Inhibition of TYRO3/Akt signaling participates in hypoxic injury in hippocampal neurons
Yan-zhen Zhu, Wei Wang, Na Xian, Bing Wu
School of Basic Medical Sciences, Fujian Medical University, Fuzhou, Fujian Province, China
RESEARCH ARTICLE
Inhibition of TYRO3/Akt signaling participates in hypoxic injury in hippocampal neurons
Yan-zhen Zhu*, Wei Wang, Na Xian, Bing Wu
School of Basic Medical Sciences, Fujian Medical University, Fuzhou, Fujian Province, China
Graphical Abstract
orcid: 0000-0001-6090-7497 (Yan-zhen Zhu)
In this study, we investigated the role of the TYRO3/Akt signaling pathway in hypoxic injury to hippocampal neurons. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showed that hypoxia inhibited the proliferation and viability of hippocampal neurons. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay demonstrated that hypoxia induced neuronal apoptosis in a time-dependent manner, with a greater number of apoptotic cells with longer hypoxic exposure. Immunofluorescence labeling revealed that hypoxia suppressed TYRO3 expression. Western blot assay showed that hypoxia decreased Akt phosphorylation levels in a time-dependent manner. Taken together, these findings suggest that hypoxia inhibits the proliferation of hippocampal neurons and promotes apoptosis, and that the inhibition of the TYRO3/Akt signaling pathway plays an important role in hypoxia-induced neuronal injury.
nerve regeneration; TYRO3; Akt; apoptosis; hippocampal neurons; primary culture; hypoxia; proliferation; neural regeneration
Introduction
Hypoxic-ischemic encephalopathy refers to neurological impairment or injury that occurs after the brain is deprived of an adequate oxygen supply, and is characterized by cognitive and memory impairment, apathy and language disability. The apoptosis of hippocampal neurons is a major cause of hypoxic-ischemic encephalopathy (Zhu and Zeng, 2011). Zhu and Zeng (2011) proposed that increased expression levels of p53 and Noxa play important roles in the pathogenesis of vascular dementia.
TYRO3 is a member of a family of receptor tyrosine kinases that also includes AXL and MER (Nguyen et al., 2013; Ji et al., 2014). Upon ligand binding, TYRO3 is activated by autophosphorylation. Phosphorylated TYRO3 binds to the SH2 domain of the p85 subunit of phosphatidylinositide 3-kinase (PI3K), which then activates the downstream effector Akt, thereby suppressing apoptosis, and enhancing cell proliferation, differentation and surivival (Liang et al., 2015). Zhao et al. (2012) found that TYRO3 is highly expressed in the rat hippocampus, suggesting that TYRO3 may have a key role in the pathogenesis of cognitive dysfunction associated with hypoxic-ischemic encephalopathy.
The increase in reactive oxygen species induced by hypoxia leads to neuronal death, which contributes tohypoxic-ischemic encephalopathy. Although animal experiments have provided insight into the effects of hypoxia and ischemia on the hippocampus, they have been unable to reveal the specific functional role of individual proteins in hypoxic injury to neurons. In comparison, primary cultured neurons subjected to hypoxic injury provide a model that simulates the pathophysiological changes in hippocampal neurons after hypoxic-ischemic injury (Brimson et al., 2011; Shimamura et al., 2012). While hippocampal neurons are usually prepared from rat or mouse embryos at embryonic days 18—19, the age of fetal rats cannot be easily controlled, and the location of some nuclei and functional areas cannot be accurately determined. Therefore, neonatal rats (within 24 hours of birth) have increasingly been used for cultures of hippocampal neurons.
In this study, we used neonatal Sprague-Dawley rats (obtained within 1 day of birth) for preparing primary hippocampal neuronal cultures. We examined the effects of hypoxia on neuronal proliferation and apoptosis, as well as on TYRO3/Akt signaling, to provide insight into the role of the TYRO3/Akt pathway on the pathogenesis of hypoxic-ischemic encephalopathy.
Materials and Methods
Ethics statement
The animal studies were approved by the Animal Study Committee of Fujian Medical University of China, and were performed in accordance with the Experimental Animals Administration Regulations of Fujian Province, China.
Culture and purification of hippocampal neurons from neonatal rats
A total of 140 clean neonatal Sprague-Dawley rats (within 24 hours of birth) were provided by the Experimental Animal Center of Fujian Medical University of China, animal licence No. SCXK (Min) 2012-0001. Rats were killed by cervical dislocation. The entire brain was immersed in phosphate-buffered saline (PBS). The hippocampi were isolated under an anatomical microscope (Paxinos and Watson, 2005) and placed in PBS. Hippocampi were cut into blocks with eye scissors, digested with 2 mL of trypsin solution (Sigma, St. Louis, MO, USA), triturated, and centrifuged at 900 × g for 5 minutes. After removal of supernatant, samples were incubated with the medium, triturated, filtered with 100-mesh filter, and centrifuged at 900 × g for 5 minutes. After removal of supernatant, samples were incubated with 2 mL of Dulbecco's modified Eagle's medium (DMEM), supplemented with 600 mg/mL glucose, 10% horse serum, 100 µg/mL transferrin, 5 µg/mL insulin, 20 nM progesterone, 100 µM putrescine, 30 nM selenium dioxide, 100 U/mL penicillin and 100 µg/mL streptomycin. Cells were seeded into 12-well plates, at 1 × 106/well, and placed in an incubator at 37°C, 5% CO2, and saturated humidity. On day 3, 6.7 mg/mL 5-fluorouracil (Sigma) was added to inhibit the growth of glial cells. Immunofluorescent staing of microtubule-associated protein 2 was used to identify hippocampal neurons (Bollimpelli and Kondapi, 2015).
Hippocampal neurons subjected to hypoxia
Cultured neurons were placed in a hypoxic incubator (Thermo scientific Forma, Barnstable, MA, USA) at 37°C, 94% N2, 1% O2and 5% CO2for 12 or 24 hours. Neurons in the normal group were placed in an incubator at 37°C, 5% CO2and saturated humidity. The growth and morphology of hippocampal neurons were observed with an inverted phase contrast microscope (Leica, Wetzlar, Germany).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
A total of 1 × 106neurons were incubated with 10 µL MTT solution (Shanghai Biological Engineering Technology Co., Ltd., Shanghai, China) for 4 hours. 100 µL of dimethyl sulfoxide (Shanghai Biological Engineering Technology Co., Ltd.) was added to each well for 4 hours. Optical density values were measured at 570 nm with a microplate reader (Bio-Rad, Hercules, CA, USA). Each group contained six replicate wells. The mean value was calculated. The wells without the cell suspension or drug were used as blank controls.
Terminal deoxynucleotidyl transferase(TdT)-mediated dUTP nick end labeling (TUNEL) assay
For the TUNEL assay, 1 × 106hippocampal neurons were fixed with 4% paraformaldehyde at room temperature for 30 minutes, washed with PBS, treated with 50 µL of NeuroPore for 30 minutes, and then rinsed with DNAse-free wash solution for 2 minutes (twice). Quenching solution containing 3% H2O2/methanol was added at room temperature for 5 minutes. After PBS washes, samples were incubated with TdT buffer at room temperature for 5 minutes, incubated with reaction mixture (TdT dNTP Mix, TdT Enzyme, Mn2+) in a wet box at 37°C for 1 hour. The reaction was terminated with TdT stop buffer at room temperature for 5 minutes. After PBS washes, 2 × 2 minutes, streptavidin-horseradish peroxidase was added at room temperature for 10 minutes, followed by PBS washes, 2 × 3 minutes. Samples were visualized with 3,3′-diaminobenzidine at room temperature for 5 minutes, and washed with running water. After nuclear counterstaining, samples were washed with running water, treated with ammonia water, washed again with running water, mounted, and observed with a light microscope (Leica). Reagents were purchased from Roche (Basel, Switzerland).
Immunofluorescence staining
A total of 1 × 106hippocampal neurons were washed with PBS, fixed in 4% paraformaldehyde at room temperature for 30 minutes, incubated with 0.3% Triton X-100 for 20 minutes, and then with 30 µL 0.3% H2O2in methanol at room temperature for 10 minutes to inactivate endogenous peroxidase activity. 30 µL of normal goat serum (Beijing Zhongshan Biological Reagent Company, Beijing, China) was added to the cells at room temperature for 10 minutes, and then washed with PBS. Afterwards, samples were incubated with mouse anti-TYRO3 polyclonal antibody (1:50; Santa Cruz Biotechnology, Dallas, CA, USA) at 4°C overnight, and then with fluorescein isothiocyanate (FITC)-labeledgoat anti-mouse IgG (1:100; Beijing Zhongshan Biological Reagent Company) at 37°C for 1 hour. Cells were observed by laser scanning confocal microscopy (Leica). 0.01 M PBS, instead of primary antibody, served as negative control.
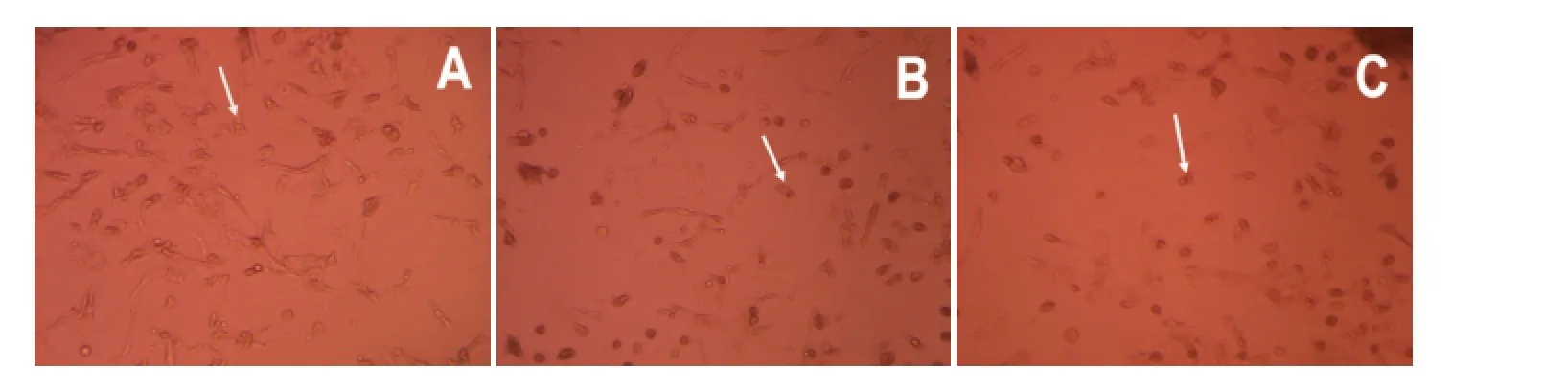
Figure 1 Effects of hypoxia on the morphology of hippocampal neurons from neonatal rats (inverted phase contrast microscope, × 20).

Figure 2 Effects of hypoxia on hippocampal neuronal viability (MTT assay).

Figure 6 Correlation between TYRO3 expression and Akt phosphorylation levels in hippocampal neurons.


Figure 3 Effects of hypoxia on hippocampal neuronal apoptosis.
Western blot assay


Figure 4 Effects of hypoxia on TYRO3 expression in hippocampal neurons.

Figure 5 Effects of hypoxia on Akt phosphorylation levels in hippocampal neurons (western blot assay).
1 × 106hippocampal neurons were digested with trypsin, incubated with medium containing 10% horse serum, and triturated into a single-cell suspension. Cells were placed in a 15-mL Eppendorf tube, and centrifuged. The supernatant was discarded. Cells were washed twice with PBS, and centrifuged. After removal of the supernatant, radioimmunoprecipitation assay lysis buffer (strong) containing 1 mM phenylmethylsulfonyl fluoride was added. After homogenization, the lysate was centrifuged at 14,000 r/min at 4°C for 10 minutes. A 5-µL aliquot of supernatant was used to detect protein concentration with the bicinchoninic acid assay. The remaining supernatant was added to 5× loading buffer, boiled, and SDS-PAGE was performed. Proteins were then wet-transferred onto a membrane. Blots were incubated with rabbit anti-Akt(Ab-473) polyclonal antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-rat Phospho-Akt(Ser473) monoclonal antibody (1:1,000; Cell Signaling Technology) or rabbit anti-rat GAPDH monoclonal antibody (1:1,000; Cell Signaling Technology) at 4°C overnight, and then with horseradish peroxidase-labeled goat anti-rabbit IgG (1:4,000; Beijing Zhongshan Biological Reagent Company) at room temperature for 2 hours. The membranes were washed three times with PBS/Tween 20, and treated with enhanced chemiluminescence reagent (Santa Cruz Biotechnology), and then exposed to X-ray film in a dark room. Phoretix 1D software (TotalLab Ltd., Newcastle Upon Tyne, UK) was used for quantification of band intensities. The level of Akt phosphorylation was corrected with GAPDH, and represented as p-Akt/Akt.
Statistical analysis
All data are expressed as the mean ± SD, and were statistically anallyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance and the least significant difference test were performed for intergroup comparisons. Pearson correlation analysis was used to analyze TYRO 3 expression and Akt phosphorylation levels. A value of P < 0.05 was considered statistically significant.
Results
Morphology of hippocampal neurons subjected to hypoxic injury
Normal neurons were large, plump, elliptical or conical, with a smooth surface, a strong refractive index and distinct processes. Normal neurons made connections with each other and formed neural networks (Figure 1A). In the 12-hour hypoxia group, hippocampal neurons appeared deformed and injured, with a reduced refractive index and vacuoles. The cellular processes were distorted, broken, and decreased in number or missing (Figure 1B). In the 24-hour hypoxia group, neurons were reduced in number, and apoptotic and necrotic cells were seen (Figure 1C).
Hypoxia reduced the proliferation and viability of hippocampal neurons
MTT assay revealed that, compared with the normal group, neuronal viability was decreased by approximately 37% in the 12-hour hypoxia group (P < 0.01), and approximately 55% in the 24-hour hypoxia group (P < 0.01). Neuronal viabilitywas lower in the 24-hour hypoxia group than in the 12-hour hypoxia group (P < 0.05; Figure 2).
Hypoxia promoted hippocampal neuronal apoptosis
A few TUNEL-positive neurons were visible in the normal group. The number of TUNEL-positive neurons was significantly greater in the 12-hour hypoxia group than in the normal group (P < 0.01). The number of apoptotic cells was higher in the 24-hour hypoxia group than in the 12-hour hypoxia group (P < 0.05; Figure 3).
Hypoxia decreased TYRO3 expression in hippocampal neurons
Laser scanning confocal microscopy revealed TYRO3 expression (green fluorescence) in the cytoplasm, cell membrane and processes. TYRO3 expression was significantly lower in the 12-hour and 24-hour hypoxia groups compared with the normal group (both P < 0.01). No significant difference in TYRO3 expression was observed between the 12-hour and 24-hour hypoxia groups (P > 0.05; Figure 4).
Hypoxia diminished Akt phosphorylation levels in hippocampal neurons
Akt is an important downstream effector of TYRO3. Akt phosphorylation contributes to cell proliferation and suppresses apoptosis (He et al., 2011; Demarest et al., 2013). Western blot assay showed that Akt phosphorylation levels were reduced by hypoxia in a time-dependent manner. Compared with the normal group, Akt phosphorylation levels were reduced by 30% in the 12-hour hypoxia group (P< 0.01) and by 47% in the 24-hour hypoxia group (P < 0.01). Akt phosphorylation levels were significantly lower in the 24-hour hypoxia group than in the 12-hour hypoxia group (P< 0.05; Figure 5).
Correlation between TYRO3 expression and Akt phosphorylation levels in hippocampal neurons
TYRO3 expression was positively correlated with Akt phosphorylation levels in hippocampal neurons (r = 0.759, P = 0.002; Figure 6).
Discussion
Hippocampal neuronal injury is a key event in the pathogenesis of cognitive dysfunction after hypoxia-ischemia (Lee et al., 2015; Mezuki et al., 2015). Animal studies have shown that apoptosis in hippocampal neurons appears on day 7 and peaks on day 14 after bilateral common carotid artery ligation. A large number of apoptotic neurons can still be seen on day 21.
In the present study, we investigated damage to hippocampal neurons subjected to hypoxic injury. We found that hypoxia reduced neuronal viability and promoted neuronal apoptosis, which were increasingly aggravated with prolonged exposure to hypoxia. These effects of hypoxia may underlie cognitive dysfunction in hypoxic-ischemic encephalopathy. The mechanisms of hypoxia-induced neuronal apoptosis likely include glutamate neurotoxicity, calcium influx, activation of apoptotic proteins and mitochondrial dysfunction.
The TYRO3 receptor is extensively expressed in the nervous system, and has received much attention in the field of nerve regeneration, particularly because of its ability to promote cell proliferation and suppress apoptosis (Nguyen et al., 2013; Lew et al., 2013). Lingand binding by TYRO3 induces dimerization or oligomerization of the protein and activates the catalytic domain, resulting in tyrosine autophosphorylation, which ultimately activates downstream signaling molecules (Zhong et al., 2010). Our present results show that hypoxia suppresses TYRO3 expression in hippocampal neurons. This suggests that the inhibition of the TYRO3 receptor plays an important role in hippocampal neuronal injury induced by hypoxia.
Akt is an important downstream effector of PI3K. PI3K activation induces Akt phosphorylation at Thr308 and Ser473, and results in Akt activation (Li et al., 2016). Akt activation contributes to cell proliferation, survival and differentiation, and it also modulates the cell cycle and suppresses apoptosis (Liu et al., 2010; Ju et al., 2015). Previous studies (Cosemans et al., 2010; Demarest et al., 2013) have shown that TYRO3 activation leads to Akt phosphorylation, thereby enhancing cell survival and proliferation. Indeed, Akt phosphorylation levels were substantially lower in the hypoxia groups than in the normal group, indicating that the inhibiton of Akt activity is probably an important mechanism of hypoxia-induced apoptosis in hippocampal neurons.
The suppression of neuronal proliferation and the induction of apoptosis following hypoxia/ischemia may involve the following mechanisms: (1) TYRO3 negatively regulates microglial cells and antigen presenting cells, and prevents injury to hippocampal neurons induced by proinflammatory cytokine overproduction (Seitz et al., 2007). Hypoxia inhibits TYRO3 expression in hippocampal neurons, thereby exacerbating the neurotoxic effects of proinflammatory cytokines. (2) TYRO3 facilitates neuronal survival, proliferation and differentiation by regulating endogenous neurotrophic factors (e.g., increasing nerve growth factor expression). Consequently, diminished TYRO3 levels lead to lowered neurotrophic factor expression, thereby reducing the proliferation and differentiation of hippocampal neurons. (3) Increased levels of inactive Akt lead to the activation of pro-apoptotic factors and inhibit expression of the anti-apoptotic factor Bcl-2 (Guo et al., 2011; Kim et al., 2012).
In summary, we found that a reduction in TYRO3 expression and a decrease in Akt phosphorylation levels are two important events in hypoxic injury to hippocampal neurons. Inhibition of the TYRO3/Akt signaling pathway plays an important role in the hypoxia-induced apoptosis of hippocampal neurons. Our findings suggest that the TYRO3/Akt pathway is a potential drug target for the prevention and treatment of hypoxic-ischemic encephalopathy.
Acknowledgments: We are very grateful to Zhi-hong Zheng and Xiao-zhen Zhao from Center of Neurobiology, Fujian Medical University, China for instructing empirical methods.
Author contributions: As a principal investigator, YZZ had full access to all data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. NX and BW were responsible for western blot assay. WW provided study guidance. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Bollimpelli VS, Kondapi AK (2015) Enriched rat primary ventral mesencephalic neurons as an in-vitro culture model. Neuroreport 26:728-734.
Brimson JM, Tencomnao T (2011) Rhinacanthus nasutus protects cultured neuronal cells against hypoxia induced cell death. Molecules 16:6322-6338.
Cosemans JM, Van Kruchten R, Olieslagers S, Schurgers LJ, Verheyen FK, Munnix IC, Waltenberger J, Angelillo-Scherrer A, Hoylaerts MF, Carmeliet P, Heemskerk JW (2010) Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J Thromb Haemost 8:1797-1808.
Demarest SJ, Gardner J, Vendel MC, Ailor E, Szak S, Huang F, Doern A, Tan X, Yang W, Grueneberg DA, Richards EJ, Endege WO, Harlow E, Koopman LA (2013) Evaluation of Tyro3 expression, Gas6-mediated Akt phosphorylation, and the impact of anti-Tyro3 antibodies in melanoma cell lines. Biochemistry 52:3102-3118.
Guo H, Barrett TM, Zhong Z, Fernández JA, Griffin JH, Freeman RS, Zlokovic BV (2011) Protein S blocks the extrinsic apoptotic cascade in tissue plasminogen activator/N-methyl D-aspartate-treated neurons via Tyro3-Akt-FKHRL1 signaling pathway. Mol Neurodegener 6:13.
He K, Jang SW, Joshi J, Yoo MH, Ye K (2011) Akt-phosphorylated PIKE-A inhibits UNC5B-induced apoptosis in cancer cell lines in a p53-dependent manner. Mol Biol Cell 22:1943-1954.
Ji R, Meng L, Jiang X, Cvm NK, Ding J, Li Q, Lu Q (2014) TAM receptors support neural stem cell survival, proliferation and neuronal differentiation. PLoS One 9:e115140.
Jin DQ, Lim CS, Hwang JK, Ha I, Han JS (2005) Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem Biophys Res Commun 331:1264-1269.
Ju DT, Liao HE, Shibu MA, Ho TJ, Padma VV, Tsai FJ, Chung LC, Day CH, Lin CC, Huang CY (2015) Nerve regeneration potential of protocatechuic acid in RSC96 schwann cells by induction of cellular proliferation and migration through IGFR-PI3K-Akt signaling. Chin J Physiol 58:412-419.
Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim JR, Ha CM, Choi YK, Lee SJ, Kim JY, Harris RA, Jeong D, Lee IK (2012) α-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med 16:273-286.
Lee JC, Cho GS, Kim IH, Park JH, Cho JH, Ahn JH, Bae EJ, Ahn JY, Park CW, Cho JH, Kim YM, Won MH, Lee HY (2015) p63 expression in the gerbil hippocampus following transient ischemia and effect of ischemic preconditioning on p63 expression in the ischemic hippocampus. Neurochem Res 40:1013-1022.
Lew ED, Oh J, Burrola PG, Lax I, Zagórska A, Través PG, Schlessinger J, Lemke G (2014) Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife 3.
Li H, Kang T, Qi B, Kong L, Jiao Y, Cao Y, Zhang J, Yang J (2016) Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3inducing rats model of Alzheimer's disease. J Ethnopharmacol 179:162-169.
Liang F, Yue J, Wang J, Zhang L, Fan R, Zhang H, Zhang Q (2015) GPCR48/LGR4 promotes tumorigenesis of prostate cancer via PI3K/ Akt signaling pathway. Med Oncol 32:49.
Liu JJ, Liu WD, Yang HZ, Zhang Y, Fang ZG, Liu PQ, Lin DJ, Xiao RZ, Hu Y, Wang CZ, Li XD, He Y, Huang RW (2010) Inactivation of PI3k/ Akt signaling pathway and activation of caspase-3 are involved in tanshinone I-induced apoptosis in myeloid leukemia cells in vitro. Ann Hematol 89:1089-1097.
Mezuki S, Fujimoto S, Matsuki T, Suzuki S, Ishitsuka T, Kitazono T (2015) Two cases of ischemic cerebrovascular disease with only memory disturbance as neurological symptom and abnormal MR findings. Rinsho Shinkeigaku 55:145-150.
Nguyen KQ, Tsou WI, Kotenko S, Birge RB (2013) TAM receptors in apoptotic cell clearance, autoimmunity, and cancer. Autoimmunity 46:294-297.
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. London: Academic Press.
Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK (2007) Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol 178:5635-5642.
Shimamura M, Taniyama Y, Katsuragi N, Koibuchi N, Kyutoku M, Sato N, Allahtavakoli M, Wakayama K, Nakagami H, Morishita R (2012) Role of central nervous system periostin in cerebral ischemia. Stroke 43:1108-1114.
Zhao QM, Li LM, Zhang C, Guo R (2012) Expression and localization of receptor tyrosine kinase Tyro3 in rat brain. Beijing Da Xue Xue Bao 44:905-910.
Zhong Z, Wang Y, Guo H, Sagare A, Fernández JA, Bell RD, Barrett TM, Griffin JH, Freeman RS, Zlokovic BV (2010) Protein S protects neurons from excitotoxic injury by activating the TAM receptor Tyro3-PI3K-Akt pathway through its SHBG-like region. J Neurosci 30:15521-15534.
Zhu Y, Zeng Y (2011) Electroacupuncture protected pyramidal cells in hippocampal CA1 region of vascular dementia rats by inhibiting the expression of p53 and Noxa. CNS Neurosci Ther 17:599-604.
Copyedited by Patel B, Stow A, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.182701 http://www.nrronline.org/
How to cite this article: Zhu YZ, Wang W, Xian N, Wu B (2016) Inhibition of TYRO3/Akt signaling participates in hypoxic injury in hippocampal neurons. Neural Regen Res 11(5)∶752-757.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81001541; the Natural Science Foundation of Fujian Province of China, No. 2013J01331.
Accepted: 2016-01-06
*Correspondence to: Yan-zhen Zhu, zhuyanzhen1976@126.com.
- 中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells

