Superovulatory response and embryonic progressive in Iranian Qezel ewes treated with two different concentrations of bovine somatotropin
Amir Hossein Asgari Safdar, Ali Asghar Sadeghi
Department of Animal Science, Faculty of Agriculture and Natural Resources, Tehran Science and Research Branch, Islamic Azad University, Tehran, Iran.
Superovulatory response and embryonic progressive in Iranian Qezel ewes treated with two different concentrations of bovine somatotropin
Amir Hossein Asgari Safdar*, Ali Asghar Sadeghi
Department of Animal Science, Faculty of Agriculture and Natural Resources, Tehran Science and Research Branch, Islamic Azad University, Tehran, Iran.
ARTICLE INFO
Article history:
Received
Received in revised form Accepted
Available online
Insulin-like growth factor 1
Embryo transfer
Bovine somatotropin
Insulin
Qezel ewes
Objective: This study was conducted in order to the administration of 50 and 100 mg of bovine somatotropin (bST) at the beginning of the estrus synchronization and natural mating of the sheep to evaluate the improvement of the ovulation rate, embryonic development and pregnancy rate of the transferred embryos. Methods: Forty eight donors were treated with three diff erent types of treatment; Group A: treated with bST-100 (n = 15), received 100 mg of bST at the beginning of the synchronization and natural mating, Group B: treated with 50 mg of bST (n = 15) same as the previous group and control (n = 18) did not receive any type of bST. Each recipient received two embryos, (n = 108): 30 recipients received the embryos from bST-100s, 45 recipients received the embryos from bST-50 and 33 recipients received embryos from the control group. Using SAS related GENMOD method, rate of superovulatory, recovered structure percentage, cleavage rate, transferable embryo percentage, quality of embryos, rates of pregnancy and embryonic development were analyzed. Using GLM procedure, numbers of corpus luteum and blastocyst cells were analyzed. Results: The bST administration had no signif icant ef fect on rate of superovulatory, number of CL and recovered structures (P ≥ 0.05). Number of transferable embryos and embryos that had access to the blastocyst in bST-50 (P ≤ 0.01) was more than bST-100 and control group. Conclusions: The treatment 50 mg bovine somatotropins enhance the ratio and growth of the transferable embryos. Embryos of bST-50 treatment indicated an improved embryonic development but bST did not aff ect the pregnancy rates of transferred embryos.
1. Introduction
Multiple ovulation and embryo transfer (MOET) is an implement to maximize the sheep population of fi ttest race. However, high diversity in superovulatory rate affects the efficiency of high quality embryo production[1]. External administration of bovine somatotropin (bST) increases the circulating concentrations of insulin and insulin-like growth factor 1 (IGF-1) in sheep[2,3]. Studies indicate that insulin and IGF-1 are in charge of bST effects on reproduction[4-6]. These eff ects include an increase in the recruited follicles[2,7] and development of produced embryos and increase their number[3,8].
Insulin and IGF-1 receptors in follicles of sheep have been identified[9]. In follicular cells, insulin increases the glucose and amino acid metabolism, stimulates cell growth and proliferation, inhibits follicular steroid secretion[10] and regulates the activity of gonadotropin receptors[11,12]. In granulosa cells, IGF-1 cooperates (synergistic) with FSH[13] to improve hormonal activities such as secretion of follistatin, an activator and inhibitor, proliferation and diff erentiation of granulosa cells, estradiol production and regulation of aromatase activity[14]. Generally, IGF-1 protects the oocyte and improves its maturity[15]. Researches about bST in ruminates indicates that this hormone improves the embryonic development, as a result, increases the reproduction efficiency[16]. bST and obtained concentrations of IGF-1, increase nuclear maturation rate and pyruvate metabolism and have anti-apoptosis eff ect on in-vitro bovine embryos[17] and increase the number of blastocyst[18,19]. Moreover, these hormones regulate the PGF2α synthesis[20]. In studies that are related to sheep, commercially available amount of bovine hormone is 500 mg that would be divided to four 125 mg parts. This amount was also used in several other studies before the mating or during the mating. The results of these studies indicate improvement in the embryo size[12] and embryonic development in sheep[3,8]. Also, another study by using the same amount in goatsthat were at anestrus cycle, showed an increase in pregnancy rate[21]. However, several researches show contradictory effects about superovulatory responses and pregnancy rate after the administration of bST[22-25]. Other scientists suggested that this diversity could be due to several factors such as bST levels, physical condition and serum concentrations of IGF-1[16,26,27]. For example, treating with bST helps to improve the pregnancy rate in dairy cows[28] decreases the pregnancy rate in non-lactating cows[28]. Detrimental eff ect of bST on pregnancy rate of non-lactating cows could be related to overstimulation of blood insulin and IGF-1 secretion[28]. In sheep, various responses may be attributed to the amount of bST. The purpose of this study was to assess the administration of 50 and 100 mg of bovine somatotropin in ewes with multiply ovulation at the beginning of synchronization and during the natural mating to see that if it improves the ovulation rate, embryonic development and pregnancy rate of transferred embryos or not.
2. Materials and methods
2.1. Animals and treatments
This study was conducted in Kashan during the breeding season (autumn) with an average elevation of 982 m and the average annual temperature of 35 °C (The latitude of the location of the experiment was: 32.637 524 532°38’15.09’’N), Qezel Ewes Research Center. All experimental procedures were approved by the Kansas State University. Donors 48 cyclic, adult Qezel ewes (3 years old) and recipients include 108 cyclic, adult Qezel ewes (3 years old). Donors and recipients were kept at the same place and they were fed hay, barley, maize, soybean meals and minerals. All of the sheep had the delivery experience (at least once) and their physical condition score was between 3 and 4 (in scale of 5)[29]. Ewes were synchronized using intra-vaginal CIDR (DEC manufacturing, Hamilton, New Zealand), the tool remained inside the vagina for 12 d. Intramuscular injection of selenium (0.05 mg/kg of body weight) and vitamin E (0.05 mg/kg of body weight) was conducted for all of the sheep, simultaneous by application of CIDR (day zero) (Mu-Se, MSD Animal Health, Mexico). After removing the intra-vaginal CIDR, intramuscular injection of 250 IU equine chorionic gonadotropins conducted for all of the sheep (Pregnecol, Bioniche Life Sciences Inc., Australia). At the day 0 (beginning day), donors were treated by three types of diff erent treatments, randomly: (A) treatment with bST (n = 15), received 100 mg of bST as subcutaneous injection at the beginning of synchronization and the second injection was carried out at the time of mating (Boostin-S, MSD Animal Health, Mexico), (B) treatment with bST-50 (n = 15), received a subcutaneous injection of 50 mg of bST with the same program as previous group (Boostin-S, MSD Animal Health, Mexico) and the control group (n = 18) received saline instead of bST. Ten days after placing the CIDR, superovulatory started. Superovulatory was stimulated using 164 mg of pFSH (Folltropin-V, Bioniche, Ontario, Canada) that was conducted as eight reductive doses (1 for every 12 h). Two days after removing the CIDRs, ewes were mated with male sheep that were approved for fertilizing ability, starting at 8 am, each ewe was mated 5 times (with 1 h intervals).
2.2. Embryo transfer
Actually, for each sheep three CLs or more is considered as a response to superovulatory[30]. Embryos were collected using semilaparoscopic method[31]. Embryos were classifi ed according to the growth level and morphology (Quality grade 1: excellent or good, Quality grade 2: fair, Quality grade 3: weak, Quality grade 4: dead or corrupt)[32].
Collecting and transferring the embryos was conducted 6 d after mating. Embryos were transferred using semi-laparoscopic method[31]. This method introduces two fresh embryos in the presence of CL to the ovary, inside the uterus. Only embryos of the first and second grades of quality were used. Embryos were transferred to 108 recipients: 30 recipients received the embryos from bST-100 treated, 45 recipients received the embryos from bST-50 treated and 33 recipients received the embryos from the control group. At the 40thday after embryo transfer, pregnancies were identifi ed using a 5.0 MHz real-time ultrasound device (Logic 100 PRO VET, GE Medical, Bangalore, India).
2.3. Blood sampling and measurement of hormones
From each group, fi ve female sheep were selected randomly and blood samples were collected inside the clot-activator associated tubes (BD Vacutainer, Becton, DikinSon, Copany, Franklin Lakes, NJ, US). These samples were collected each 48 h after the placement of CIDR (day 0) until the embryo collecting day (day 20), from the jugular vein. All of the samples were collected at 8 am, before feeding the sheep. Blood samples were centrifuged at 1 500× g for 10 min in order to denotative the serum, then separated blood serum were stored at -20 °C for further laboratory analysis. Concentrations of blood insulin were measured using commercial radioimmunoassay (RIA) kits (Insulin-CT, Cis-Bio International, Gif sur Yvette, France) with the coeffi cient of variation of internal testing of 3.5%. Serum IGF-1 concentration was measured for up and down control groups using double antibody radioimmunoassay with the coeffi cient of variation of internal testing of 0.65% and 14.8%, respectively (IGF-1-RIACT, Cis-Bio international, Gif sur Yvette, France).
2.4. Cell count
Totally, 102 embryos were counted (34 embryos in each group). These cells were counted by making expanded blastocysts permeable and marking them with 0.2% of Triton®X-100 (Cat. No. 93443; Sigma-Aldrich) solvent in preservative solution containing 30 μg/mL propidium iodide in 20 s (EmCare, ICPBio Limited, Auckland, New Zealand). Immediately after the marking, embryos were washed in preservative solution and placed in methanol ice containing 10 μg/mL bisBenzimide H33258 (Cat. No. B1155; Sigma- Aldrich) for 10 min. These embryos were transferred to 50:50 methanol-glycerol solution and they were mounted on small drops of this solution[33]. Mounted embryos were compacted slowly using a shrouding cover so that they would become spread in order to be counted (number of cells).Cells were counted using a Leica epifl uorescent microscope (DMIL Leica Microsystems, Wetzelar, Germany).
2.5. Statistical analysis
The superovulatory rate, recovered structures percentage (oocytes or embryos), cleavage rate, transferrable embryos percentage, classifi ed embryos percentage and their quality, number of embryos in each growth stage and pregnancy rate in transferred embryos were analyzed using GENOD method (SAS/STAT version 9.3, SAS Institute Inc., Cary, NC). Number of CLs and number of blastocyst cells were analyzed using SAS related GLM procedure. Insulin and IGF-1 concentrations among groups were analyzed using ANOVA in order to measure the repeats. The area under the curve (AUC) of insulin and IGF-1 was calculated using trapezoidal rule. AUC data for both hormones was analyzed using ANOVA. The differences between the groups were evaluated using t-test.
3. Results
Superovulatory rate (P = 0.14), number of CLs (P = 0.13) and recovered structures percentage (P = 0.07) were not aff ected by any type of treatments (50 and 100 mg of bST). However, cleavage division rate in bST-100 group (P = 0.000 1) was lower than bST-50 and control group. Transferable embryos percentage in each donor (P = 0.01) and the percentage of embryos reached the blastocyst stage (expanded and hatched) (P < 0.001) in bST-50 group was more than bST-100s and control group. The number of blastocyst cells (P = 0.15) and pregnancy rate of donors were not aff ected by any type of bST treatment (P = 0.21) (Table 1).
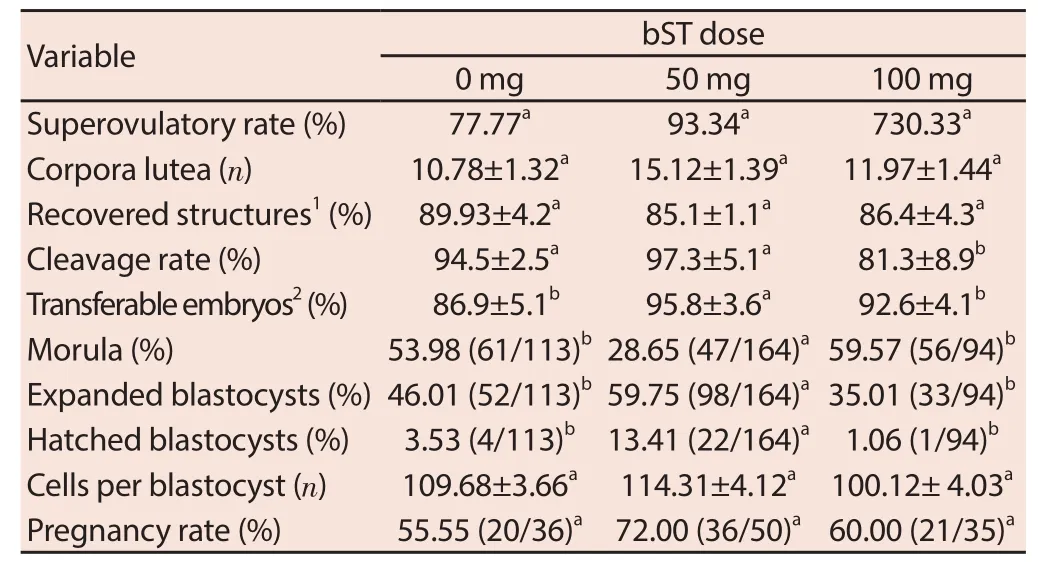
Table 1 Superovulatory rate, embryo development and pregnancy rate of embryos obtained from superovulated ewes treated of 0, 50 and 100 mg of bST at the beginning of estrous synchronization and mating.
After 48 h of the first hormone injection, insulin concentration in bST treated sheep increased. Treatment with bST-100 created the highest concentration of insulin (P = 0.02) after treatment with bST-50 (P = 0.02). After 6 d from the first injection in both groups, insulin concentration returned to the baseline. Similarly, the concentration returned to the baseline level 96 h after the second injection (Figure 1). However, after the second injection both groups experienced an increase of IGF-1 concentration, but this increase in comparison to the fi rst injection, was less obvious (Figure 2).
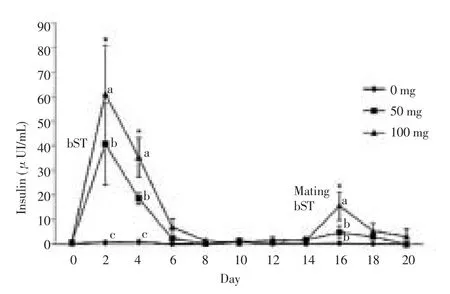
Figure 1. Insulin concentration (mean±standard error) in ewes of control group, 50 or 100 mg of bST treated at the beginning of estrus synchronization and at the time of mating.
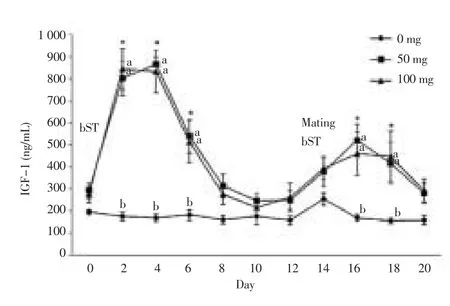
Figure 2. IGF-1 concentration (mean±standard error) in sheep of control group, 50 or 100 mg of bST treated at the beginning of estrus synchronization and at the time of mating.
AUC amounts for insulin (P = 0.02) and IGF-1 at the first injection was more than the second injection for both groups. AUC amounts for insulin in bST-50 treated was (121.5±64.6) μUI/(d·mL) and (24.9±25.8) μUI/(d·mL) for the first and second injection, respectively. Similarly, AUC amounts for IGF-1 for the first and second injections were (4.159 0±0.529 0) mg/(d·mL) and (2.529 7±0.815 4) mg/(d·mL), respectively. In bST-100 treated group, AUC amounts for insulin in the first and second injection was (203.5±115.9) μUI/(d·mL) and (46.8±35.9) μUI/(d·mL), respectively. Similarly, AUC amounts for IGF-1 in the fi rst and second injection was (4.129 0±0.105 1) and (2.479 0±0.102 1) mg/(d·mL), respectively.
4. Discussion
Administration of bST increased IGF-1 and external insulin concentrations in accordance with the results of other studies[2-6]. Interestingly, in this study serum IGF-1 concentrations for both values (50 and 100 mg) were the same. On the other hand, serum insulin concentration in bST-100s was higher. Generally, insulin and IGF-1 concentration after the fi rst injection was higher than that after the second injection. However, the reason of higher concentrations of insulin and IGF-1 as a response to the first bST injection was not discovered. Some of the researchers have discovered the same pattern for insulin concentration in non-lactating cows[22]. Spencer et al. discovered the same pattern for IGF-1 in lambs however the bST hormone had been used daily[34].
These fi ndings indicate that the fi rst injection of bST can stimulate the production of antibodies against this hormone therefore these antibodies can easily surmount the bST of the second injection, easily. Statistical analysis of the recent study didn’t show any improvement of superovulatory rate in both amounts of bST (50 and 100 mg) treatment. In contrast, Navarrete-Sierra et al. reported an improvement as a response to the administration of 125 mg of bST at the end of the treatment[35].
However, the diff erence between the groups was not statistically signifi cant, but numerical diff erences indicated that superovulatory rate in bST-50 group was higher than bST-100 and control group (21% and 13% more). Similarly, number of CLs of donors or recovered structures (oocytes or embryos) had no diff erence among the groups. In this regard, previous studies suggested that bST has a benefi cial eff ect on number of follicles or CLs[2,8,30,35]. However, other studies indicate that administration of bST does not increase the number of CLs[6,24,36] or recovered structures of ewes[8].
In this study, administration of 100 mg of bST in donors led to a declined percentage of cleavage of embryos, that is an adverse eff ect and this could be related to the increase in insulin concentration after the administration of 100 mg bST. Reports from the laboratory studies indicate that adding 5 μg/mL of insulin to the follicles medium, decreases the divided embryos (cleavage) percentage[37]. Administration of 50 mg of bST increased the transferable embryos percentage, considerably. This increase was related to the observed percentages of bST-100 and control group. Navarrete-Sierra et al. reported an increase of transferable embryos as a response to the administration of 100 mg of bST[35]. However, Montero-Pardo et al.[3] and Mejia et al.[8] didn’t observe this increase of transferable embryos as a response to the administration of 125 mg of bST. Similarly, embryos of bST-50 treatment indicated an improved embryonic development, because in comparison to other groups, most of the embryos were more advanced in term of embryonic stage (developed or hatched blastocyst). This diff erence, considering the fact that most advanced stage of growth, i.e., hatched blastocyst (hatched or expanded) was evaluated, makes it even more prominent, because the number of hatched blastocysts was approximately 5 times more than bST-50 treated and control group and approximately 11 times more than bST-100 treated group. Mejia et al. discovered that administration of 125 mg of bST to each donor at the mating time, increases the number of more advanced embryos in terms of advanced stages of development[8]. This case was similar to the results that Montero-Pardo et al.[3] reported. They used the same amount (125 mg) 5 d before the application of progestin.
Anyway, in this study, the number of cells in each blastocyst in both treated groups and the control group was the same. Montero-Pardo et al. reported that the administration of 125 mg of bST at 5 d before removing the sponge in sheep with multiplied ovulation increased the number of cells of embryo[3], although these writers reported fewer cells than the number of cells that was observed in the present study. On the contrary, Block et al. discovered that adding 100 ng/ mL of IGF-1 to the medium had no eff ect on the total number of cells in bovine embryo[38], which would suggest that IGF-1 eff ects on in-vivo embryo survival, likely is the result of diff erences in the gene expression, instead of being a result of changing the number of cells. Reports from several studies on the cows[39,40] indicate that administration of bST on the donors increases the pregnancy of the obtained embryos. However, administration of bST did not aff ect the pregnancy rate. These results are consistent with the reported results of Folch et al.[30] about sheep and Neves et al.[41] about cattle.
Variability among the experiments could be due to the bST applied amounts and the resultant of IGF-1, because IGF-1 concentration should be kept in a specifi c physiological range (approximately 200 ng/mL)[27,28]. Threshold concentration of IGF-1 can increase the fertility and pregnancy rates[28] but exceeding from this threshold concentration of IGF-1 can induce negative effects[22]. Recently, Ribeiro et al. reported that one time treatment with low amounts of bST (325 mg) at artifi cial insemination, was not enough to change the embryonic development and pregnancy[16]. However, two sequential therapies of 325 mg of bST at AI and 14 d later increased the pregnancy of the dairy cows and reduced the fertility decline that notes the importance of GH and IGF-1 during the primary growth of the embryo.
In the present study, production of transferable embryos and embryonic development of 50 mg of bST treated cows had better responses in comparison to the 100 mg of bST treated sheep. Embryos that were exposed to high concentrations of insulin and IGF-1, undergo apoptosis. As a result, apoptosis aff ects the embryo implantation and therefore, embryo would be reabsorbed[42,43]. Chi et al. showed that adding high concentrations of insulin to the mouse blastocysts medium, increases the apoptosis by DNA division[43]. Apoptosis is “dose-dependent”, because average amount (35 nmol/L) and large amount (700 nmol/L) cause 50% and 70% apoptosis, respectively. Similarly, Mihalik et al. reported that adding insulin to the bovine embryo medium has no effect on embryonic development[44]. In a study on cows with normal physical condition (3.4 on the scale of 6), Adamiak et al. reported that high concentrations of insulin, produces fewer follicles and blastocysts after the in-vitro fertilization[45]. Fouladi-Nashta and Campbell[37] showed that adding 5 μg/mL of insulin to the bovine antral medium, decreases the divided embryo ratio that were grown to transform into blastocyst and quality of embryos has no diff erence among thegroups (evaluated by total cell number). These writers suggested that decrease in the division rate is related to the primary cytoplasmic changes, that indicates oocyte were exposed to over-maturation or they have grown too old and this decreased the fertility rate. However, serum IGF-1 concentration in 50 and 100 mg bST treated groups was similar, while serum insulin concentration among the 100 mg treated group was higher, because insulin and IGF-1 can have cross-reactions with related receptors[46]. These results can show opposite eff ects, due to over-stimulation by 100 mg of bST that can increase the IGF-1R expression[47] and glucose uptake (insulindependent) by embryos[48] to impact reversely on them.
In in-vitro systems, consumption and decomposition of IGF-1 takes place without peptide renewal. On the other hand, in settings with high concentrations of IGF-1 (as an example in administration of bST) embryos are exposed to abnormal high concentrations of IGF-1 which may exacerbate apoptosis and hypertrophic ICM[47].
Declare of interest statement
The authors declare that they have no confl ict of interest.
Acknowledgments
This study was supported by the Ms. Arefe Eslalt Nejad. The authors thank to Mr. Kiani for assistance in the determination of hormone levels.
[1] Oliveira MEF. State of the art in the superovulation of ewes. Acta Sci Vet 2011; 39: 29-35.
[2] Gong JG, Campbell BK, Bramley TA, Webb R. Treatment with recombinant bovine somatotropin enhances ovarian follicle development and increases the secretion of insulin-like growth factor-I by ovarian follicles in ewes. Anim Reprod Sci 1996; 41: 13-26.
[3] Montero-Pardo A, Hernández-Céron J, Rojas-Maya S, Valencia J, Rodríguez-Cortez A, Gutiérrez CG. Increased cleavage and blastocyst rate in ewes treated with bovine somatotropin 5 days before the end of progestin-based estrous synchronization. Anim Reprod Sci 2011; 125: 69-73.
[4] Camacho LE, Benavidez JM, Hallford DM. Pregnancy rates and serum insulin-like growth factor-1, triiodothyronine, and progesterone profi les in rambouillet ewes treated with recombinant bovine somatotropin before breeding. Proceedings, Western Section. Am Soc Anim Sci 2008; 59: 249-252.
[5] Carrillo F, Hernández-Cerón J, Orozco V, Hernández JA, Gutiérrez CG. A single dose of bovine somatotropin 5 days before the end of progestinbased estrous synchronization increase prolifi cacy in sheep. Anim Reprod Sci 2007; 102: 31-37.
[6] Joyce IM, Khalid M, Haresign W. Growth hormone priming as an adjunct treatment in superovulatory protocols in the ewe alters follicle development but has no eff ect on ovulation rate. Theriogenology 1998; 50: 873-884.
[7] Ramon UJP, Folch PJ, Cocero MJ, Fernández-Arias A, Alabart JL, Garbayo JM. Embryo transfer to recipient ewes treated with growth hormone, eff ects on embryonic viability. Vet Mex 1998; 29: 137-145.
[8] Mejia O, Palma-Irizarry M, Rosas J, Madrid-Marina V, Valencia MJ, Zarco L. Administration of recombinant bovine somatotropin (rsBT) at the time of breeding in superovulated fertile and subfertile ewes. Small Rumin Res 2012; 102: 51-56.
[9] Scaramuzzi RJ, Brown HM, Dupont J. Nutritional and metabolic mechanisms in the ovary and their role in mediating the eff ects of diet on folliculogenesis: A perspective. Reprod Dom Anim 2010; 45: 32-41.
[10] Gallet C, Dupont J, Campbell BK, Monniaux D, Guillaume D, Scaramuzzi RJ. The infusion of glucose in ewes during the luteal phase increases the number of follicles but reduces oeestradiol production and some correlates of metabolic function in the large follicles. Anim Reprod Sci 2011; 127: 154-163.
[11] Munoz-Gutierrez M, Blache D, Martin GB, Scaramuzzi RJ. Folliculogenesis and ovarian expression of mRNA encoding aromatase in anoestrous sheep after 5 days of glucose or glucosamine infusion or supplementary lupin feeding. Reproduction 2002; 124: 721-731.
[12] Somchit A, Campbell BK, Khalid M, Kendall NR, Scaramuzzi RJ. The eff ect of short-term nutritional supplementation of ewes with lupin grain (Lupinus luteus), during the luteal phase of the estrous cycle on the number of ovarian follicles and the concentrations of hormones and glucose in plasma and follicular fluid. Theriogenology 2007; 68: 1037-1046.
[13] Beg MA, Ginther OJ. Follicle selection in cattle and horses: role of intrafollicular factors. Reproduction 2006; 132: 365-377.
[14] Silva JRV, Figueiredo JR, van den Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009; 71: 1193-1208.
[15] Neira JA, Tainturier D, Pena MA, Martal J. Effect of the association of IGF-I, IGF-II, bFGF. TGF-1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010; 73: 595-604.
[16] Ribeiro ES, Bruno RGS, Farias AM, Hernández-Rivera JA, Gomes GC, Surjus R, et al. Low doses of bovine somatotropin enhance conceptus development and fertility in lactating dairy cows. Biol Reprod 2014; 90: 1-12.
[17] Stefanello JR, Barreta MH, Porciuncula PM, Arruda JN, Oliveira JF, Oliveira MA, et al. Eff ect of angiotensin II with follicle cells and insulinlike growth factor-I or insulin on bovine oocyte maturation and embryo development. Theriogenology 2006; 66: 2068-2076.
[18] Moreira F, Paula-Lopes FF, Hansen PJ, Badinga L, Thatcher WW. Eff ects of growth hormone and insulin-like growth factor-I on development of in-vitro derived bovine embryos. Theriogenology 2002; 57: 895-907.
[19] Sirisathien S, Hernandez-Fonseca HJ, Brackett BG. Influences of epidermal growth factor and insulin-like growth factor-1 on bovine blastocyst development in vitro. Anim Reprod Sci 2003; 77: 21-32.
[20] Badinga L, Guzeloglu A, Thatcher WW. Bovine somatotropin attenuates phorbol ester-induced prostaglandin F2α production in bovine endometrial cells. J Dairy Sci 2002; 85: 537-543.
[21] Martinez AM, Gutiérrez CG, Domínguez HYM, Hernández CJ. Estrous response and pregnancy rate in seasonal anoestrous goats treated with progestogens and bovine somatropin. Rev Mex Cienc Pecu 2011; 2: 221-227.
[22] Bilby TR, Guzeloglu A, Kamimura S, Pancarci SM, Michel F, Head HH, et al. Pregnancy and bovine somatotropin in nonlactating dairy cows: I. Ovarian, conceptus, and insulin-like growth factor system responses. J Anim Sci 2004; 87: 3256-3267.
[23] Eckery DC, Moeller CL, Nett TM, Sawyer HR. Recombinant bovine somatotropin does not improve superovulatory response in sheep. J Anim Sci 1994; 72: 2425-2430.
[24] Hasler JF, Bilby CR, Collier RJ, Denham SC, Lucy MC. Effect of recombinant bovine somatotropin on superovulatory response and recipient pregnancy rates in a commercial embryo transfer program. Theriogenology 2003; 59: 1919-1928.
[25] Rivera F, Narciso C, Oliveira R, Cerri RLA, Correa-Calderón A, Chebel RC, et al. Eff ect of bovine somatotropin (500 mg) administered at tenday intervals on ovulatory responses, expression of estrus, and fertility in dairy cows. J Dairy Sci 2010; 93: 1500-1510.
[26] Block J, Rivera M, Drost M, Jousan FD, Looney CR, Silvestre FT, et al. Eff ects of bovine somatotropin and timed embryo transfer on pregnancy rates in non-lactating cattle. Vet Rec 2005; 156: 175-176.
[27] Velazquez MA, Zaraza J, Oropeza A, Webb R, Niemann H. The role of IGF1 in the in vivo production of bovine embryos from super-ovulated donors. Reproduction 2009; 137: 161-180.
[28] Bilby TR, Sozzi A, Lopez MM, Silvestre FT, Ealy AD, Staples CR, et al. Pregnancy, bovine somatotropin, and dietary n-3 fatty acids in lactating dairy cows: I. Ovarian, conceptus, and growth hormone-insulin-like growth factor system responses. J Dairy Sci 2006; 89: 3360-3374.
[29] Russel AJF, Doney JM, Gunn RG. Subjective assessment of body fat in live sheep. J Agric Sci 1969; 72: 451-454.
[30] Folch J, Ramon JP, Cocero MJ, Alabart JL, Beckers JF. Exogenous growth hormone improves the number of transferable embryos in superovulated ewes. Theriogenology 2001; 55: 1777-1785.
[31] Bari F, Khalid M, Haresign W, Murray A, Merrell B. Effect of mating system, fl ushing procedure, progesterone dose and donor ewe age on the yield and quality of embryos within a MOET program in sheep. Theriogenology 2000; 53: 727-742.
[32] Stringfellow DA, Seidel GE. Manual of the International Embryo Transfer Society (IETS). 3rd Ed. Savory: International Embryo Transfer Society 2000; p. 175-178.
[33] Fouladi-Nashta AA, Gutierrez CG, Gong JG, Garnsworthy PC, Webb R. Impact of dietary fatty acids on oocyte quality and development in lactating dairy cows. Biol Reprod 2007; 77: 9-17.
[34] Spencer GSG, Schurmann A, Berry C, Wolff JE, Napier JR, Hodgkin son SC, et al. Comparison of the eff ects of recombinant ovine, bovine and porcine growth hormones on growth, efficiency and carcass characteristics in lambs. Livest Prod Sci 1994; 37: 311-321.
[35] Navarrete-Sierra LF, Cruz-Tamayo AA, González-Parra EI, Pina-Aguilar RE, Sangines-García JR, Toledo-López V, et al. Effect of recombinant growth hormone (rbST) application on superovulatory response and embryo viability in hair ewes. Rev Cient 2008; 18: 175-179.
[36] Driancourt MA, Disenhaus C. Lack of effects of growth hormone administration on ovarian function of lactating goats. Anim Reprod Sci 1997; 46: 123-132.
[37] Fouladi-Nashta AA, Campbell KHS. Dissociation of oocyte nuclear and cytoplasmic maturation by the addition of insulin in cultured bovine antral follicles. Reproduction 2006; 131: 449-460.
[38] Block J, Wrenzycki C, Niemann H, Herrmann D, Hansen PJ. Effects of insulin-like growth factor-1 on cellular and molecular characteristics of bovine blastocysts produced in vitro. Mol Reprod Dev 2008; 75: 895-903. [39] Lee HJ, Hwang S, Yoon JT. Effects of bovine somatotropin (bST) administration combined with controlled internal drug release (CIDR) on embryo quality and pregnancy of Hanwoo (Korean Native Beef Cattle) during commercial embryo transfer program. Asian-Aust. J Anim Sci 2007; 20(2): 194-199.
[40] Moreira F, Badinga L, Burney C, Thatcher WW. Bovine somatotropin increases embryonic development in superovulated cows and improves post-transfer pregnancy rates when given to lactating recipient cows. Theriogenology 2002; 57: 1371-1387.
[41] Neves EF, Ramos AF, Marques JAP. Pre-tratamento com somatotropina bovina (rBST) na superovulaςão de doadoras da raςa Holandesa. Arq Bras Med Vet Zootec 2005; 57: 205-209.
[42] Betancourt-Alonso MA, Flores-Pérez FI, Rosas-Velasco C, Pérez-Martínez M. Role of cytokines in embryo implantation in domestic mammals. Vet Méx 2006; 37: 335-350.
[43] Chi MM-Y, Schlein AL, Moley KH. High insulin-like growth factor 1 (IGF-1) and insulin concentration trigger apoptosis in the mouse blastocyst via down-regulation of the IGF-1 receptor. Endocrinology 2000; 141: 4784-4792.
[44] Mihalik J, Rehák P, Koppel J. The influence of insulin on the in-vitro development of mouse and bovine embryos. Physiol Res 2000; 49: 347-354.
[45] Adamiak SJ, Mackie K, Watt RG, Webb R, Sinclair KD. Impact of nutrition on oocyte quality: cumulative eff ects of body composition and diet leading to hyperinsulinemia in cattle. Biol Reprod 2005; 73: 918-926. [46] Augustin R, Pocar P, Wrenzycki C, Niemann H, Fischer B. Mitogenic and anti-apoptotic activity of insulin on bovine embryos produced invitro. Reproduction 2003; 126: 91-99.
[47] Velazquez MA, Hermann D, Kues WA, Niemann H. Increased apoptosis in bovine blastocysts exposed to high levels of IGF1 is not associated with down regulation of the IGF1 receptor. Reproduction 2011; 141: 91-103.
[48] Velazquez MA, Hadeler KG, Herrmann D, Kues WA, Rémy Becker’s JF, Niemann H. In vivo oocyte IGF-1 priming increases inner cell mass proliferation of in vitro-formed bovine blastocysts. Theriogenology 2012; 78: 517-527.
ent heading
10.1016/j.apjr.2016.04.003
*Corresponding author: A.H. Asgari Safdar, Department of Animal Science, Faculty of Agriculture and Natural Resources, Tehran Science and Research Branch, Islamic Azad University, Tehran, Iran.
Tel: +98-55-234-100
Fax: +98-55-234-101
E-mail: amir9002001@yahoo.com
 Asian Pacific Journal of Reproduction2016年3期
Asian Pacific Journal of Reproduction2016年3期
- Asian Pacific Journal of Reproduction的其它文章
- A rare cause of infertility: A late complication of female genital mutilation
- Corpus callosum agenesis: Role of fetal magnetic resonance imaging
- Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG)
- Establishment, characterization and cryopreservation of Fars native goat fetal fibroblast cell lines
- Somatic embryogenesis and in vitro flowering in Hybanthus enneaspermus (L.) F. Muell.-a rare multipotent herb
- Pollutant exposure in Manila Bay: Effects on the allometry and histological structures of Perna viridis (Linn.)
