Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
PERSPECTIVE
Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
Neuronal degeneration is a social and health problem, because of high incidence of acute injury, stroke, and neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease. Neural regeneration is crucial for promoting functional recovery after neural injury (Yiu and He, 2006). Human central nervous system, however, shows low ability to regenerate injured neurons. Strategies to promote neural regenerations are required for developing therapeutics of spinal cord injury, traumatic brain injury, and neurodegenerative disorders. Here, we describe physiological roles of apolipoprotein (apo) E-containing lipoproteins (LpEs) and polyunsaturated fatty acids (PUFAs) on neural outgrowth, axonal extension, and neural protection, and their implications to promote neural regeneration.
Glial cells have critical contributions to the growth, function, and regulation of neurons. For example, glial cells secrete neurotrophic factors such as nerve growth factors, which stimulate neuronal growth. Glial cells supply lipids to neurons and contribute to lipid metabolism in the central nervous system. Disturbance of lipid metabolism is implicated in the pathogenesis of neurodegenerative disorders such as Alzheimer's disease and Niemann-Pick C disease. LpEs, secreted from glial cells, primarily astrocytes, transport lipids such as cholesterol and phospholipids to neurons (Vance and Hayashi, 2010). LpEs are present in the cerebrospinal fluid and are the size and density of high-density lipoprotein (HDL) in the periphery. The lipid components of LpEs are cholesterol and phospholipids such as HDL, while the major protein component of LpEs is apoE rather than apoA-I in HDL. There are subtypes of apoE, including apoE2, apoE3, and apoE4. Of the subtypes, apoE4 expression is the strongest genetically-mediated risk factor for developing Alzheimer's disease. ApoE and LpEs are involved in amyloid β deposition and apoE synthesis increases after nerve injury. ApoE is synthesized in astrocytes and lipidated by two ABC transporters, ABCA1 and ABCG1 (Figure 1). These transporters mediate the efflux of cholesterol and phospholipids, resulting in LpE formation (Matsuo, 2010). However, it is not clear whether apoE is lipidated within cells, or following secretion. LpEs secreted from astrocytes bind to receptors, including low-density lipoprotein receptor, low-density lipoprotein receptor-related protein (LRP), and apoER2. These receptors are expressed on the plasma membrane of neurons and binding initiates downstream signalling pathways. LpEs are endocytosed with the receptors, and lipids from the LpEs are reabsorbed by neurons. Lipids from the LpEs are recycled into the membrane component of neurons or participate in regulating neural functions.
The roles of LpEs on neuronal functions have been studied, with results indicating that LpEs protect neurons from apoptosis induced by removal of neurotrophic factors (Hayashi et al., 2009). LpEs bind to LRP1 in rat retinal ganglion cells and affect GSK3β and calcineurin signalling pathways, thereby preventing apoptosis. However, protection of neurons from apoptosis does not require LpE uptake by LRP1. Neurite outgrowth and axonal extensions require lipids to expand membranes; therefore, LpEs are involved in neural regeneration. For example, synaptogenesis is promoted by cholesterol that originates from LpEs. We examined the effects of LpEs on axonal extension after axotomy using a compartmented culture system (Matsuo et al., 2011). LpEs stimulated axonal extension of retinal ganglion cells, which are central nervous system neurons. We also demonstrated that LpEs bind to LRP1 in distal axons, because the addition of LpEs to distal axons but not cell bodies and proximal axons had stimulatory effects on axonal extension. Furthermore, suppression of LRP1 abolished the stimulatory effect, although α2-macroglobulin, a LRP1 ligand, did not have effects. These findings suggest that the LRP1-mediated signalling pathway is not sufficient for LpE-dependent axonal extension. Phospholipids exported to LpEs by ABC transporters, especially ABCG1, in astrocytes are required for enhancement of axonal extension, and the lipid composition of LpEs affects axonal extension stimulated by LpEs. These results suggest that the phospholipids in LpEs are endocytosed into neurons and that these phospholipids are essential to enhancement of axonal extension by LpEs. Furthermore, LpEs isolated from cerebrospinal fluid LRP-dependently stimulate neurite outgrowth in a neuronal cell line in the presence of exogenously added apoE, but not without apoE administration (Holtzman et al., 1995; Fagan et al., 1996). These studies suggest that LpE may be involved in the neuroprotection from the stress and the neuroplasticity.
PUFAs are a group of fatty acids that have plural double bonds in their carbon chains. n-3PUFAs, including eicosapentaenoic acid (20:5 n-3, EPA) and docosahexaenoic acid (22:6 n-3, DHA), have double bonds (C=C) at the third carbon atom from the end of the carbon chain. In contrast, n-6PUFAs, including arachidonic acid (20:4 n-6, AA), have a double bond at the sixth carbon atom. PUFAs have many crucial roles in the human body, including as precursors of membrane lipids, as anti-oxidants, and as ligands to transcription factors such as peroxisome proliferator-activated receptor and retinoid X receptor. PUFAs also activate cell signalling pathways and affect the functions of membrane proteins. AA is a precursor of eicosanoids, which are involved in functions such as inflammation and regulation of blood pressure. n-3 PUFAs, which are found in high concentrations in fish oils, suppresses the risk of atherosclerosis via anti-inflammatory and anti-arrhythmic effects. PUFAs are obtained from dietary sources and produced in the liver from precursor fatty acids. They are then circulated throughout the body, including the brain. PUFAs, especially DHA, are highly enriched in the brain, but they cannot be synthesized de novo there. Glial cells and neurons are minor producers of PUFAs; therefore, PUFAs must be transported across the blood-brain barrier. For example, DHA in the form of lysophosphatidylcholine is transported across the blood-brain barrier by Mfsd2a (Nguyen et al., 2014). PUFAs have many physiological effects in the central nervous system, such as affecting neurotransmission and synaptic plasticity, as well as contributing to cognition, learning, and memory (Zhang et al., 2011). EPA and DHAare associated with a reduced risk of Alzheimer's disease and also protect neurons from apoptosis and the effects of cerebral ischemia. DHA concentration decreases in Alzheimer's disease patient compared with healthy control, indicating that DHA is physiologically critical. Additionally, the administration of DHA in free fatty acid form to neurons stimulates neurite outgrowth (Cao et al., 2005). There are conflicting reports regarding AA and EPA; several reports have demonstrated that AA and EPA stimulates neurite outgrowth, while other reports indicated that they suppress it.
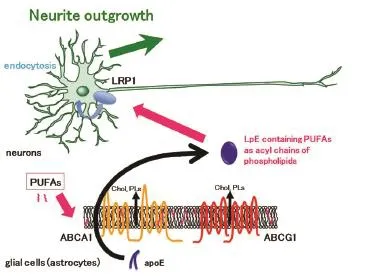
Figure 1 Apolipoprotein (apo) E-containing lipoproteins (LpEs) formation and incorporation of polyunsaturated fatty acids (PUFAs) into LpEs as phospholipids by ABCA1 and ABCG1 in glial cells.
Cells incubated with PUFAs internalize the PUFAs (Figure 1). PUFAs translocate from the outer leaflet to the inner leaflet of membrane bilayers by flip-flopping and interact with fatty acid binding proteins. Then, PUFAs are used for the synthesis of diacylglycerol, and are finally incorporated into phospholipids as acyl chains. PUFAs are located predominantly at the sn-2 position of phospholipids. ABCA1 and ABCG1 efflux membrane phospholipids to form LpEs as described above. Therefore, we hypothesize that PUFA administration to astrocytes affects both the lipid compositions of LpEs and neuronal functions. Our recently published study examined the effects of PUFAs on neurite outgrowth in hippocampal neurons, via LpE (Nakato et al., 2015). Mass spectrometry demonstrated that administration of AA, EPA, and DHA to the medium of astrocytes resulted in their inclusion into phosphatidylcholine as acyl chains of LpEs secreted from astrocytes. Additionally, LpEs secreted from astrocytes treated with EPA contained metabolites of PUFAs such as docosapentaenoic acid (22:5 n-3), suggesting that PUFAs are metabolized in astrocytes by elongation. When control LpEs purified by sucrose gradient ultracentrifugation were added to hippocampal neurons, neurite outgrowth was stimulated. Furthermore, LpEs containing AA, EPA, or DHA stimulated neurite outgrowth more compared to control LpEs. However, LpEs containing saturated fatty acid or monounsaturated fatty acid stimulated neurite outgrowth similarly to control LpEs. These results indicate that LpEs enhance neurite outgrowth and that PUFAs have a stimulatory effect on this enhancement.
We also analysed the molecular mechanisms for internalization of PUFAs in LpEs, as well as the mechanisms forthe effects on neurite outgrowth. Receptor-associated protein, a protein that competes with LpEs for binding to LRP, and an antibody against LRP1 abolished the stimulatory effects of LpEs containing PUFAs, indicating a critical role for LRP1. α2-macroglobulin, which binds to LRP1 and initiates signalling pathways, did not affect neurite outgrowth, suggesting that LRP1-mediated signalling pathways are not sufficient to stimulate neurite outgrowth. Furthermore, blockade of LpEs endocytosis by cytochalasin D, an actin filament disruptor, abolished the stimulatory effects of LpEs. These results suggest that LRP1-dependent endocytosis of LpEs contributes to PUFA internalization and stimulation of neurite outgrowth. Control LpEs enhanced neurite outgrowth by increasing the length of neurites and the number of branches, and PUFAs in LpEs stimulated neurite outgrowth by increasing the number of branches. Hippocampal neuron mRNA levels of growth-associated protein 43 (GAP-43), which contributes to growth cone formation, increased with LpE treatment containing AA, EPA, or DHA compared to control LpEs. This finding suggests that GAP-43, which is localized to neurites and induces neurite outgrowth, is responsible for the stimulatory effects of PUFAs in LpEs. It is likely PUFAs exert their effects through multiple mechanisms.
Our study revealed a novel role of LpEs, as well as a novel pathway in which PUFAs are supplied to neurons (Figure 1). LpEs have stimulatory effects on neurite outgrowth, and neurons receive PUFAs from LpEs as phospholipids. The contribution of indirect pathways via LpEs must be considered, in addition to the direct pathway supplying PUFAs to neurons, in order to fully elucidate the physiological effects of PUFAs. We also found free fatty acid forms of PUFAs and their metabolites in LpEs, using mass spectrometry, although the concentrations were quite low. These low concentrations likely resulted from astrocytes being washed with buffer in order to remove the remaining PUFAs on the plasma membrane. More free PUFAs would be expected without washing, and free acid forms of PUFAs would occur in LpEs in vivo, owing to the fact that PUFAs are attached to albumin and lipoproteins in peripheral circulation. In addition to the PUFAs in phospholipids, free PUFAs conjugated with LpEs may be delivered to neurons. Therefore, there may be three pathways of delivering PUFAs to neurons: direct supply of free PUFAs from cerebrospinal fluid, free PUFAs from LpEs, and PUFAs originating from phospholipids. The contributions of these three pathways to neurite outgrowth require further investigation.
Neurons are injured by ischemic stress, spinal cord injuries, and neurodegenerative disorders. Therefore, neuronal regeneration is an important consideration in developing treatments to prevent or alleviate neuronal dysfunction. Synthesis of apoE in glial cells markedly increases after nerve injury (Ignatius et al., 1986) and LpEs stimulate both axonal extension and neurite outgrowth; therefore, we propose a possible application of LpEs to neural regeneration. Compounds that induce apoE synthesis in glial cells may be useful for increasing production of LpEs. Furthermore, developing compounds that increase synthesis and activities of ABCA1 and ABCG1 may increase LpEs levels. Increased PUFAs to astrocytes may result in LpEs containing PUFAs that have more potency to repair injured neurons by stimulating neurite outgrowth. Therefore, increased formation of LpEs and PUFA availability may improve neural regeneration after injury.
This work was supported by Grant-in-Aid for Scientific Research (2480139 and 26660071) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), by the Bio-Oriented Technology Research Advancement Institution of Japan, and by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry from the Ministry of Agriculture, Forestry and Fisheries of Japan. This work was also supported by grants from Kyoto Women's University and the Research Institute for Production Development.
Michinori Matsuo*
Department of Food and Nutrition, Faculty of Home Economics, Kyoto Women’s University, Kyoto, Japan
*Correspondence to: Michinori Matsuo, Ph.D., matsuomi@kyoto-wu.ac.jp.
Accepted: 2016-03-15
orcid: 0000-0001-8905-8898 (Michinori Matsuo)
Cao D, Xue R, Xu J, Liu Z (2005) Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutri Biochem 16:538-546.
Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM (1996) Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem 271:30121-30125.
Hayashi H, Campenot RB, Vance DE, Vance JE (2009) Protection of neurons from apoptosis by apolipoprotein E-containing lipoproteins does not require lipoprotein uptake and involves activation of phospholipase Cγ1 and inhibition of calcineurin. J Biol Chem 284:29605-29613.
Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL (1995) Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci U S A 92:9480-9484.
Ignatius MJ, Gebicke-Härter PJ, Skene JH, Schilling JW, Weisgraber KH, Mahley RW, Shooter EM (1986) Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A 83:1125-1129.
Matsuo M (2010) ATP-binding cassette proteins involved in glucose and lipid homeostasis. Biosci Biotechnol Biochem 74:899-907.
Matsuo M, Campenot RB, Vance DE, Ueda K, Vance JE (2011) Involvement of low-density lipoprotein receptor-related protein and ABCG1 in stimulation of axonal extension by apoE-containing lipoproteins. Biochim Biophys Acta 1811:31-38.
Nakato M, Matsuo M, Kono N, Arita M, Arai H, Ogawa J, Kioka N, Ueda K (2015) Neurite outgrowth stimulation by n-3 and n-6 PUFAs of phospholipids in apoE-containing lipoproteins secreted from glial cells. J Lipid Res 56:1880-1890.
Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509:503-506.
Vance JE, Hayashi H (2010) Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim Biophys Acta 1801:806-818.
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617-627.
Zhang W, Li P, Hu X, Zhang F, Chen J, Gao Y (2011) Omega-3 polyunsaturated fatty acids in the brain: metabolism and neuroprotection. Front Biosci 16:2653-2670.
10.4103/1673-5374.182686 http∶//www.nrronline.org/
How to cite this article: Matsuo M (2016) Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration. Neural Regen Res 11(5):715-716.
- 中国神经再生研究(英文版)的其它文章
- Self-assembling peptide nanofibrous hydrogel as a promising strategy in nerve repair after traumatic injury in the nervous system
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells

