Cyanidin suppresses amyloid beta-induced neurotoxicity by inhibiting reactive oxygen speciesmediated DNA damage and apoptosis in PC12 cells
Yi Wang, Xiao-ting Fu, Da-wei Li Kun Wang Xin-zhi Wang Yuan Li Bao-liang Sun Xiao-yi Yang Zun-cheng Zheng, Nam Chun Cho
1 Department of Ophthalmology, Chonbuk National University Medical College, Jeonju-si, Jeollabuk-do, Republic of Korea
2 Key Lab of Cerebral Microcirculation in Universities of Shandong, Taishan Medical University, Taian, Shandong Province, China
3 Department of Rehabilitation, Taian Central Hospital, Taian, Shandong Province, China
RESEARCH ARTICLE
Cyanidin suppresses amyloid beta-induced neurotoxicity by inhibiting reactive oxygen speciesmediated DNA damage and apoptosis in PC12 cells
Yi Wang1,2,#, Xiao-ting Fu2,#, Da-wei Li2, Kun Wang2, Xin-zhi Wang2, Yuan Li2, Bao-liang Sun2, Xiao-yi Yang2, Zun-cheng Zheng3,*, Nam Chun Cho1,*
1 Department of Ophthalmology, Chonbuk National University Medical College, Jeonju-si, Jeollabuk-do, Republic of Korea
2 Key Lab of Cerebral Microcirculation in Universities of Shandong, Taishan Medical University, Taian, Shandong Province, China
3 Department of Rehabilitation, Taian Central Hospital, Taian, Shandong Province, China
Amyloid beta (Aβ)-induced oxidative stress is a major pathologic hallmark of Alzheimer's disease. Cyanidin, a natural flavonoid compound, is neuroprotective against oxidative damage-mediated degeneration. However, its molecular mechanism remains unclear. Here, we investigated the effects of cyanidin pretreatment against Aβ-induced neurotoxicity in PC12 cells, and explored the underlying mechanisms. Cyanidin pretreatment significantly attenuated Aβ-induced cell mortality and morphological changes in PC12 cells. Mechanistically, cyanidin effectively blocked apoptosis induced by Aβ, by restoring the mitochondrial membrane potential via upregulation of Bcl-2 protein expression. Moreover, cyanidin markedly protected PC12 cells from Aβ-induced DNA damage by blocking reactive oxide species and superoxide accumulation. These results provide evidence that cyanidin suppresses Aβ-induced cytotoxicity, by preventing oxidative damage mediated by reactive oxide species, which in turn inhibits mitochondrial apoptosis. Our study demonstrates the therapeutic potential of cyanidin in the prevention of oxidative stress-mediated Aβ neurotoxicity.
nerve regeneration; cyanidin; amyloid-beta; oxidative damage; reactive oxide species; apoptosis; neural regeneration
#These authors contributed equally to this study.
orcid: 0000-0002-4021-4084 (Zun-cheng Zheng)
Accepted: 2015-12-22
Introduction
Alzheimer's disease (AD) is a chronic, age-related, progressive neurodegenerative disease. Deposition of amyloid beta (Aβ) leads to senile plaques, which subject the neuron to oxidative stress and disrupt intracellular connections, resulting in neuronal death. This is considered the main mechanism of AD pathogenesis (Selkoe, 1998; Huang and Jiang, 2009; Eckert et al., 2010; Pagani and Eckert, 2011). Reactive oxygen species (ROS) are small, reactive molecules that play an important role in causing human diseases. Aβ-mediated oxidative stress can attack proteins, membrane lipids and DNA, induce apoptotic cell death, and eventually result in the dysfunction of the neuronal network (Acquaviva et al., 2003). Therefore, blocking Aβ-induced oxidative stress is becoming recognized as an effective strategy by which to combat AD, and potent antioxidant agents show potential in the treatment of neurodegenerative diseases.
Cyanidin, a pigment found in red berries, belongs to the flavonoid family, and has attracted much attention owing to its antioxidant and biological properties (Acquaviva et al., 2003; King et al., 2013). Acquaviva et al. (2003) reported that cyanidin showed marked protective potential against ROS-mediated oxidative damage. Further evidence also suggested that a cyanidin analogue could eliminate overproduced ROS (Chen et al., 2003; Guerra et al., 2005). Cyanidin was also identified as a neuroprotective constituent against oxidative stress (Kim et al., 2005). However, the protective efficiency and mechanism remain to be evaluated in detail. Ye et al. (2010) reported that purple sweet potato anthocyanins could inhibit Aβ-induced cytotoxicity in PC12 cells through inhibition of oxidative stres. Therefore, in the present study, we used the PC12 cell line as an in vitro model for evaluating oxidative damage in neurons (Paavlica et al., 2005), to investigate the potential of cyanidin to protect PC12 cells from Aβ-induced neurotoxicity and apoptosis.
Materials and Methods
Cell culture and drug treatment
PC12 cells (ATCC, MD, USA) were maintained in high glucose Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 100 U/mL penicillin and 50 U/mL streptomycin under 5% CO2at 37°C. Cells were pre-cultured in a 96-wellplate (1 × 104cells per well) for 24 hours. Aβ25—35(Sigma, San Francisco, CA, USA) was dissolved in phosphate-buffered saline (PBS) and incubated at 37°C for 3 days prior to treatment. A dose-finding study was performed in which PC12 cells were exposed to cyanidin (5—80 µM) for 24 hours followed by Aβ25—35(5, 10, 20, 40 and 80 µM) for 24 hours. For the protection experiment, PC12 cells were pre-incubated with cyanidin (20, 40, 80 µM; Sigma) for 24 hours followed by Aβ25—35(10 µM) for 24 hours.
MTT assay
Cell viability was assayed using MTT (Fan et al., 2014). After exposure to cyanidin and Aβ25—35, 20 µL MTT solution (Sigma; 5 mg/mL) was added to each well for 5 hours at 37°C. The medium was removed, and 100 µL DMSO was added to each well and the plate was incubated for 15 minutes. Color intensity was measured at 570 nm using a SpectraMax M3 (Molecular Devices, Sunnyvale, CA, USA). Results were expressed as percent MTT reduction, assuming that the absorbance of control cells was 100%. Cells were viewed under a microscope (Nikon Eclipse 80i; Nikon, Tokyo, Japan).
TUNEL/DAPI double staining
A TUNEL-DAPI double staining kit (Beyotime, Beijing, China) was used to measure cell apoptosis, as described previously (Fan et al., 2014). Cells cultured in chamber slides were fixed, permeabilized with 0.1% Triton X-100 in PBS for 2 minutes, treated with TUNEL reaction mixture and terminal deoxynucleotidyl transferase, washed with PBS, and observed under a microscope (Nikon).
Measurement of mitochondrial membrane potential (Δψm) Δψm was evaluated using JC-1 dye, as described previously (Fan et al., 2014). In brief, PC12 cells were incubated with 80 µM cyanidin for 1 hour, co-incubated with 10 µM Aβ25—35for 2 hours, and then treated with JC-1 probe (10 µg/mL) for 10 minutes. The cells were washed with PBS and visualized using fluorescence microscopy (200× magnification).
Caspase-3 activity
After cyanidin and Aβ25—35treatment, PC12 cells were collected and lysed in buffer, then centrifuged. Protein concentration was measured, and caspase-3 activity was examined as described previously (Fan et al., 2014). Briefly, the caspase-3 substrate Ac-Asp-Glu-Val-Asp-AMC (Ac-DEVD-AMC; Cell Signaling Technology, Boston, USA) and 100 µg total protein samples were added to each well of a 96-well plate. The plate was incubated at 37°C in the dark for 2 hours, and caspase-3 activity was measured at excitation and emission wavelengths of 380 nm and 440 nm, respectively.
Detection of ROS and superoxide
Relative levels of ROS were determined using a fluorometric method, the dichloro-dihydro-fluorescein diacetate assay (DCFH-DA; Beyotime). Because free radical generation is an early event, ROS and superoxide were detected at 2 hours, as previously reported (Wang et al., 2015; Fu et al., 2016). Briefly, cells were seeded at 1 × 104cells/well in a 96-well plate, then incubated for 1 hour with or without 20—80 µM cyanidin, and co-incubated with 10 µM Aβ25—35for 2 hours. DCFH-DA (10 µM) was added to the cells, and ROS generation was monitored at excitation and emission wavelengths of 488 nm and 525 nm, respectively. The superoxide was detected by a mitochondria-targeted fluorogenic dye, MitoSOX (Beyotime).
Western blot assay
Protein expression was evaluated by western blot assay as previously described (Wang et al., 2015; Fu et al., 2016). Briefly, cells were collected and lysed, and protein was quantified using a BCA assay kit. The protein was mixed with buffer, boiled, and 40 µg protein was used for SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to a nitrocellulose membrane, which was then blocked with 5% non-fat milk (Sigma) for 2 hours and incubated with primary antibodies (1:2,000) for 12 hours at 4°C. Primary antibodies were caspase-3, Bcl-xl, Bcl-2, Bas, Bad, Ser15-p53, total-p53, Ser139-histone and β-actin (all from Cell Signaling Technology). The membrane was then incubated with secondary antibodies (1:2,000) (Cell Signaling Technology) for 2 hours at 37°C. Proteins were visualized on X-ray film and quantified using Quantity-One software (Bio-Rad, Hercules, CA, USA). Target protein expression was normalized to β-actin (a reference control).
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± SD. The difference between two groups was analyzed by a two-tailed Student's t-test. Differences among three or more groups were analyzed by one-way analysis of variance and multiple comparisons. P < 0.05 was considered statistically significant.
Results
Cyanidin protected PC12 cells from Aβ-induced cytotoxicity
Cell growth was first assessed in the presence of cyanidin and Aβ alone. The MTT assay showed that PC12 cell viability did not change after cyanidin treatment at concentrations of 5—80 µM (Figure 1A). Exposure to Aβ alone (5—80 µM) decreased cell viability in a concentration-dependent manner, and with the greatest toxicity seen at 80 µM (Figure 1B). Therefore, we chose a concentration of 10 µM Aβ, which induced approximately 40% cell death, for subsequent experiments. Cell viability was 60.2% after exposure to 10 µM Aβ (Figure 1C). However, cyanidin pretreatment effectively blocked the Aβ-induced decrease in PC12 cell viability. At 40 and 80 µM, cyanidin increased cell viability to 79.2% and 94.4%, respectively, from 60.2%. The results demonstrated that cyanidin pretreatment could effectively block Aβ-induced cytotoxicity. Furthermore, cells exposed to Aβ showed apoptotic bodies and were fewer in number compared with control cells. As expected, cyanidin pretreatment markedly improved the morphological changes induced by Aβ (Figure 1D). No apoptotic bodies were observed and cell number was markedly greater in the cyanidin pretreatment groupthan in the group exposed to Aβ alone. These changes in cell morphology and number further validated the protective potential of cyanidin (Figure 1D).
Cyanidin blocked Aβ-induced apoptosis in PC12 cells
The prevention of cyanidin against Aβ-induced apoptosis was first detected using TUNEL and DAPI staining. Apoptotic cells generate many 3′-OH ends, which fluoresce green when subjected to the TUNEL reaction. Thus, green fluorescence represents apoptotic cells. PC12 cells exposed to Aβ alone showed notable apoptotic changes, such as chromatin condensation and nuclear fragmentation (Figure 2A). Furthermore, caspase-3 cleavage and activity were also examined. Caspase-3 activity and cleavage were notably elevated after Aβ treatment (Figure 2B, C; P < 0.05). However, cyanidin pretreatment protected PC12 cells from Aβ-induced apoptosis, shown by a decrease in fluorescence intensity, and significantly attenuated caspase-3 activity and cleavage (P < 0.05). Collectively, the results affirmed that cyanidin pretreatment markedly suppressed Aβ-induced cytotoxicity by inhibiting Aβ-induced cell apoptosis involving caspase-3 activation.
Cyanidin inhibited Aβ-induced loss of Δψm by regulating Bcl-2
In view of the importance of mitochondria in drug-induced apoptosis, Δψm was examined by JC-1 probe. JC-1 probe can go through the cell mitochondria and show obvious red fluorescence. But, in response to apoptotic stimuli, the probe can transfer into cytoplasm and show green fluorescence. Hence, the fluorescent changes can indirectly reflect the loss of Δψm. The Δψm in PC12 cells was notably decreased after Aβ exposure alone, shown by the enhanced green fluorescence (Figure 3A). Cells treated with cyanidin alone caused no change in the Δψm. Pretreatment of cells with cyanidin completely reversed Aβ-induced depletion of Δψm. The result suggests that cyanidin blocked Aβ-induced mitochondrial dysfunction by regulating Δψm. The Bcl-2 family plays a key role in regulating Δψm and launching mitochondria-mediated apoptosis. Bcl-2 family members (including pro-apoptotic and pro-survival members) were examined by western blot assay (Figure 3B). Aβ treatment significantly upregulated the expression of Bax and Bad, but downregulated the expression of Bcl-XL and Bcl-2, which indicated that Aβ induced PC12 cell apoptosis by regulation of the Bcl-2 family. However, cyanidin pretreatment markedly blocked Aβ-induced imbalance of Bcl-2 family. Together, these results revealed that cyanidin inhibited Aβ-induced apoptosis and the loss of Δψm by modulation of the Bcl-2 family.
Cyanidin blocked ROS-mediated DNA damage in Aβ-exposed cells
ROS accumulation was measured by DCFH-DA assay. Intracellular ROS was significantly increased to 226.3% of control after treatment with 10 µM Aβ for 1 hour (Figure 4). However, pretreatment with cyanidin (20, 40 and 80 µM) caused a marked dose-dependent decline in ROS (to 198.3%, 153.8% and 118.1%, respectively) in cells exposed to Aβ. Furthermore, the superoxide in live cells was also detected by MitoSOX, a mitochondria-targeted red dye. PC12 cells showed notable superoxide overproduction after Aβ treatment in mitochondria, but cyanidin pretreatment markedly blocked this (Figure 4B), as evidenced by the weakness of the red fluorescence. These results suggest that cyanidin protects PC12 cells from Aβ-induced cytotoxicity via inhibition of ROS and superoxide.
ROS accumulation can damage DNA, even causing apoptotic cell death. Therefore, we examined the effect of cyanidin on Ser15-p53 and Ser139-H2A phosphorylation, both DNA damage markers. Phosphorylated Ser139-H2A and Ser15-p53 were markedly upregulated in PC12 cells exposed to Aβ (P < 0.05). As expected, pretreatment with cyanidin completely suppressed phosphorylation of Ser139-H2A and Ser15-p53, to the level observed in control cells (P < 0.05), indicating that cyanidin pretreatment prevented Aβ-induced DNA damage. Taken together, the results provide evidence that cyanidin pretreatment markedly attenuates Aβ-induced DNA damage by repressing ROS accumulation.
Discussion
Aβ-induced oxidative stress and redox dysregulation contribute to the etiology and pathology of neurodegenerative diseases (Selkoe, 1998; Huang and Jiang, 2009; Eckert et al., 2010; Pagani and Eckert, 2011). Oxidative stress may damage neuronal cells and is accepted as a major cause of neurotoxicity, and plays an important role in the occurrence and development of human neurodegenerative disorders (Crispo et al., 2010; Tapias et al., 2014). ROS can easily go through the cytoplasmic membrane, bind DNA, and lead to disorders in DNA function (Gao et al., 2013). Antioxidant treatments are beneficial in controlling these diseases (Arai et al., 2007). Therefore, agents with antioxidant activities are usually accepted as chemopreventive agents in epidemiological studies to regulate the progression of oxidative stress-mediated diseases. Increasing evidence indicates that cyanidin, a widely used natural product, exhibits novel anti-oxidant activity and can directly scavenge free radicals. However, its mechanism remains unclear.
Caspases represent the cysteine proteases that have a positive effect in launching cell apoptosis. Caspase-3 can be cleaved and activated in response to apoptosis (Kong et al., 2003; Zhang et al., 2008). Our results indicate that cyanidin blocks PC12 cells from Aβ-induced activation and decreases the expression levels of caspase-3. The changes in caspase-3 activity and caspase-3 expression both validated that cyanidin had the potential to inhibit Aβ-induced apoptosis. ROS is an important small molecule in regulating and inducing cell apoptosis. The balance between ROS accumulation and the cellular defense system ultimately decides the survival or death of the cell (Pabla et al., 2008; Fan et al., 2014). Cyanidin can protect neuronal cells from oxidative damage-induced neurotoxicity by decreasing the generation of ROS (Li et al., 2013; Mokni et al., 2013). In this study, Aβ significantly increased ROS accumulation and activated DNA damage in PC12 cells. However, cyanidin effectively terminated Aβ-induced ROS production and DNA damage. The findings indicated the potential of cyanidin to scavenge free radicals and thus prevent PC12 cells from ROS-mediated oxidative stress and apoptosis.
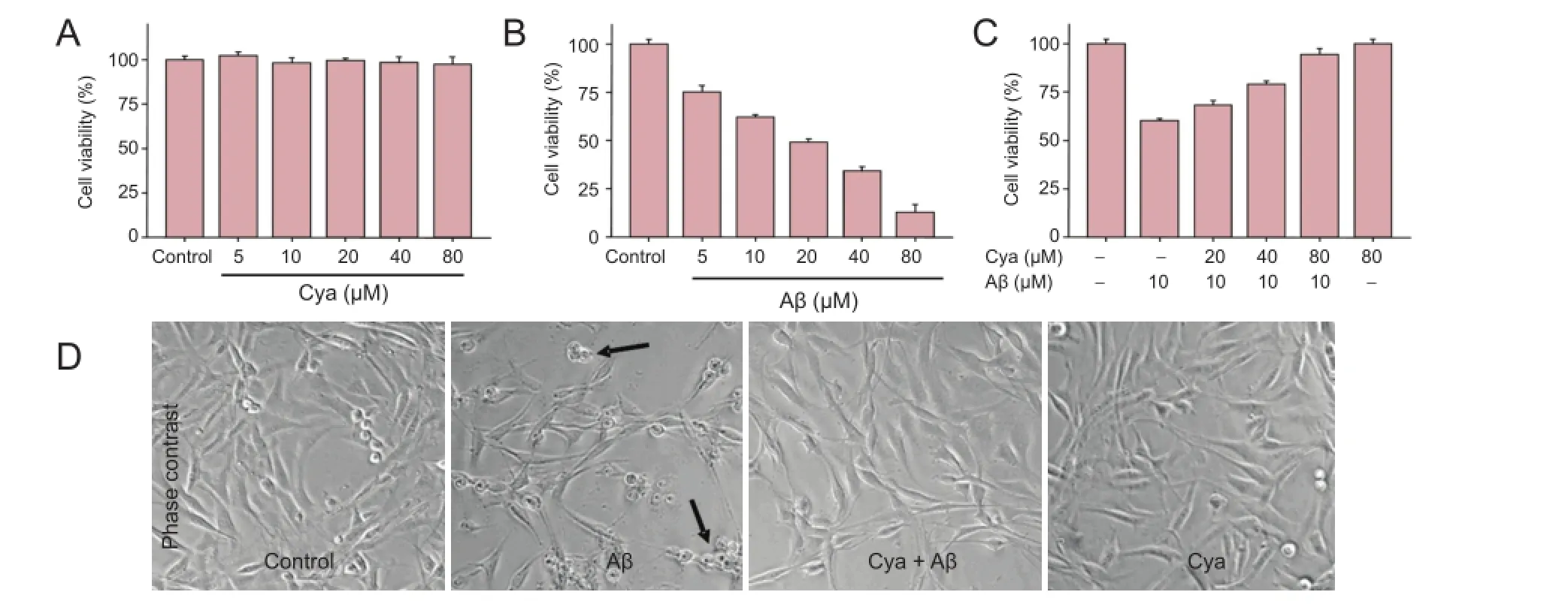
Figure 1 Cyanidin (Cya) suppressed amyloid-beta (Aβ)-induced cytotoxicity in PC12 cells.

Figure 2 Cyanidin (Cya) prevented amyloid-beta Aβ)-induced apoptosis in PC12 cells.


Figure 3 Cyanidin (Cya) inhibited amyloid-beta (Aβ)-induced loss of mitochondrial membrane potential (Δψm) by modulating Bcl-2 family proteins.
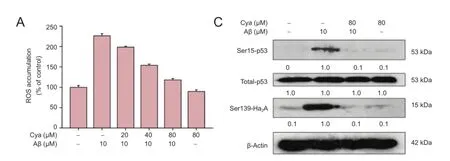

Figure 4 Cyanidin (Cya) prevented amyloid-beta (Aβ)-induced DNA damage by suppressing reactive oxygen species (ROS) accumulation.
The antioxidant enzyme system is the endogenous free radical scavenger (Mokni et al., 2013). These enzymes can transfer H2O2to water without any toxicity, block the process of lipid peroxide in the body and make the lipid peroxide reduction into a harmless polyol (Kalpana et al., 2007). Therefore, we speculated that the addition of cyanidin may affect the endogenous anti-oxidase system, eliminate the Aβ-induced ROS and ultimately suppress Aβ-induced PC12 cell apoptosis, providing a new idea for development of antioxidant drugs in the treatment of neurological disorders.
In conclusion, we evaluated the protective potential of cyanidin against Aβ-induced neurotoxicity in PC12 cells and investigated the underlying mechanism. Our results indicate that cyanidin suppresses Aβ-induced cytotoxicity by suppressing ROS-mediated oxidative damage and inhibiting mitochondria-mediated apoptosis (Figure 5).
Author contributions: NCC and ZCZ conceived the whole study. YW, XTF, DWL, KW and YL performed the experiments. XZW, BLS, and XYY analyzed the results. YW and KW wrote the paper. All authors read and approved the final version of this paper for publication.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
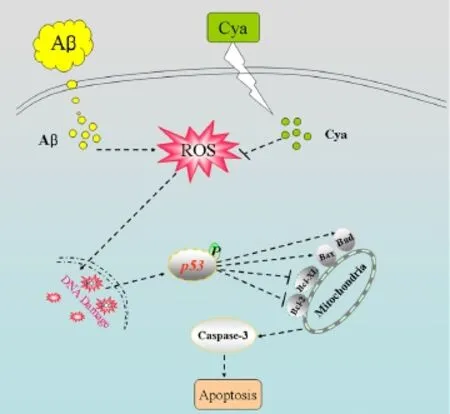
Figure 5 Scheme illustrating potential signaling pathways.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Acquaviva R, Russo A, Galvano F, Galvano G, Barcellona ML, Li Volti G (2003) Cyanidin and cyanidin 3-O-beta-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biol Toxicol 19:243-252.
Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y (2007) Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 59:27-33.
Chun OK, Kim DO, Lee CY (2003) Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem 51:8067-8072.
Crispo JA, Piche M, Ansell DR, Eibl JK, Tai IT, Kumar A (2010) Protective effects of methyl gallate on H2O2-induced apoptosis in PC12 cells. Biochem Biophys Res Commun 393:773-778.
Eckert A, Schulz KL, Rhein V, Gotz J (2010) Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Mol Neurobiol 41:107-114.
Fan C, Zheng W, Fu X, Li X, Wong YS, Chen T (2014) Strategy to enhance the therapeutic effect of doxorubicin in human hepatocellular carcinoma by selenocystine, a synergistic agent that regulates the ROS-mediated signaling. Oncotarget 5:2853-2863.
Fu XY, Yang MF, Cao MZ, Li DW, Yang XY, Sun JY (2016) Strategy to suppress oxidative damage-induced neurotoxicity in PC12 cells by curcumin, the role of ROS-mediated DNA damage and MAPKs and AKT pathways. Mol Neurobiol 53:369-378.
Gao S, Chen T, Choi MY, Liang Y, Xue J, Wong YS (2013) Cyanidin reverses cisplatin-induced apoptosis in HK-2 proximal tubular cells through inhibition of ROS-mediated DNA damage and modulation of the ERK and AKT pathways. Cancer Lett 333:36-46.
Guerra MC, Galvano F, Bonsi L, Speroni E, Costa S, Renzulli C (2005) Cyanidin-3-O-beta-glucopyranoside, a natural free-radical scavenger against aflatoxin B1- and ochratoxin A-induced cell damage in a human hepatoma cell line (Hep G2) and a human colonic adenocarcinoma cell line (CaCo-2). Br J Nutr 94:211-220.
Huang HC, Jiang ZF (2009) Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer's disease. J Alzheimers Dis 16:15-27.
Kalpana C, Sudheer AR, Rajasekharan KN, Menon VP (2007) Comparative effects of curcumin and its synthetic analogue on tissue lipid peroxidation and antioxidant status during nicotine-induced toxicity. Singapore Med J 48:124-130.
Kim DO, Heo HJ, Kim YJ, Yang HS, Lee CY (2005) Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem 53:9921-9927.
King AE, Southam KA, Dittmann J, Vickers JC (2013) Excitotoxin-induced caspase-3 activation and microtubule disintegration in axons is inhibited by taxol. Acta Neuropathol Commun 1:59.
Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R (2003) Analysis and biological activities of anthocyanins. Phytochemistry 64:923-923.
Li S, Shen C, Guo W, Zhang X, Liu S, Liang F (2013) Synthesis and neuroprotective action of xyloketal derivatives in Parkinson's disease models. Mar Drugs 11:5159-5189.
Mokni M, Hamlaoui S, Karkouch I, Amri M, Marzouki L, Limam F (2013) Resveratrol provides cardioprotection after ischemia/reperfusion injury via modulation of antioxidant enzyme activities. Iran J Pharm Res 12:867-875.
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994-1007.
Pagani L, Eckert A (2011) Amyloid-beta interaction with mitochondria. Int J Alzheimers Dis doi: 10.4061/2011/925050.
Pavlica S, Gebhardt R (2005) Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic Res 39:1377-1390.
Selkoe DJ (1998) The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol 8:447-453.
Tapias V, Cannon JR, Greenamyre JT (2014) Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson's disease. Neurobiol Aging 35:1162-1176.
Wang K, Fu XY, Fu XT, Hou YJ, Fang J, Zhang S (2015) DSePA antagonizes high glucose-induced neurotoxicity: evidences for dna damage-mediated p53 phosphorylation and mapks and akt pathways. Mol Neurobiol DOI: 10.1007/s12035-015-9373-1.
Ye JL, Meng XJ, Yan CL, Wang CB (2010) Effect of purple sweet potato anthocyanins on β-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochem Res 35:357-365.
Zhang YB, Lu GW, Yang MF, Niu JZ, Sun BL (2008) Changes in Bcl-2 and Caspase-3 expressions in cortex of hypoxic preconditioning mice. Sheng Li Xue Bao 60:249-253.
Copyedited by Slone-Murphy J, Raye W, Li CH, Song LP, Zhao M
10.4103/1673-5374.182707 http://www.nrronline.org/
How to cite this article: Wang Y, Fu XT, Li DW, Wang K, Wang XZ, Li Y, Sun BL, Yang XY, Zheng ZC, Cho NC (2016) Cyanidin suppresses amyloid beta-induced neurotoxicity by inhibiting reactive oxygen species-mediated DNA damage and apoptosis in PC12 cells. Neural Regen Res 11(5)∶795-800.
Funding: The study was supported by the Natural Science Foundation of Shandong Province of China, No. ZR2014HM046 (to ZCZ).
*Correspondence to: Zun-cheng Zheng, M.D or Nam Chun Cho, Ph.D., zhengzc1965@126.com or cnauo@jbnu.ac.kr.
- 中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells

