelli and Chiono, 2006; Stang et al., 2009; Huang et al., 2012). Sustained-release microsphere membrane is a new biodegradable material, and it not only has the advantage of microspheres and collagen, but can also reduce degradation of the drug.
In the present study, we assessed the inhibitory effect of methylprednisolone microsphere sustained-release membrane (MMSRM) on scar formation in the lesioned nerve.
Materials and Methods
Animals
A total of 120 Sprague-Dawley male rats, 3—4 months of age, weighing 200—250 g, were provided by the Animal Laboratory of the Affiliated Dongnan Hospital of Xiamen University, Zhangzhou, China (license No. SCXK (Min) 2012-0001). All rats were maintained and housed under controlled conditions (22°C), with a 12-hour light/dark cycle, and were provided with food and water ad libitum. All efforts were made to minimize the number of animals used and to reduce their suffering. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1986).
Grouping and surgical procedures
Twelve hours before surgery, all animals were deprived of food and water. Before surgery, the animals were anesthetized by intraperitoneal injection of 10% chloral hydrate (300 mg/kg per day). The rats were randomly divided into four groups (n = 30 per group) and underwent transection of the proximal left sciatic nerve and subsequent end-toend suture under a microscope (Langone et al., 1995; Ozturk, 2015). After this procedure, the rats in the four groups were treated with one of the following: (1) MMSRM (Affiliated Dongnan Hospital of Xiamen University, Zhangzhou, China), with a drug release rate of 1 mg/kg per day, which was used to wrap the nerve stump followed by suturing; (2) blank control microsphere sustained-release membrane (BMSRM group; Affiliated Dongnan Hospital of Xiamen University); (3) local injection of methylprednisolone (15 mg/0.5 mL, purity > 99%, batch No. 041006, provided by Zhejiang Hisun Pharmaceutical Co., Ltd., Taizhou, Zhejiang Province, China; methylprednisolone group); (4) local injection of normal saline (0.5 mL, provided by Affiliated Dongnan Hospital of Xiamen University; normal saline group). Subsequently, the skin incision was sutured and disinfected using 75% alcohol following hemostasis. The rats were fed the same diet under the same environmental conditions.
Specimen collection
As previously reported (Sun et al., 2009), adhesive tissues around the nerve stump were separated carefully to avoid nerve injury at 4, 8 or 12 weeks post-operation. After electromyography testing, a nerve tissue sample, 1 cm distal to the center of nerve anastomosis, was taken and subjected to 10% formalin fixation and paraffin embedding for histological analysis. Experimental data were recorded in detail.
General observation
At 4, 8 or 12 weeks post-operation, 10 rats from each group were taken for analysis. An incision was made layer by layer after anesthesia to observe the healing of the skin and muscle, and the degree of tissue adhesion between the nerve anastomotic ends and adjacent muscular lacuna was evaluated as previously reported (Petersen et al., 1996).
Histological observation
A 1.5-cm-long nerve segment comprising the center of the nerve anastomosis was excised and fixed with 4% paraformaldehyde for 24 hours, and then subjected to paraffin embedding.
Masson staining
Masson staining was performed to stain collagen fibers blue (Calvi et al., 2014).
Osmic acid staining
Osmic acid staining was used to evaluate peripheral nerve myelin formation (Wolthers et al., 2005; Saceda et al., 2011) and to assess nerve fiber diameter and the thickness of the myelin sheath. Image analysis using computer scanning system (Media Cybernetics, Rockville, MD, USA) was used for quantitative analysis of myelin hyperplasia. Myelin tissues were colored blue-brown or black.
Immunohistochemical staining for collagens
Immunohistochemical staining for type I and type III collagen was performed using a kit according to the manufacturer's instructions (Xi Tang Biological Technology Co., Ltd., Shanghai, China and Ao Xing Biological Technology Co., Ltd., Zhejiang, China). The relative expression levels of type I and type III collagen fibers, which were stained brown-yellow, were analyzed with a computer.
Image analysis
Masson staining, osmic acid staining and type I and type III collagen immunohistochemical staining were analyzed by light microscopy (100×; Olympus, Tokyo, Japan). Five fields of vision were chosen in each image followed by image analysis and processing using Image-Pro Plus 7.0 software (Media Cybernetics) (Cheng et al., 2011).
Neurological function evaluation
For neurological functional testing, we used a homemade footprint walking box, 50 cm long, with white tracing paper lining the bottom. The hind feet were dyed using carbon ink, and the rat was placed in the box. Then, 3—4 clear hind footprints were recorded. The bilateral footprints were recorded every 2 weeks, 4—12 weeks postoperatively (normal group, N; experimental group, E). The following three variables were measured (Figure 1): (1) Footprint length (PL), the distance from the heel to the toe (the longest PL was selected in this study); (2) Footprint width (TS), the distance from the first toe to the fifth toe (the longest TS was selected in this study); (3) Middle digit distance (IT), the distance from the second toe to the fourth toe (the longest IT was selected in this study). All measurements were accurate to the mm. These three variables were entered into the Bain formula (de Medinaceli et al., 1982; Shen and Zhu, 1993) to calculate the sciatic function index (SFI) for each group. SFI = -38.3[(EPL-NPL)/NPL] + 109.5[(ETSNTS)/NTS] + 13.3[(EIT-NIT)/NIT] - 8.85. The SFI value ranged from 0 (normal) to -100 (complete loss of nerve function) (Azuma et al., 2007).
Statistical analyses
Data are expressed as the mean ± SD and were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Statistical analysis was performed using homogeneity test of variance. Rank sum test was used for ordered multi classification data, and the Mann-Whitney U-test used for data processing. Two-factor and two-level factorial design was used for Masson staining and for type I and type III collagen analysis (these comparisons were only made between the MMSRM group and the methylprednisolone group). The SFI, the number of nerve fibers and the thickness of the myelin sheath were analyzed using one-way analysis of variance and Bonferroni analysis. P-values less than 0.05 were considered statistically significant.
Results
General observation
Because of infection-induced death, nine rats from the BMSRM group were taken at each time point after operation, and nine and eight rats from the methylprednisolone group and normal saline group, respectively, were taken at 4 weeks after operation. Rank sum test analysis showed that the healing of the skin and fascia muscle was good in the four groups, and there was no significant difference among them (P > 0.05). Compared with the BMSRM and normal saline groups, tissue adhesion was milder in the MMSRM group and methylprednisolone group 8 weeks after operation (P < 0.05), but there was no significant difference between the MMSRM group and the methylprednisolone group or between the BMSRM group and the normal saline group. Compared with the methylprednisolone group, tissue adhesion was milder in the MMSRM group 12 weeks after operation (P < 0.05). The rank sum test was used to evaluate differences in tissue adhesion (Table 1). Mann-Whitney U-test was performed to compare the MMSRM and methylprednisolone groups, revealing a significant difference between the two groups (P < 0.05). The MMSRM group had milder tissue adhesion compared with the methylprednisolone group (Figure 2).
Masson staining
The factorial design was used for statistical analysis. A significant interaction between time and treatment (P = 0.032) was found (Table 2). As shown in Table 2, the collagen content increased significantly (P = 0.017) in the methylprednisolone group at 12 weeks compared with 4 weeks. As shown in Table 3, there was no significant difference in collagen content between 4 and 12 weeks in the MMSRM group (P = 0.739). At 4 weeks, the collagen content in the MMSRM group increased significantly compared with the methylprednisolone group (P = 0.020). However, at 12 weeks, there was no statistical difference in collagen content between the MMSRM group and the methylprednisolone group (P = 0.463).
At 12 weeks, Masson staining in the MMSRM group revealed the presence of nerve growth cones (Figure 3A). Epineurial collagen was less, and the outer membrane was thinner (Figure 3B). In the methylprednisolone group, a disordered arrangement of nerve fibers was observed, with no growth cone formation and much collagen deposition (Figure 3C). There was much collagen deposition on the epineurium, which was comparatively thicker (Figure 3D).
Morphological observation and axon regeneration assessment using osmic acid staining
Because the data recorded at 4 and 8 weeks after surgery were lost, only the data for week 12 were analyzed. The number of nerve fibers near the site of nerve anastomosis was counted under a microscope using a 100× objective (Table 4). The number of nerve fibers was much higher in the MMSRM group and local methylprednisolone group than in the BMSRM group or the normal saline group (P < 0.05). There was no significant difference between the MMSRM group and the methylprednisolone group or between the BMSRM group and the normal saline group (P > 0.05). These results suggest that local applications of drug-loaded microsphere sustained-release membrane and methylprednisolone both increase the number of nerve fibers after nerve anastomosis. There was no significant difference between the MMSRM and methylprednisolone groups.
At 12 weeks after surgery, regenerated myelin sheaths had an irregular shape, distribution and thicknesses, and there were great differences in their shape, diameter and thickness. The regenerated myelin sheath was significantly thicker in the MMSRM and methylprednisolone groups compared with the BMSRM group or the normal saline group (P <0.05). Moreover, there was a significant difference between the MMSRM group and the methylprednisolone group (P = 0.022; Table 5). Nerve regeneration was the lowest in the BMSRM and normal saline groups, and the regenerated myelin sheath was thin (P = 0.887). Overall, the thickness of the regenerated myelin sheath was greater in the MMSRM groupthan in the other three groups.
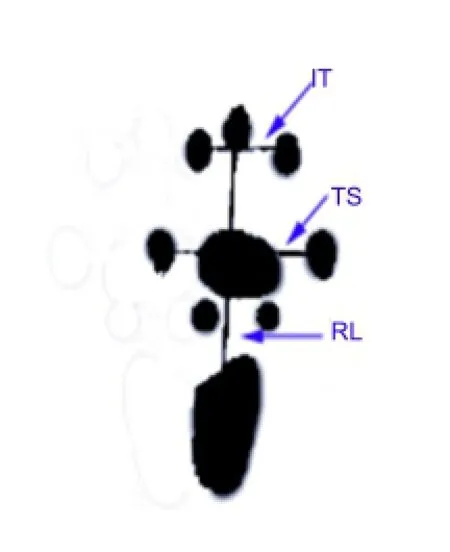
Figure 1 Schematic diagram of SFI measurement (right footprints).
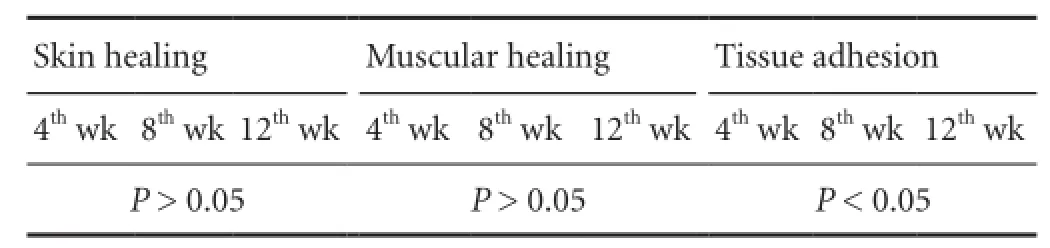
Table 1 Differences in tissue adhesion scores evaluated using the rank sum test
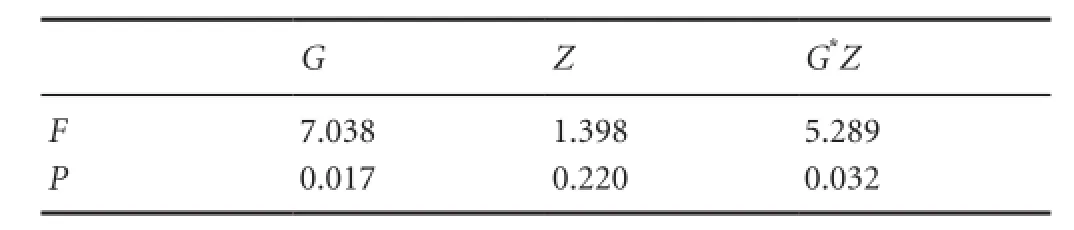
Table 2 Two-factor two-level factorial design analysis of the collagen content at the site of nerve anastomosis in the MMSRM group and the methylprednisolone group

Figure 2 Tissue adhesion at the site of nerve anastomosis (arrows) 12 weeks post-operation.
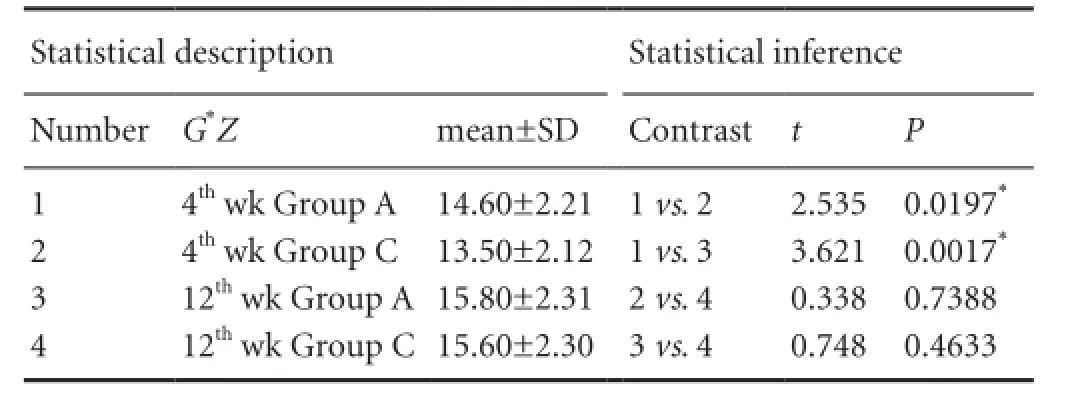
Table 3 Single effect analysis of Masson staining for collagen content at the site of nerve anastomosis in the MMSRM group and the methylprednisolone group
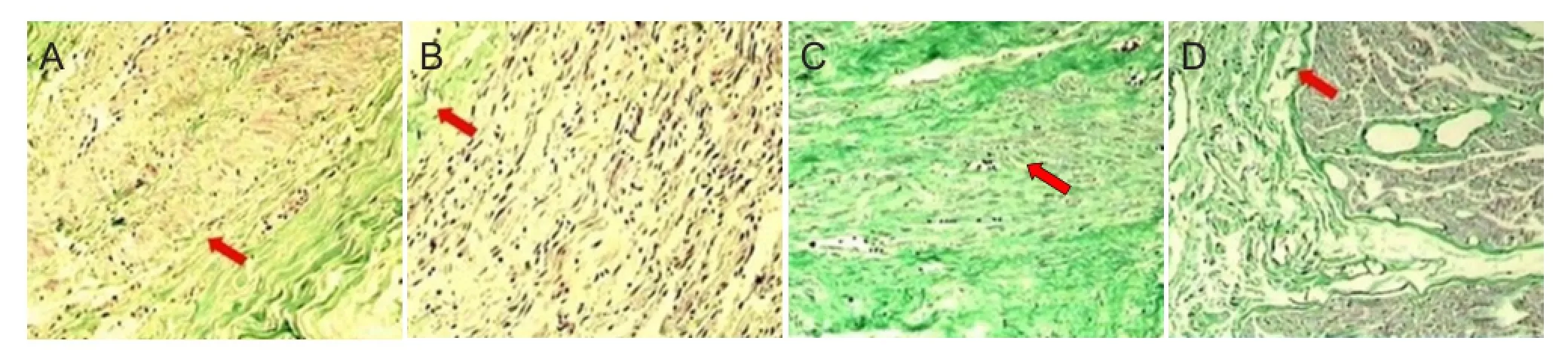
Figure 3 Masson staining of collagen fibers (× 100).

Table 4 Bonferroni analysis of the number of nerve fibers 12 weeks after surgery

Table 7 Two-factor two-level factorial design analysis for type III collagen at the site of nerve anastomosis in the MMSRM and methylprednisolone groups
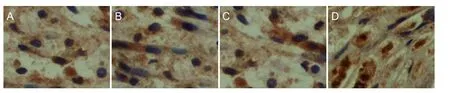
Figure 4 Type I collagen immunohistochemical staining in the MMSRM and methylprednisolone groups 4 and 12 weeks after surgery (× 100).
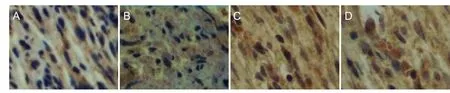
Figure 5 Type III collagen immunohistochemical staining in the MMSRM and methylprednisolone groups 4 and 12 weeks after surgery (× 100).

Figure 6 Comparison of sciatic function index (SFI) at different time points after surgery.

Table 6 Two-factor two-level factorial design analysis for type I collagen at the site of nerve anastomosis in the MMSRM and methylprednisolone groups
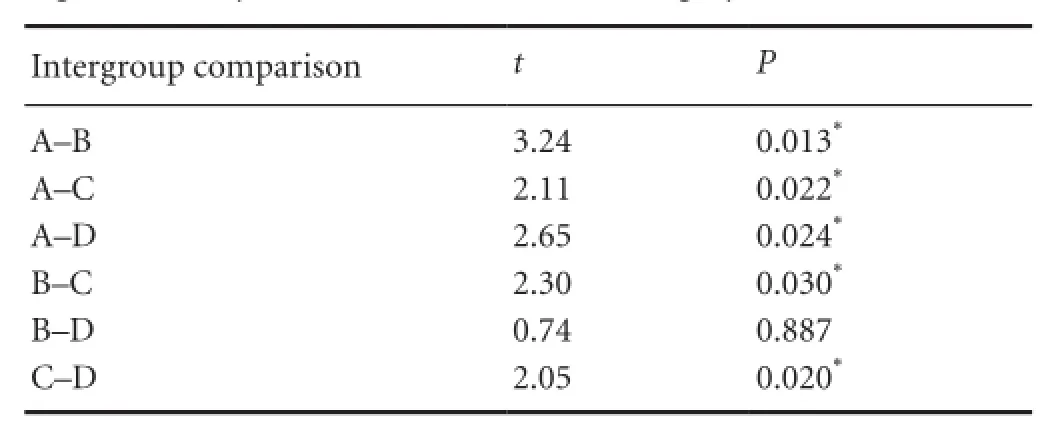
Table 5 Bonferroni analysis used to compare the thickness of the regenerated myelin sheath 12 weeks after surgery
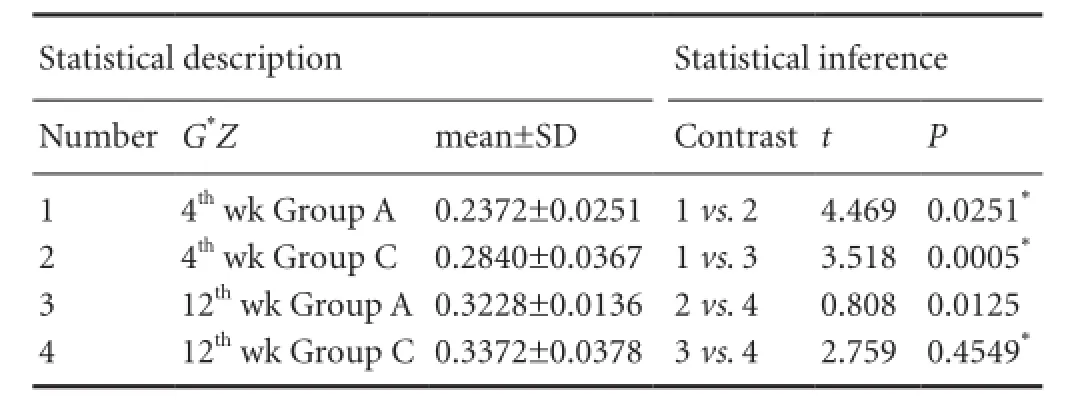
Table 8 Single effect analysis of Masson staining for III collagen content at the site of nerve anastomosis in the MMSRM and methylprednisolone groups

Table 9 Bonferroni analysis of the sciatic function index in the various groups
Relative expression levels of type I and type III collagens
Type I collagen content analysis
As shown in Table 6, there was no interaction between the two factors, time and treatment (P = 0.906). The effects of these two factors were independent of each other, and it was necessary to analyze their main effect independently. As shown in Table 6 and Figure 4, type I collagen content in the two groups was higher at 12 weeks compared with 4 weeks (P = 0.034). Type I collagen content was significantly lower in the MMSRM group than in the methylprednisolone group (P = 0.003).
Type III collagen content analysis
As shown in Table 7, there was an interaction between the two factors, time and treatment (P = 0.042). As shown in Table 8 and Figure 5, type III collagen content in the MMSRM group was higher at 12 weeks than at 4 weeks (Figure 5A, C) (P = 0.0125). The type III collagen content in the methylprednisolone group was higher at 12 weeks than at 4 weeks (Figure 5B, D) (P = 0.0005). At 4 weeks, the type III collagen content was higher in the MMSRM group than in the methylprednisolone group (P = 0.0251). However, there was no difference between the two groups at 12 weeks (P = 0.4549).
Evaluation of SFI
At 8 weeks after surgery, the SFI value was -11.08 in the normal saline group, -5.02 in the MMSRM group, -16.36 in the BMSRM group and -5.24 in the methylprednisolone groups (Table 9). There were significant differences between the BMSRM and MMSRM groups and between the BMSRM and methylprednisolone groups (P < 0.05). The BMSRM group exhibited lower neurological recovery compared with the MMSRM group or the methylprednisolone group. At 10 and 12 weeks after surgery, there was a significant difference between the MMSRM and methylprednisolone groups (Table 9), indicating that neurological recovery was greater in the MMSRM group than in the methylprednisolone group. The overall mean analysis showed that the MMSRM group showed better neurological recovery than the other three groups, especially during long-term postoperative observation (Figure 6).
Discussion
Methylprednisolone dosage used in this study
Methylprednisolone treatment for acute spinal cord injury (Pfister et al., 2007) is recommended within 8 hours of injury (Hanks et al., 1996; Francis et al., 2007; de Ruiter et al., 2008). The first dosage is 30 mg/kg, and the maintenance dose is 5.4 mg/kg per hour. The dose conversion coefficient used to convert the human dose to the rat dose is W = 6.25. Therefore, the first dose of methylprednisolone for the rat is 30 mg/kg × 6.25, i.e. 187 mg/kg, and the maintenance dose is 33 mg/kg per hour. In this study, intramuscular injection instead of continuous intravenous infusion was used in rats. As the average weight of the rats was approximately 250 g, W = 5 was used as the dose conversion coefficient. Previous studies have shown that rats given a high dose, but less than the human-rat converted volume (15—30 mg/kg), develop psychiatric symptoms and local severe infection. The negative impact of infection was largely offset by the enhanced nerve regeneration and by the cytoprotective effect of the drug. Because the dose used in the present study was 5—10 times less than the doses used in most studies, the hormone had no negative effects. Therefore, systemic application of moderate doses of methylprednisolone, i.e. 15—30 mg/kg, effectively promotes the regeneration of peripheral nerves in rats. The SFI value was significantly better than in the normal saline group, showing that the drug promotes the regeneration of peripheral nerves. The local administration of the drug can have the same positive effects of systemic administration while reducing systemic side effects.
Intravenous delivery of high-dose methylprednisolone in patients with peripheral nerve injury is problematic because of systemic side effects. Thus, it has limited clinical potential. Our present findings show that local application of methylprednisolone effectively promotes peripheral nerve repair and regeneration after injury in the rat. The high local methylprednisolone concentration during the acute phase of injury, allowed by the MMSRN, likely inhibits inflammatory reactions and protects cells after injury. Furthermore, local drug application likely avoids serious side effects, especially infections, caused by the systemic application of high-dose methylprednisolone. Future clinical studies should examine whether localized application can promote nerve regeneration, similar to systemic administration.
MMSRM versus local methylprednisolone
The local application of the MMSRM showed anti-inflammatory and sustained-release effects, and promoted peripheral nerve functional recovery in rats compared with a single local injection of methylprednisolone. Although the SFI is not a fully objective scoring method, it can reflect neurological functional status. Our next goal is to evaluate whether the MMSRM can improve nerve fiber conduction and enhance systemic functional recovery.
Conclusion
In this study, the degree of tissue adhesion was significantly lower in the MMSRM group than in the other groups. Furthermore, other indices, including nerve functional recovery, collagen content, thickness of the myelin sheath and the number of nerve fibers, were significantly ameliorated compared with the other groups. Our results demonstrate that MMSRM promotes nerve regeneration. Our findings lay the foundation for future studies on peripheral nerve regeneration and repair.
Author contributions: KJL designed the study. QL, XCC, and TL performed experiments and wrote the paper. DQL was responsible for data analysis. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Abrams M, Widenfalk J (2004) Emerging strategies to promote improved functional outcome after peripheral nerve injury. Restor Neurol Neurosci 23:367-382.
Azuma C, Tohyama H, Nakamura H, Kanaya F, Yasuda K (2007) Antibody neutralization of TGF-beta enhances the deterioration of collagen fascicles in a tissue-cultured tendon matrix with ex vivo fibroblast infiltration. J Biomech 40:2184-2190.
Calvi E, Nahas F, Barbosa M, Calil J, Ihara S, Juliano Y, Ferreira L (2014) Collagen fibers in the rectus abdominis muscle of cadavers of different age. Hernia 18:527-533.
Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA (2011) Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol 30:126-134.
Ciardelli G, Chiono V (2006) Materials for peripheral nerve regeneration. Macromol Biosci 6:13-26.
de Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77:634-643.
de Ruiter GC, Onyeneho IA, Liang ET, Moore MJ, Knight AM, Malessy MJ, Spinner RJ, Lu L, Currier BL, Yaszemski MJ (2008) Methods for in vitro characterization of multichannel nerve tubes. J Biomed Materials Res Part A 84:643-651.
Francis JS, Olariu A, Kobayashi E, Leone P (2007) GFP-transgenic Lewis rats as a cell source for oligodendrocyte replacement. Exp Neurol 205:177-189.
Hanks CT, Wataha JC, Sun Z (1996) In vitro models of biocompatibility: a review. Dent Mater 12:186-193.
Huang W, Begum R, Barber T, Ibba V, Tee N, Hussain M, Arastoo M, Yang Q, Robson L, Lesage S (2012) Regenerative potential of silk conduits in repair of peripheral nerve injury in adult rats. Biomaterials 33:59-71.
Jeong B, Gutowska A (2002) Lessons from nature: stimuli-responsive polymers and their biomedical applications. Trends Biotechnol 20:305-311.
LacKamp A, Zhang GC, Mao LM, Fibuch E, Wang J (2009) Loss of surface N-methyl-D-aspartate receptor proteins in mouse cortical neurones during anaesthesia induced by chloral hydrate in vivo. Br J Anaesth 102:515-522.
Langone F, Lora S, Veronese FM, Caliceti P, Parnigotto PP, Valenti F, Palma G (1995) Peripheral nerve repair using a poly (organo) phosphazene tubular prosthesis. Biomaterials 16:347-353.
Mukudai S, Matsuda KI, Nishio T, Sugiyama Y, Bando H, Hirota R, Sakaguchi H, Hisa Y, Kawata M (2014) Differential responses to steroid hormones in fibroblasts from the vocal fold, trachea, and esophagus. Endocrinology 156:1000-1009.
Ngeow WC (2010) Scar less: a review of methods of scar reduction at sites of peripheral nerve repair. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:357-366.
Ozturk C (2015) Peripheral Nerve Surgery Models Sciatic Nerve Crush Injury Model. Plast Reconstr Surg 513-517.
Petersen J, Russell L, Andrus K, MacKinnon M, Silver J, Kliot M (1996) Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery 38:976-984.
Pfister LA, Papaloïzos M, Merkle HP, Gander B (2007) Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst 12:65-82.
Saceda J, Isla A, Santiago S, Morales C, Odene C, Hernández B, Deniz K (2011) Effect of recombinant human growth hormone on peripheral nerve regeneration: experimental work on the ulnar nerve of the rat. Neurosci Lett 504:146-150.
Shea JE, Garlick JW, Salama ME, Mendenhall SD, Moran LA, Agarwal JP (2014) Side-to-side nerve bridges reduce muscle atrophy after peripheral nerve injury in a rodent model. J Surg Res 187:350-358.
Shen NJ, Zhu JK (1993) The application of sciatic nerve function index in the evaluation of neural function. Zhonghua Xianwei Waike Zazhi 16:284-287.
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146-156.
Stang F, Keilhoff G, Fansa H (2009) Biocompatibility of different nerve tubes. Materials 2:1480-1507.
Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H, Chen B, Xiao Z, Dai J (2009) The effect of collagen-binding NGF-β on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials 30:4649-4656.
Wolthers M, Moldovan M, Binderup T, Schmalbruch H, Krarup C (2005) Comparative electrophysiological, functional, and histological studies of nerve lesions in rats. Microsurgery 25:508-519.
Yin ZS, Zhang H, Gao W (2010) Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats. AJNR Am J Neuroradiol 31:509-515.
Copyedited by Patel B, Norman C, Wang J, Wang L, Li CH, Song LP, Zhao M
10.4103/1673-5374.182713 http://www.nrronline.org/
How to cite this article: Li Q, Li T, Cao XC, Luo DQ, Lian KJ (2016) Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion. Neural Regen Res 11(5)∶835-841.
Funding: This study was supported by the Technology Fund of Zhangzhou City in China, No. Z2010086.
Accepted: 2015-12-22
*Correspondence to: Ke-jian Lian, gkxiaohe@163.com.
- 中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells

