Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy
Han-yu Dong, Xin-mei Jiang, Chun-bo Niu, Lin Du, Jun-yan Feng, Fei-yong Jia,,
1 Department of Pediatric Neurology and Rehabilitation, First Hospital of Jilin University, Changchun, Jilin Province, China
2 Institute of Jilin Neurological Research, First Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Pathology, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
RESEARCH ARTICLE
Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy
Han-yu Dong1, Xin-mei Jiang2, Chun-bo Niu3, Lin Du1, Jun-yan Feng1, Fei-yong Jia1,2,*
1 Department of Pediatric Neurology and Rehabilitation, First Hospital of Jilin University, Changchun, Jilin Province, China
2 Institute of Jilin Neurological Research, First Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Pathology, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
Graphical Abstract
orcid: 0000-0002-8309-1395 (Fei-yong Jia)
To examine the effects of Cerebrolysin on the treatment of diabetic peripheral neuropathy, we first established a mouse model of type 2 diabetes mellitus by administering a high-glucose, high-fat diet and a single intraperitoneal injection of streptozotocin. Mice defined as diabetic in this model were then treated with 1.80, 5.39 or 8.98 mL/kg of Cerebrolysin via intraperitoneal injections for 10 consecutive days. Our results demonstrated that the number, diameter and area of myelinated nerve fibers increased in the sciatic nerves of these mice after administration of Cerebrolysin. The results of several behavioral tests showed that Cerebrolysin dose-dependently increased the slope angle in the inclined plane test (indicating an improved ability to maintain body position), prolonged tail-flick latency and foot-licking time (indicating enhanced sensitivity to thermal and chemical pain, respectively, and reduced pain thresholds), and increased an index of sciatic nerve function in diabetic mice compared with those behavioral results in untreated diabetic mice. Taken together, the anatomical and functional results suggest that Cerebrolysin ameliorated peripheral neuropathy in a mouse model of type 2 diabetes mellitus.
nerve regeneration; peripheral neuropathy; diabetes mellitus; Cerebrolysin; neurological function; sciatic nerve; neural regeneration
Introduction
Diabetic peripheral neuropathy (DPN) is the most common chronic complication of diabetes mellitus and is the main cause of incapacitation in these patients (Qiu et al., 2010). At least 50% of the patients with a history of diabetes mellitus for over 10 years will develop peripheral neuropathy to varying degrees (Feldman, 2003). The first and the most common pathological change is damage to small nerve fibers, such as sensory nerves (Llewelyn, 2003). Other symptoms in patients with DPN include hypoesthesia and sensory deficits. Dysfunction of motor and autonomic nerves is also observed in individuals with diabetes.
Although numerous studies have examined the etiology of DPN, its pathogenesis remains unclear. Previous studies (Cameron et al., 2001; El-Mesallamy et al., 2011) determined that the formation of advanced glycation end products may be an important underlying mechanism contributing to DPN. Furthermore, metabolic dysfunction (Kryvko IuIa et al., 2001), vascular injury (Yagihashi, 2002), nerve growth factor deficiency (Kanbayashi et al., 2002; Yasuda et al., 2003; Yuan et al., 2005) and oxidative stress (Coppey et al., 2000; Piotrowski et al., 2001; Cameron and Cotter, 2002; Feldman,2003) caused by high blood glucose levels are also factors associated with DPN (Verrotti et al., 2001). Currently, there is no effective clinical treatment for DPN.
Cerebrolysin, hydrolysated cerebroproteins containing free amino acids and neurotrophic substances, is widely used in treatment of stroke (Ladurner et al., 2005) because of its protective effect in the central nervous system (Hutter-Paier et al., 1998; Masliah and Díez-Tejedor, 2012) and its ability to easily penetrate the blood-brain barrier (Hartbauer et al., 2001; Sharma et al., 2010). Recent studies have shown that Cerebrolysin also improves function after peripheral nerve injuries (Bai et al., 2004; Xu et al., 2006; Fu et al., 2011; Hamed, 2011). However, few studies have examined whether Cerebrolysin may be beneficial in the treatment of DPN.
Therefore, we examined whether Cerebrolysin ameliorated peripheral neuropathy in a mouse model of diabetes. A series of behavioral tests were used to assess the functions of mice using this model. In addition, pathological changes in the sciatic nerves of mice were examined in the presence and absence of Cerebrolysin treatment.
Materials and Methods
Establishment of the mouse models of DPN
Clean Kunming mice, aged 4-5 weeks (50 males and 50 females) and weighing 19-23 g, were provided by the Laboratory Animal Center of Jilin University in China (license No. SYXK (Ji) 2008-0011). Mice were allowed free access to water and food during the experiments and were maintained in an environment at 18-20°C with a relative humidity of 50-60%. This study was approved by the Animal Ethics Committee of the First Hospital of Jilin University, China.
A control group of 10 mice was fed a normal diet (supplied by the Laboratory Animal Center of Jilin University, Changchun, Jilin Province, China). The remaining 90 mice were used to establish an animal model of DPN. A high-fat, high-sugar diet consisting of a mixture of 10% lard, 20% white granulated sugar and regular chow (supplied by Laboratory Animal Center of Jilin University, China) was fed to Kunming mice for 4 weeks to induce insulin resistance (Carpentier et al., 2000; Lam et al., 2002). Then, after fasting for 12 hours, mice were intraperitoneally injected with 150 mg/kg of streptozotocin (Changchun Baoxin Biological Technology Co., Ltd., Changchun, Jilin Province, China). Streptozotocin was freshly prepared and dissolved in a citrate buffer solution (0.1 M, pH 4.4). Five days later, blood was obtained from the caudal (tail) vein and fasting blood glucose levels were determined using a glucometer (Sinocare Inc., Changsha, Hunan Province, China). A diabetic mouse was defined as one having fasting blood glucose levels ranging from 199.8-300.6 mg/dL (Gurley et al., 2006; Zhang et al., 2008). The diabetic mice were then fed a normal diet for 6 weeks. The general condition of each mouse was recorded along with blood glucose levels and body weights once every 2 weeks. After 4-8 weeks, a mouse model of diabetes mellitus was successfully established with hallmarks of DPN observed similar to those in humans. It has been previously shown that 8 weeks of high blood glucose may lead to nerve injury (Yagihashi et al., 1990). Thus, we considered the Kunming mouse model of DPN successfully established (Xu, 2009). Several behavioral measurements were conducted and pathological changes in the sciatic nerve were observed after treatment with Cerebrolysin for 10 days.
Cerebrolysin administration
Although 90 mice were initially used to establish the mouse models of DPN, some of the mice failed to be defined as diabetic, displayed unstable blood glucose levels, or were underor overweight. Therefore, 40 diabetic mice in good general condition with similar body weights and stable blood glucose levels were selected and randomly divided into a untreated model group and three groups treated with low, moderate, or high doses of Cerebrolysin (n = 10 mice per group).
Mice in the control and model groups were intraperitoneally injected with 0.5 mL physiological saline. Based on the equivalent dose conversion from animals to humans (US Food and Drug Administration, 2002), mice in the low-, moderate-, and high-dose Cerebrolysin groups were intraperitoneally injected with 1.80, 5.39, and 8.98 mL/kg, respectively, of Cerebrolysin per day for 10 consecutive days.
Behavioral tests
A series of behavioral measurements were obtained using the slope test, hot water tail-flick test, formalin algesia test, and walking tracks test to assess behavioral manifestations of the mouse models and treatments.
Slope test
The inclined plane method is often used to measure the ability of mice to maintain their body position in experiments examining the effects and treatments of motor nerve injury in four limbs (Rivlin and Tator, 1977). We previously published a modified protocol for this test (Chai et al., 2013). Briefly, a glass flume (60 cm long, 30 cm wide and 60 cm high) with a crude rubber mat inside was used. The mice were placed in the middle of the flume on the mat with their body axis perpendicular to that of the plane (i.e., the slope) for 3 minutes to allow them to become accustomed to the environment. Then, the maximum inclination of the plane at which a mouse could maintain body position for 5 seconds was recorded and represented the animal’s functional ability (Rivlin and Tator, 1977). After that, the angle of the inclined plane was increased at a uniform rate, and the angle of the slope was recorded at which all four limbs of the mouse slipped 2 cm. Each mouse was tested three times and the mean value was calculated.
Hot water tail-flick test
The sensory function of the animals was evaluated using a thermal stimulus (Crawley, 1999) to measure pain threshold. Two types of nerve fibers conduct pain information. Myelinated nerve fibers called Aδ fibers conduct the rapid, “first”pain signals, whereas unmyelinated nerve fibers called C fibers conduct the slower, longer latency “second” pain signals. The Aδ fiber-mediated “first” pain is often described as sharpand pinprick-like, in contrast to the dull, long-lasting and burning C-fiber-mediated “second” sensation of pain (Forss et al., 2005). The tail-flick test examines the Aδ fiber-conducted nociceptive information.
Tail-flick latency, the time from when the tail tip was dipped into hot water until the mouse withdrew the tail, was recorded to assess pain threshold in mice. The bottom third of the mouse’s tail was dipped into a water bath maintained at a constant temperature of 53°C. If the mouse did not flick its tail from the water within 15 seconds, tail-flick latency was recorded as 15 seconds, and the tail was removed from the water to avoid tissue damage. Each mouse was tested three times with a 15-minute interval.
Formalin algesia test
The formalin test is a chemical assay generally used to measure the sensory function of nerve fibers. In this test, 20 µL of 4% formalin solution was subcutaneously injected into the surface of the right hindpaw. The total amount of time each mouse spent foot licking within 5 minutes after the injection was recorded (Tjølsen et al., 1992).
Walking track analysis
The walking track test, first described by de Medinaceli et al. (1982), was used for the functional assessment of sciatic nerve function. The test provides a sciatic nerve functional index after measuring specific aspects of the rodent’s footprint. A piece of white recording paper (8 cm wide) was placed on the floor of a footprint walking box (40 cm long, 8 cm wide and 8 cm high). A mouse with its metapodium pad smeared with black ink was placed into one side of the box and allowed to walk through the box. The distances from the second toe to the forth toe (inter-toe distance, IT), from the first toe to the fifth toe (toe spread, TS), and from the heel to the toe (print length, PL,) were measured on the recorded footprints three times, and the mean values were calculated. The mean values of the IT, TS, and PL of mice in the control group were calculated and regarded as the normal values (NIT, NTS, and NPL), whereas the mean values of those measures for mice in the DPN model groups were considered the experimental values (EIT, ETS, and EPL). The sciatic nerve function index was calculated using Bain’s formula (Sciatic function index = 109.5(ETS − NTS)/NTS − 38.3 (EPL −NPL)/NPL + 13.3(EIT − NIT)/NIT − 8.8) (Bain et al., 1989). Nerve function was considered normal, without damage, when the sciatic function index was 0 and considered wholly damaged when the sciatic function index was −100.
Sciatic nerve pathology
At the conclusion of the behavioral tests, mice were deeply anesthetized using an intraperitoneal injection of 10% chloral hydrate (350 mg/kg). Each mouse was then pinned to a dissecting board in the supine position. The skin was disinfected with 70% ethanol. A small vertical incision (approximately 5.0 mm) was made along the thigh using scissors, the skin was retracted laterally, and the muscles of the posterior thigh (including the hamstring muscles) were moved to expose the entire length of the sciatic nerve, which appears as a thick whitish cord, in the thigh region. The nerve was gently lifted using forceps and excised at the proximal and distal ends to obtain the middle portion with a length of approximately 0.5 cm. The sciatic nerves were pre-fixed with 3% glutaraldehyde for 3 hours, post-fixed with osmic acid for 2 hours, dehydrated through a gradient of alcohol with propylene oxide, and embedded in epoxy resin. Subsequently, semi-thin sections 1 µm thick were sliced using a Leica semithin microtome (Leica Microsystems, Wetzlar, Germany). The sections were stained with 1% toluidine blue for 5-8 minutes and dried over an alcohol burner. Images of sciatic nerve sections were captured using a microscope (BX51; Olympus, Tokyo, Japan) with the CellSens Dimension cell image analysis system (Olympus Co., Ltd., Shanghai, China) and then analyzed using the Image-Pro Plus medical image measurement software (IPP Image Analysis Software Co., Ltd., Shanghai, China). The average diameter, circumference, and area of the sciatic nerve were calculated. Macrofibrils and fibrils were differentiated by a fiber diameter of 3 µm for the former, and the proportion of fibrils was calculated based on the total nerve fiber and fibril counts (Zhao and Ding, 2009).
Statistical analysis
The SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA) was used to process and analyze the data. The data are expressed as the mean ± SD. Data comparisons of multiple groups were performed using one-way analysis of variance, with Bonferroni corrections used to adjust for multiple pairwise comparisons. Differences with P < 0.05 were regarded as statistically significant.
Results
Overall condition of diabetic mice after administration of Cerebrolysin
Mice in the control group were in good general condition, with marked increases in their body weights. By contrast, mice in the model group as well as those in the low-, moderate-, and high-dose Cerebrolysin groups developed symptoms such as polydipsia, polyphagia and diuresis, and their body weights increased at a slower rate than those in the control group (P < 0.01). The weight gain (difference in body weights before intraperitoneal injection of streptozotocin and after Cerebrolysin administration) and fasting blood glucose levels (after the drug intervention) are shown in Figure 1.
Behavioral changes in diabetic mice after administration of Cerebrolysin
Slope angle
The slope angle test was conducted to examine the ability of mice to maintain their body position (Rivlin and Tator, 1977). The slope angles for mice in the moderate- and high-dose Cerebrolysin groups were significantly greater than those in the model group (P < 0.05, P < 0.01, respectively). The slope angle for mice in the low-dose Cerebrolysin group was greater than that in the model group without reaching statisticalsignificance (P > 0.05; Figure 2). These data indicate that Cerebrolysin improves the ability of diabetic mice to maintain their body position in a dose-dependent manner.
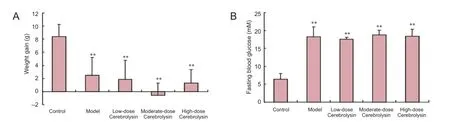
Figure 1 Body weight gain and fasting blood glucose levels in diabetic mice after administration of three doses of Cerebrolysin.
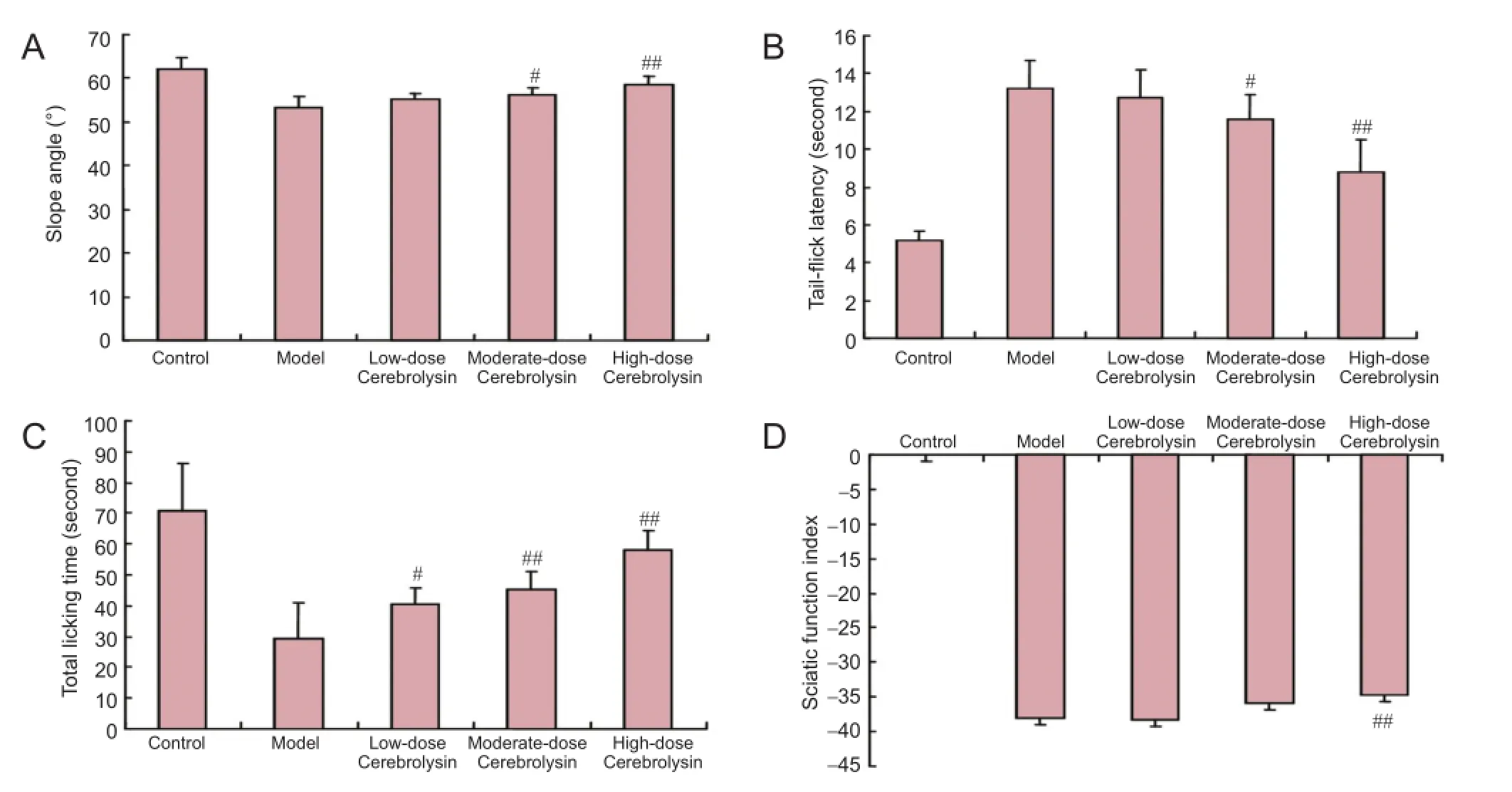
Figure 2 Behavioral changes in diabetic mice after administration of three doses of Cerebrolysin.
Tail-flick latency
Tail-flick latency can be used to reflect the pain threshold for a physical stimulus (Yasphal et al., 1982). The fail-flick latencies for mice in the moderate- and high-dose Cerebrolysin groups were significantly higher than that in the model group (P < 0.05 and P < 0.01, respectively); however, the tail-flick latency for mice in the low-dose Cerebrolysin group was not significantly different than that in the model group (P > 0.05). These data suggest that diabetic mice treated with Cerebrolysin are more sensitive to hot water stimulation and their pain thresholds are reduced compared with untreated diabetic mice (Figure 2).
Total licking time
The total time spent licking for mice injected with formalin represents the level of sensitivity to a chemical stimulus (Cao et al., 1998). The total time spent licking for mice in the low, moderate-, and high-dose Cerebrolysin groups was significantly longer than that for mice in the model group (P <0.05 or P < 0.01). These data demonstrate that diabetic mice treated with Cerebrolysin are more sensitive to formalin stimulation and show reduced pain thresholds compared with untreated diabetic mice (Figure 2).
Sciatic function index
The index of sciatic nerve function for mice in the high-dose Cerebrolysin-treated DPN model group was significantly higher than that for mice in the untreated DPN model group (P < 0.01). These data reveal that high-dose Cerebrolysinameliorates sciatic nerve dysfunction (Figure 2).
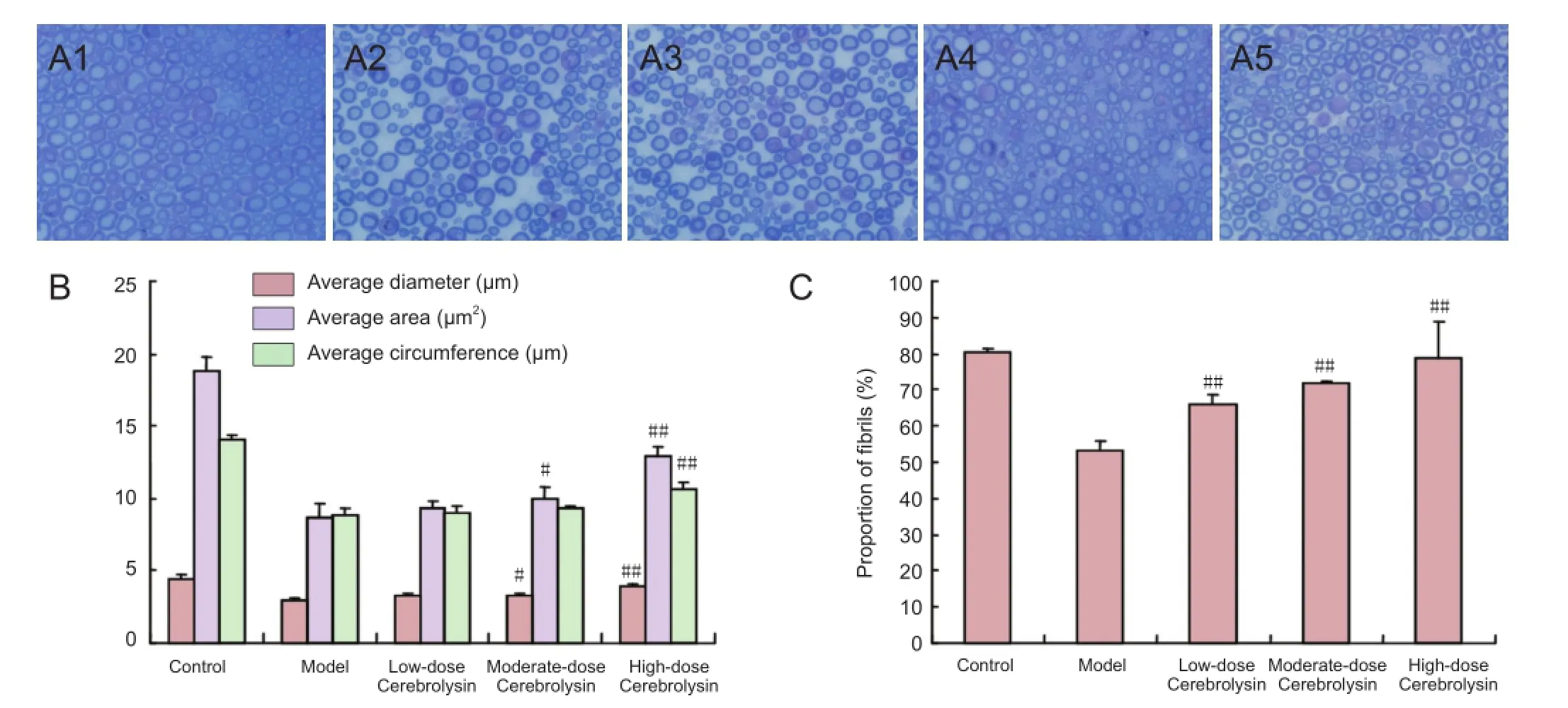
Figure 3 Neuropathological changes in sciatic nerves of diabetic mice after administration of three doses of Cerebrolysin.
Neuropathological changes in sciatic nerves of diabetic mice after administration of Cerebrolysin
Sciatic nerve sections stained with toluidine blue were observed under a microscope. In the control group, the myelinated nerve fibers were uniformly distributed, the sheaths were uniformly dyed, and the axons were saturated with dye. By contrast, in the model group, the myelinated nerve fibers were sparsely distributed, the thickness of sheaths was uneven, and the axons were bulky. The myelinated nerve fibers in the Cerebrolysin-treated mice were more sparsely distributed than those in the control group but were more abundant than those in the untreated DPN model group. The average diameter and average area of the sciatic nerves from mice in the moderate- and high-dose Cerebrolysin-treated groups were significantly higher than those in the untreated DPN model group (P < 0.05 or P < 0.01). In addition, the average perimeter of the sciatic nerves from mice in the high-dose Cerebrolysin-treated group was significantly greater than that in the untreated DPN model group (P <0.01). The proportion of fibrils in the low-, moderate-, and high-dose Cerebrolysin-treated groups was significantly higher than that in the model group (P < 0.01). Taken together, these results show that Cerebrolysin helps repair medullated fibers in sciatic nerves damaged in DPN (Figure 3).
Discussion
Cerebrolysin is neurotrophic, neuroprotective and neuroregenerative, clearly functioning to protect the central nervous system. Using high pressure liquid chromatography, 17 different amino acids have been detected in Cerebrolysin (Ning and Li, 2002) in addition to neurotrophic factors such as glial cell-derived neurotrophic factor and insulin-like growth factors 1 and 2. These nutritionally active substances assist in the neurotrophic effect of Cerebrolysin (Chen et al., 2007). An animal experiment provided evidence that Cerebrolysin can promote the combination of nerve cells and keratin (Shtrygol’OIu et al., 2000) to enhance the outward growth of dorsal root ganglion and sympathetic trunk axon. Eder et al. (2001) reported that Cerebrolysin plays a neurotrophic role by increasing the density of glutamate receptor 1. Cerebrolysin also affects free radicals by promoting catalase and superoxide dismutase activity to enhance the oxygen free radical scavenger system and reduce free radical reactions to protect the mitochondria of nerve cells from damage by toxicants (Han et al., 2004). In addition, Cerebrolysin inhibits the abnormal metabolism of nitric oxide to reduce apoptosis. Furthermore, Cerebrolysin reduces the intake of calcium ions and the activation of calpain to ameliorate the calcium overload in nerve cells (Wronski et al., 2000; Eder et al., 2001). In its neuroprotective role, Cerebrolysin decreases the expression of amyloid precursor protein by regulating amyloid-beta degradation to reduce the deposition of amyloid protein (Rockenstein et al., 2006). Cerebrolysin also controls the expression of interleukin-1 to reduce the degree of inflammation (Alvarez et al., 2000).
DPN is a common complication in patients with diabetes and is an unsolved clinic problem. The dysfunction of peripheral nerves in patients with diabetes often leads to pain, abnormal sensory functions, and even disabilities that together generate potential physical and psychological burdens for the patients and their families. Unfortunately, noeffective medication has been developed to ameliorate DPN. Cerebrolysin has been widely used in patients with central nervous system diseases, such as stroke and dementia, because of its neurotrophic effects. However, studies examining the effects of Cerebrolysin on peripheral damage are rare; in particular, no studies have investigated the effects of Cerebrolysin in an animal model of DPN.
Abnormal sensory and motor dysfunctions are the primary clinical manifestation in patients with DPN. The effects of Cerebrolysin on sciatic nerve dysfunction in diabetic mice were examined in the present study. During the experiment, many mice bit their feet, likely because of the pain induced by this animal model of DPN. Moreover, some mice died of infection and others died perhaps due to ketoacidosis or other complications of diabetes. These deaths suggested that we had successfully developed a mouse model of diabetes mellitus.
Cerebrolysin dose-dependently improved the ability of these mice to maintain their body position as assessed in the inclined plane slope test. Cerebrolysin also increased their chemical and physical pain thresholds as assessed by their responses in the formalin and tail-flick tests, respectively. In addition, the high dose of Cerebrolysin improved an index of sciatic nerve function in these mice as assessed in the analysis of their walking tracks. Taken together, these results suggest that Cerebrolysin may be effective in ameliorating sensory and motor dysfunctions in patients with DPN. Furthermore, administration of Cerebrolysin improved indexes of neuropathology (the average diameter, perimeter, and area) as well as the morphology of sciatic nerves obtained from diabetic mice. These anatomical data provide insight, at least in part, for the mechanism of the improved function in these diabetic mice. We also showed that the effects of Cerebrolysin were dose-dependent; the higher the dose, the better was the therapeutic effect, consistent with a previous report (Ladurner et al., 2005; Sanchez-Vega et al., 2015).
Previous studies have reported that Cerebrolysin might improve the symptoms associated with peripheral nerve dysfunction in humans. Hamed (2011) concluded that Cerebrolysin is associated with more rapid neurological recovery after various peripheral nerve lesions than other therapies, including steroids and supportive therapies such as vitamins and antioxidants. Those results provided evidence supporting the therapeutic efficacy of Cerebrolysin in the treatment of acquired peripheral nervous system diseases. Xu et al. (2006) reported that Cerebrolysin also improves the postoperative recovery of peripheral nerve dysfunction in patients with cerebral palsy. That study showed that the remission rate for pain and numbness significantly increased and suggested that Cerebrolysin may be a neurotransmitter itself or a precursor of a neurotransmitter to improve the recovery of neurological function. Fu et al. (2011) concluded that Cerebrolysin is more effective than conventional treatments for acute idiopathic facial paralysis and, more relevant to the present study, that Cerebrolysin has an effect on peripheral nerve injury caused by DPN. Bai et al. (2004) found that intravenous injection of cerebroprotein hydrolysate, which acted similar to an intramuscular injection of Methycobal combined with an intravenous injection of Actovegin, improved nervous system abnormalities in patients, including asymmetric body sweat, arduous micturition, slow urination or uroschesis, and alternating diarrhea and constipation. Despite the evidence for a therapeutic effect of Cerebrolysin in human patients with peripheral nerve dysfunction, its efficacy and mechanism of action have not been previously examined in an animal model of DPN. To the best of our knowledge, the present study is the first to report the effects of Cerebrolysin administration on the sciatic nerve in a mouse model of DPN. Our results provide theoretical support and new insight for the use of Cerebrolysin in the treatment of DPN and offer further evidence supporting previous reports that Cerebrolysin ameliorates peripheral nerve dysfunction. However, the specific therapeutic mechanism(s) and clinical effects of Cerebrolysin in the treatment of DPN will require further investigation.
In summary, Cerebrolysin dose-dependently ameliorated the sciatic nerve dysfunction and pathological changes associated with diabetes in a mouse model of DPN, suggesting that a clinical trial is warranted for the use of Cerebrolysin in the treatment of diabetic peripheral neuropathy.
Acknowledgments: We thank Laboratory Animal Center of Jilin University in China for providing laboratory animals and rearing condition. We also thank the professionals from Translational Medicine Research Institute of Jilin University in China for their helps in the pathological experiment.
Author contributions: HYD and XMJ conceived and designed the study. HYD, LD and CBN performed the experiment. HYD, LD and JYF wrote the paper. JYF and FYJ reviewed and edited the paper. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Alvarez XA, Lombardi VR, Fernández-Novoa L, García M, Sampedro C, Cagiao A, Cacabelos R, Windisch M (2000) Cerebrolysin reduces microglial activation in vivo and in vitro: a potential mechanism of neuroprotection. J Neural Transm Suppl 59:281-292.
Bai H, Xu M, Han M, Wu N (2004) Effect of cerebroprotein hydrolysate on diabetic peripheral neuropathy. Zhongguo Kangfu Lilun yu Shijian 10:41-42.
Bain JR, Mackinnon SE, Hunter DA (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83:129-138.
Cameron NE, Cotter MA (2002) Effects of protein kinase Cβ inhibition on neurovascular dysfunction in diabetic rats: interaction with oxidative stress and essential fatty acid dysmetabolism. Diabetes Metab Res Rev 18:315-323.
Cameron NE, Eaton SEM, Cotter MA, Tesfaye S (2001) Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44:1973-1988.
Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI (1998) Primary afferent tachykinins are required to experience moderate to intense pain. Nature 392:390-394.
Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF (2000) Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 49:399-408.
Chai ZJ, Chen H, Fan XL, Jiang XM (2013) Experimental study of the effect of Cerebrolysin on peripheral nerve regeneration. Zhongfeng yu Shenjing Jibing Zazhi 30:151-153.
Chen H, Tung YC, Li B, Iqbal K, Grundke-Iqbal I (2007) Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol Aging 28:1148-1162.
Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA (2000) Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res 1:131-143.
Crawley JN (1999) Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests1. Brain Res 835:18-26.
de Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77:634-643.
Eder P, Reinprecht I, Schreiner E, Skofitsch G, Windisch M (2001) Increased density of glutamate receptor subunit 1 due to Cerebrolysin treatment: an immunohistochemical study on aged rats. Histochem J 33:605-612.
El-Mesallamy HO, Hamdy NM, Ezzat OA, Reda AM (2011) Levels of soluble advanced glycation end product-receptors and other soluble serum markers as indicators of diabetic neuropathy in the foot. J Invest Med 59:1233-1238.
Feldman EL (2003) Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest 111:431-433.
Forss N, Raij TT, Seppä M, Hari R (2005) Common cortical network for first and second pain. Neuroimage 24:132-142.
Fu R, Dai W, Meng R, Zhao XH, Huang D (2011) Cerebrolysin injection in the treatment of acute idiopathic facial paralysis: a randomized controled trial. Zhongguo Shenjng Jingshen Jibing Zazhi 37:295-297. Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM (2006) Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290:F214-222.
Hamed SA (2011) Cerebrolysin as a nerve growth factor for treatment of acquired peripheral nervous system diseases. Neural Regen Res 6:1415-1420.
Han ZT, Liu J, Zhang BL, Zhang JL, Li WB, Tao GS (2004) Experimental study of influence on SOD and MDA of mice on the treatment of cerebrolysin. Zhongguo Laonian Xue Zazhi 24:447-448.
Hartbauer M, Hutter-Paier B, Skofitsch G, Windisch M (2001) Antiapoptotic effects of the peptidergic drug cerebrolysin on primary cultures of embryonic chick cortical neurons. J Neural Transm 108:459-473.
Hutter-Paier B, Steiner E, Windisch M (1998) Cerebrolysin protects isolated cortical neurons from neurodegeneration after brief histotoxic hypoxia. J Neural Transm Suppl 53:351-361.
Kanbayashi H, Itoh H, Kashiwaya T, Atoh K, Makino I (2002) Spatial distribution of nociceptive neuropeptide and nerve growth factor depletion in experimental diabetic peripheral nervous system. J Int Med Res 30:512-519.
Kryvko IuIa, Kozyts’kyĭ ZIa, Serhiienko OO, Kuchmerovs’ka TM, Velykyĭ MM (2001) Diabetic neuropathies. Metabolism of sorbitol in sciatic nerve tissue in streptozotocin diabete. Ukr Biokhim Zh 73:69-74.
Ladurner G, Kalvach P, Moessler H (2005) Neuroprotective treatment with Cerebrolysin in patients with acute stroke: a randomised controlled trial. J Neural Transm 112:415-428.
Lam TK, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, Giacca A (2002) Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-delta. Am J Physiol Endocrinol Metab 283:E682-691.
Llewelyn J (2003) The diabetic neuropathies: types, diagnosis and management. J Neurol Neurosurg Psychiatry 74:ii15-19.
Masliah E, Díez-Tejedor E (2012) The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs Today (Barc) 48 Suppl A:3-24.
Ning ZC, Li B (2002) The determining of the amino acid contents and the peptide mapping of injection of cerebroprotein hydrolysate. Heilongjiang Yiyao 15:430-432.
Piotrowski P, Wierzbicka K, Smiałek M (2001) Neuronal death in the rat hippocampus in experimental diabetes and cerebral ischaemia treated with antioxidants. Folia Neuropathol 39:147-154.
Qiu ZC, Zhang ZG, Li ZG, Gu PH, Yue DX (2010) A brief analysis of etiology and pathogenesis of DPN and discussion of therapy. Zhongguo Shiyan Fangji Xue Zazhi 16:255.
Rivlin AS, Tator CH (1977) Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 47:577-581.
Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E (2006) Cerebrolysin decreases amyloid-β production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. J Neurosci Res 83:1252-1261.
Sanchez-Vega L, Juárez I, De Jesus Gomez-Villalobos M, Flores G (2015) Cerebrolysin reverses hippocampal neural atrophy in a mice model of diabetes mellitus type 1. Synapse 69:326-335.
Sharma HS, Zimmermann-Meinzingen S, Johanson CE (2010) Cerebrolysin reduces blood-cerebrospinal fluid barrier permeability change, brain pathology, and functional deficits following traumatic brain injury in the rat. Ann N Y Acad Sci 1199:125-137.
Shtrygol’ OIu, Sadin AV, Konkina EA, Branchevskiĭ LL (2000) The efficacy of different salt diets and the modulation of the protective action of cerebrolysin in an experimental disturbance of the cerebral circulation. Eksp Klin Farmakol 63:29-32.
Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51:5-17.
US Food and Drug Administration (2002) Guidance for industry and reviewers: estimating the safe starting dose in clinical trials for therapeutics in adult healthy Volunteers: US Department of Health and Human Services, Rockville, MD, USA.
Verrotti A, Giuva T, Morgese G, Chiarelli F (2001) New trends in the etiopathogenesis of diabetic peripheral neuropathy. J Child Neurol 16:389-394.
Wronski R, Tompa P, Hutter-Paier B, Crailsheim K, Friedrich P, Windisch M (2000) Inhibitory effect of a brain derived peptide preparation on the Ca++-dependent protease, calpain. J Neural Transm 107:145-157.
Xu XL, Yu YB, Zhang L (2006) Clinical research of the dysneuria of peripheral nerve of cerebrolysin after nerve surgery of cerebral palsy. Zhongguo Linchuang Yisheng 34:33-34.
Xu Y (2009) Theoretical and experimental study of the effect of kidney-nourishing and collateral-activating therapy on the treatment of DPN. Nanjing: Nanjing University of Chinese Medicine.
Yagihashi S (2002) Pathology of diabetic neuropathy; a review from the updated literature of the last 10 years. Nihon Rinsho 60 Suppl 10:204-208.
Yagihashi S, Kamijo M, Watanabe K (1990) Reduced myelinated fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats. Am J Pathol 136:1365-1373.
Yasphal K, Wright DM, Henry JL (1982) Substance P reduces tail-flick latency: implications for chronic pain syndromes. Pain 14:155-167.
Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R (2003) Diabetic neuropathy and nerve regeneration. Prog Neurobiol 69:229-285.
Yuan BJ, Lu GC, Liu JP, Zhao GR, Wu H (2005) Promoting effect of nerve growth factor on sciatic nerve regeneration after the crush injury. Zhongguo Linchuang Kangfu 9:178-180.
Zhang L, Zhang Y, Xia Q, Zhao XM, Cai HX, Li DW, Yang XD, Wang K, Xia ZL (2008) Effective control of blood glucose status and toxicity in streptozotocin-induced diabetic rats by orally administration of vanadate in an herbal decoction. Food Chem Toxicol 46:2996-3002.
Zhao YY, Ding ZT (2009) Small fiber neuropathy. Zhongguo Linchuang Shenjing Kexue 17:534-539.
Copyedited by Smith T, Maxwell R, Yu J, Qiu Y, Li CH, Song LP, and Zhao M
10.4103/1673-5374.175063
How to cite this article: Dong HY, Jiang XM, Niu CB, Du L, Feng JY, Jia FY (2016) Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy. Neural Regen Res 11(1)∶156-162.
http://www.nrronline.org/
Accepted: 2015-07-07
*Correspondence to: Fei-yong Jia, Ph.D., erkekangfujia@163.com.
- 中国神经再生研究(英文版)的其它文章
- Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury
- Angiogenesis in tissue-engineered nerves evaluated objectively using MICROFIL perfusion and micro-CT scanning
- Dexamethasone prevents vascular damage in earlystage non-freezing cold injury of the sciatic nerve
- A novel bioactive nerve conduit for the repair of peripheral nerve injury
- Treatment with analgesics after mouse sciatic nerve injury does not alter expression of wound healingassociated genes
- Time representation of mitochondrial morphology and function after acute spinal cord injury

