Do we need a new levodopa?
PERSPECTIVE
Do we need a new levodopa?
Idiopathic Parkinson's disease (PD) is classified as a chronic progressive neurodegenerative disease. Various each other complementing, still hypothetical aetiologies describe the predominant dopaminergic neuronal death. They include genetic predisposition, exogenous environmental or endogenous toxin exposure, impaired detoxification, defects of mitochondrial function, microglial activation with chronic inflammation, infection with a still unknown pathogenic substrate, disturbed neurotransmission of biogenic amines and glutamate in particular. The essential final step to neuronal apoptosis is a disturbed balance in favour of free radical generation and antioxidant activity, commonly described as oxidative stress (Przuntek et al., 2004).
L-3,4-Dihydroxyphenylalanine (L-dopa) is the most effective treatment for PD. The introduction of chronic L-dopa application was one of the therapeutic milestones. A decreasing death rate in the PD patient population was observed during the first 15 years of prescription of L-dopa between 1960 and 1975. At that time, L-dopa was applied without blocking of enzymes involved in L-dopa metabolism. High dosing was necessary due to rapid degradation via dopa decarboxylase to dopamine and to a lesser extent via catechol-O-methyltransferase to 3-O-methyldopa (Hinz et al., 2014). Then a dopa decarboxylase inhibitor (DDI), such as carbidopa, was always added to L-dopa formulations. DDI impairs peripheral conversion of L-dopa to dopamine and enhance the L-dopa delivery to the brain. Only L-dopa but not dopamine trespasses the intact blood-brain barrier in PD. To date, this L-dopa/DDI regimen is still the most frequently applied combination despite the introduction of catechol-O-methyl transferase inhibitors (COMT-I) in the 90ties of the last century. Between 1976 and 2011 an increase of 328.7% in the general death rate of PD patients was observed again, when compared to the first interval of L-dopa therapy without DDI use (Hinz et al., 2014). The cause for this phenomenon is still unknown. One hypothesis assumes a nutritional catastrophe due to a putative, permanent DDI induced deactivation of pyrdidoxal 5′-phosphate (PLP). PLP is the active form of vitamin B6(Figure 1). PLP is required for the function of 300 enzymes, including the one responsible for the irreversible conversion of homocysteine to cysteine (Hinz et al., 2014). Pronounced cysteine deficiency in chronic L-dopa treated PD patients would confirm this theory of a slowly evolving DDI induced PLP depletion as cause for the progression of PD related with oxidative stress generation. Instead, even after overnight stop of L-dopa/ DDI therapy, a cysteine increase was demonstrated in long term L-dopa/ DDI treated PD patients with an elevation of total homocysteine in plasma above the threshold of 15 µM in particular (Müller and Kuhn, 2009). Therefore one may assume that compensatory mechanisms for PLP consumption and alternative metabolic pathways for homocysteine degradation are available in humans.
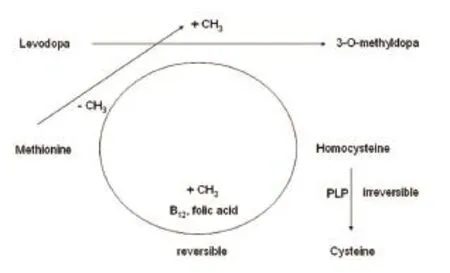
Figure 1 Simplified schematic drawing of concomitant processes during chronic O-methylation of levodopa L-dopa applied with a dopa decarboxylase inhibitor.
Homocysteine is a long living metabolite of methionine, which acts as a methyl group donor for an up regulated transformation of L-dopa to 3-O-methyldopa via COMT resulting from chronic DDI application. Turnover of many anticonvulsive and centrally acting drugs, such as valproic acid, consumes methyl groups. Accordingly, their chronic intake induces a homocysteine increase and sooner or later decay of methyl group donating vitamins, such as folic acid or vitamin B12. Both are essential for the reversible conversion of homocysteine to methionine. PLP is needed for the irreversible degration of homocysteine to cysteine. In the long run, chronic drug induced vitamin deficiency may contribute to acceleration of ageing, brain atrophy, deterioration of cognitive function, DNA hypomethylation and peripheral nerve dysfunction (Müller, 2013; Müller and Kohlhepp, 2016; Taravini et al., 2016).
Generally, high homocysteine reflect as biomarker an inappropriate or reduced capacity for methyl group consuming metabolism of drugs or toxins. Thus vulnerability for exposition against endogenous xenobiotics or exogenous substances, such as rural toxins and pesticides, elevates. Elevated homocysteine levels are also a well known risk factor for atherosclerosis, which has been found elevated in L-dopa/DDI treated PD patients in epidemiological trials (Müller, 2013). Even acute one time or repeat L-dopa/DDI application induced a rise of homocysteine, which was impaired by COMT-I. Independent of concomitant COMT inhibition also a decline of cysteine and cysteinyl-glycine (Cys-Gly) was simultaneously found (Müller and Muhlack, 2014).
Fall of Cys-Gly- and cysteine after acute L-dopa application represents an indirect biomarker for oxidative stress to balance of pro-oxidant L-dopa mechanisms (Müller and Muhlack, 2014; Müller et al., 2016). Both substrates play an important role in the oxidative stress defence provided by γ-glutamyl-cysteine-glycine. This tripeptide is available in reduced monomeric (GSH) and oxidized dimeric forms (GSSG). Exposure of GSH to reactive oxygen species (ROS) converts GSH to GSSG instead to Cys-Gly. In other words, GSH is the metabolic precursor of Cys-Gly under conditions without oxidative stress. GSH synthesis out of the amino acids cysteine, L-glycine, glutamine acid also goes up for oxidative stress compensation and therefore consumes free cysteine temporarily. This GSH mediated free radical scavenging is abundant in the cytosol, nuclei and mitochondria.
Many reports in PD research attribute nigral dopaminergic neuronal loss to oxidative stress associated with increased dopamine turnover, deficient GSH content and elevated iron content. Progression of PD makes neuronal maintenance more and more vulnerable to further oxidative challenge. Thus pro-oxidant L-dopa effects exacerbate an already compromised cellular system and aggravate a condition that it is meant to alleviate by physiologic repair mechanisms (Przuntek et al., 2004; Müller et al., 2016).

Figure 2 Simplified drawing of the impact of oxidative stress on repair mechanisms.
It is well known that ROS mediate acute or chronic neurodegenerative tissue destruction, either alone or in concert with proteases. ROS also control protein synthesis, for instance neurotrophins. These molecules initiate regeneration. Other proteins inhibit physiologicalaxon regeneration, protection of myelin and glial structures, for instance myelin-associated glycoprotein or repulsive guidance molecule A (RGMa). Furin and the proprotein convertase SKI-1 process the glycosylphosphatidylinositol-anchored RGMa protein into numerous membrane-bound and soluble fragments. This procedure is essential for the RGMa in vivo function. Various RGMa parts inhibit neurite growth by binding to the neuronal receptor Neogenin. Neogenin is a member of the immunoglobulin superfamily and consists of four N-terminal immunoglobulin-like domains (Ig), six fibronectin type III (FNIII) domains, a transmembrane domain and a C-terminal intracellular domain. Two different RGMa fragments, the N-terminal - (30 kDa) and the C-terminal fragment (40 kDa) bind to the same FNIII domain (domain 3—4) of Neogenin. Thus generally, Neogenin and RGMa are important regulators of apoptotic cell death. This interplay between both proteins prevents repair of damaged axons and supports neuronal death, probably triggered via activation of microglia. One feature of this well known phenomenon of chronic neurodegenerative processes is RGMa expression on the surface of microglia cells (Müller et al., 2016).
Simultaneously with L-dopa induced oxidative stress, higher RGMa levels were found in chronic L-dopa treated but not in L-dopa naïve patients. This RGMa elevation may contribute to an inhibition of physiologic neuronal maintenance and recovery. The missing rise of RGMa in L-dopa naïve patients may indicate the existence of a certain compensation capacity, for instance ROS scavenging due to a still adequate function of the GSH system. However, this ROS balance may be depleted during long term L-dopa exposure (Müller et al., 2016). It is well known that ROS has the potential to disrupt sulfhydryl homeostasis in cells and to alter the function of proteins with reactive cysteine residues. This condition will exacerbate if impairment takes place in one or both of the glutaredoxin or thioredoxin enzyme systems. They are responsible for reversing protein-S-glutathionylation and inter- or intramolecular disulfites, respectively. In the end, this scenario ends up in a vicious circle with elevated ROS exposure increasingly triggering apoptotic neuronal death. As a result, onset of neuronal dysfunction should appear during chronic L-dopa intake (Müller, 2013).
One aftermath, observed in the clinic, concerns the peripheral nervous system. Long term L-dopa exposure or high dosing of L-dopa induced slow or even acute evolving axonal polyneuropathy. Vitamin deficiency and homocysteine rise was concomitantly observed in PD patients. Similarities exist between the peripheral and central nervous system (Müller, 2013). One may assume analogies during chronic L-dopa exposure to the nigrostriatal dopamine generating neurons in PD patients. Long term studies in PD described a lower advance rate with dopamine agonist treatment alone compared with L-dopa/DDI monotherapy (Przuntek et al., 2004; Schapira et al., 2013). Functional imaging techniques of the dopaminergic nigrostriatal system served as biological marker for the progression of PD. Comparison of various L-dopa/DDI dosages against placebo therapy found a significant lower nigrostriatal radiotracer enrichment as marker for PD progression in the 600 mg L-dopa/carbidopa daily dose arm after nine months compared with placebo (Fahn, 2006). This outcome indicates that neuronal death promoting effects of L-dopa/carbidopa particularly appear after higher L-dopa dosing, when probability of nutritional deficiency and antioxidant depletion goes up. However, no difference was found when the effects of dopamine agonist therapy and placebo treatment were investigated with the same biomarker (Schapira et al., 2013). Treatment with placebo or dopamine agonists does not increase methyl group consumption and oxidative stress. Experimental research even describes radical scavenging properties of dopamine agonists (Schapira et al., 2013; Taravini et al., 2016).
Nearly all more recent neuropathological investigations are performed in PD brains with a previous long term L-dopa exposure. Accordingly, findings report signs of an antioxidant decrease in nigrostriatal system. Early in the disease process there may be a compensatory upregulation of dopaminergic receptors on neurons surrounding those that have died. However the disease goes on. As a result, an accumulation of dopamine within the synapses occurs, which is disproportional to the continued neuronal loss. This dopamine excess is degraded by glial monoamine oxidase (MAO) and COMT. Hydrogen peroxide accumulates as a byproduct of the MAO pathway. In conjunction with the above mentioned mechanisms associated with levodopa and dopamine exposure, the production of highly reactive hydroxyl radical goes up in this region. Depletion of antioxidant defence occurs. The likelihood of too high L-dopa and dopamine concentrations rises with fluctuating blood and brain levels during oral dosing. Temporary missing response to L-dopa results in an extra or too high dosing of L-dopa/DDI to compensate failures of gastric emptying and duodenal absorption (Müller, 2013).
This drug induced not physiological balance between free radicals and antioxidants initially damages the vicinity of neurons, impairs physiologic available repair mechanisms and ends up in glial and neuronal death. Thus, L-dopa behaves like a double edged sword. On the one hand L-dopa is most effective for amelioration of impaired motor behaviour in PD patients. On the other hand too high L-dopa dosing may accelerate neuronal death due to secondary drug induced nutritional deficiencies as a result of antioxidant defence and methyl group consumption. It is unlikely that L-dopa itself supports this degeneration, as L-dopa itself supports neuronal recovery by i.e., rise of nerve growth factor synthesis (Müller, 2013).
In conclusion these findings underline the importance of L-dopa metabolism on oxidative stress appearance and hypomethylation (Müller and Kohlhepp, 2016; Taravini et al., 2016). As a result, nutritional deficits of vitamins and antioxidants appear and accelerate the ageing process itself. Enzymatic blocking of L-dopa metabolism improved the efficacy of L-dopa on motor behaviour due to a better L-dopa bioavailability. However, the observed increased mortality rate after the supplementation of each L-dopa containing formulation may hypothetically also result from a more sustained impairment of methylation capacity and defence of oxidation processes (Müller, 2013; Hinz et al., 2014). Therefore it would make sense to supplement L-dopa/DDI formulations with blood-brain barrier trespassing methyl group donating and free radical scavenging substrates on a regular basis.
Tomas Müller*
Department of Neurology, St. Joseph Hospital Berlin-Weißensee, Berlin, Germany
*Correspondence to: Thomas Müller, M.D., th.mueller@alexius.de; thomas.mueller@ruhr-uni-bochum.de.
Accepted: 2016-04-13
Fahn S (2006) Levodopa in the treatment of Parkinson's disease. J Neural Transm Suppl 1-15.
Hinz M, Stein A, Cole T (2014) The Parkinson's disease death rate: carbidopa and vitamin B6. Clin Pharmacol 6:161-169.
Müller T (2013) Detoxification and antioxidative therapy for levodopa-induced neurodegeneration in Parkinson's disease. Expert Rev Neurother 13:707-718.
Müller T, Kuhn W (2009) Cysteine elevation in levodopa-treated patients with Parkinson's disease. Mov Disord 24:929-932.
Müller T, Muhlack S (2014) Levodopa-related cysteinyl-glycine and cysteine reduction with and without catechol-O-methyltransferase inhibition in Parkinson's disease patients. J Neural Transm 121:643-648.
Müller T, Kohlhepp W (2016) Hypomethylation in Parkinson's disease: An epigenetic drug effect? Mov Disord doi:10.1002/mds.26560.
Müller T, Trommer I, Muhlack S, Mueller BK (2016) Levodopa increases oxidative stress and repulsive guidance molecule A levels: a pilot study in patients with Parkinson's disease. J Neural Transm 123:401-406.
Przuntek H, Müller T, Riederer P (2004) Diagnostic staging of Parkinson's disease: conceptual aspects. J Neural Transm 111:201-216.
Schapira AH, McDermott MP, Barone P, Comella CL, Albrecht S, Hsu HH, Massey DH, Mizuno Y, Poewe W, Rascol O, Marek K (2013) Pramipexole in patients with early Parkinson's disease (PROUD): a randomised delayed-start trial. Lancet Neurol 12:747-755.
Taravini IR, Larramendy C, Gomez G, Saborido MD, Spaans F, Fresno C, Gonzalez GA, Fernandez E, Murer MG, Gershanik OS (2016) Contrasting gene expression patterns induced by levodopa and pramipexole treatments in the rat model of Parkinson's disease. Neuropharmacology 101:576-589.
10.4103/1673-5374.182694 http∶//www.nrronline.org/
How to cite this article: Müller T (2016) Do we need a new levodopa? Neural Regen Res 11(5):731-732.
- 中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells

