Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG)
Reda I. El-Sheshtawy, Walid S. El-Nattat, Samy I. A. Shalaby, Mohamed I. Shahba, Islam E. Al-Se'dawy
Animal Reproduction and Al Department, Veterinary Division, National Research Center, Dokki, Giza, Egypt
Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG)
Reda I. El-Sheshtawy*, Walid S. El-Nattat, Samy I. A. Shalaby, Mohamed I. Shahba, Islam E. Al-Se'dawy
Animal Reproduction and Al Department, Veterinary Division, National Research Center, Dokki, Giza, Egypt
ARTICLE INFO
Article history:
Received
Received in revised form Accepted
Available online
Buff alo
Semen
Preservation
Natural additives
Pollen grains
Objective: The benefi ts of dates palm and their pollen grains in the human nourishment system have their consideration and importance in medical and nutritional point of view. The present study aimed to investigate a new perspective for the use of date palm pollen grains (DPPG) aqueous extract or infusion in preservation of chilled and frozen buf falo semen. Methods: Pooled buff alo semen were diluted by Tris-Citrate-Fructose egg yolk (TCFY) diluent (considered as control) and diverse concentrations of clarifi ed soaked pollen grains in Tris-Citrate-Fructose (TCF) diluent in concentrations of 50, 100, 150, 200 and 250 mg DPPG / 5 mL TCF. Five concentrations were kept without egg yolk (TPG) while other f ive concentrations contained 20% egg yolk (TPGY). Each of the two dilutions was enumerated according to the concentration of DPPG. Extended semen is then chilled (for 7 d) and cryopreserved. Motility, alive sperm and intact sperm membrane (HOST) % were recorded as characteristic sperm parameters. Results: After 7 d of chilling, signifi cant (P < 0.000 1) high percent of motile sperms was recorded for TPG 50, 100, 150, 200 and 250 (66.67, 65.00, 61.67, 61.67 and 63.33, respectively). After freeze thawing, TPGY 150 and 250 showed the signifi cant (P < 0.000 1) high percent of motile sperms (43.75 and 45.00, respectively) while TPGY 100, 200, 250 and TPG 50, 150 revealed the high results concerning HOST (80.61, 83.99, 80.61, 88.33 and 83.33, respectively) whereas the highest alive sperm percentage was recorded with TPGY 150 (96.30) compared to the control. Conclusions: The aqueous cold infusion or extract of the DPPG, added to the TCF extender with or without the addition of egg yolk, proved its good preserving and maintaining capacity of chilled and thawed buf falo bull sperms which was expressed mainly by the sperm motility.
1. Introduction
The use of natural extract and infusions from fruits, vegetables and their seeds in extenders for preserving animal semen was introduced for their protective properties in preserving cattle and caprine sperms[1]. This innovative technique has resolved some problems in semen cryopreservation especially the bacterial contamination of extended semen, the presence of phytohormones in these natural products that protect the spermatozoa against the phospholipase A enzyme in the ejaculate[2]. Coconut water[3,4] and tomato juice[5,6] had given good results in semen preservation.
Palm dates pollen grains extract is also one of the potent fruits that has been investigated for its reproductive impact in rabbit bucks[7]. Gu et al.[8], Al-Farasi et al.[9] and Mansouri et al.[10] had examined the potent antioxidant activity of the aqueous extract of dates. This activity was attributed to the wide range of phenolic compounds in dates including p-coumaric, ferulic and sinapic acids, fl avonoids and procyanidins.
Admission of date palm pollen grains (DPPG) suspension into the nourishment system of male increased sperm counts and their motility, consequently it improves fertility through normalization of serum testosterone[11]. Date palm pollen is used as a traditional medicine for male fertility by improving sperm count, motility, morphology and DNA quality with increase in weight of testis and epididymis. These eff ects are due to the increase in plasmatestosterone levels as DPPG is rich in fl avonoids[12].
Hence, the present study aimed to investigate the potency of diff erent concentrations of cold aqueous infusion or extract of the DPPG, added to the Tris-citrate-fructose extender with addition of egg yolk (TPGY) or without egg yolk (TPG), in preserving and maintaining good chilled and after thawing fertile sperms.
2. Materials and methods
2.1. Preparation of different semen extenders
Tris Base Extender: Tris-citric acid-fructose without egg yolk (TCF) or with egg yolk (TCFY) diluents, were prepared according to Foote et al[13]. TCFY was used as control extender.
Tris-Pollen Grain Extender (TPG): Pollen grain extract was prepared after well grinding of DPPG in a morter to acquire a fine powder grade and sieved. 50, 100, 150, 200 and 250 mg of this powder was soaked in 2x5 test tubes each containing 5 mL of TCF diluents. All tubes were put in cooling incubator (adjusted at 10 °C) for 5 d with daily vortex and fi nally centrifuged to get the supernatant TPG extender. Five tubes were kept without egg yolk addition and enumerated TPG 50, TPG 100, TPG 150, TPG 200 and TPG 250, while the other fi ve tubes had received 20% egg yolk and enumerated TPGY 50, TPGY 100, TPGY 150, TPGY 200 and TPGY 250 later after soaking and centrifugation.
2.2. Semen collection and initial evaluation
Three mature cattle bulls maintained at Artificial Insemination Center, General Organization for Veterinary Services Ministry of Agriculture, Abbasia, Egypt, were used as semen donors. Ejaculates were collected using a bovine adapted artifi cial vagina at weekly intervals for 3 weeks. Semen samples were initially evaluated for subjective sperm motility and sperm concentration. Ejaculates fulfilling minimum standard of sperm motility (70%) and sperm morphology pooled in order to have suffi cient semen for a replicate and to eliminate the bull eff ect. The semen was given a holding time for 10 min at 37 °C in the water bath before dilution.
2.3. Semen processing
Semen samples were diluted with TCFY extender (control) and other aliquots of pooled semen samples were diluted with the different concentrations of TPG and TPGY extenders in order to provide a concentration of 60 million sperm/mL. Extended semen was slowly cooled to 5 °C and equilibrated for 2 h. Then, they were packed into 0.25 mL polyvinyl French straws. After equilibrium periods, the straws were horizontally placed on a rack and prefrozen in a liquid nitrogen vapour 4 cm above liquid nitrogen surface for 10 min and were fi nally dipped in liquid nitrogen. A fraction of extended semen from control and the diff erent concentrations of TPG TPGY was chilled for 7 d and inspected for sperm motility every 24 h.
2.4. Assessment of semen quality parameters
The assessment was undertaken on cooled and after thawing of bull spermatozoa. Also, sperm motility was evaluated for raw semen, 2 h after cooling and chilled semen daily up to 7 d. Frozen straws were thawed at 37 °C/ 1 min. The parameters studied were subjective semen characteristics (motility, alive and hypoosmotic swelling test (HOST) %)[14].
2.5. Statistical analysis
Statistical analysis were analyzed using the SAS[15] computerized program v. 9.2 to calculate the analysis of variance (ANOVA) for the diff erent parameters between control and additives replications. Signifi cant diff erence between means was calculated using Duncan multiple range test at P < 0.05.
3. Results
3.1. Effect of different concentrations of TCF enriched with DPPG on sperm motility of cooled and chilled buffalo bull semen
Sperm motility was signifi cantly higher with all concentrations of DPPG used after 2 h of cooling if compared to control. Although, after 7 d of chilling, the TPG 50, 100, 150, 200 and 250 revealed a signifi cantly (P < 0.000 1) higher percent of motile sperms compared to the control and the other TPGY concentrations (Table 1).
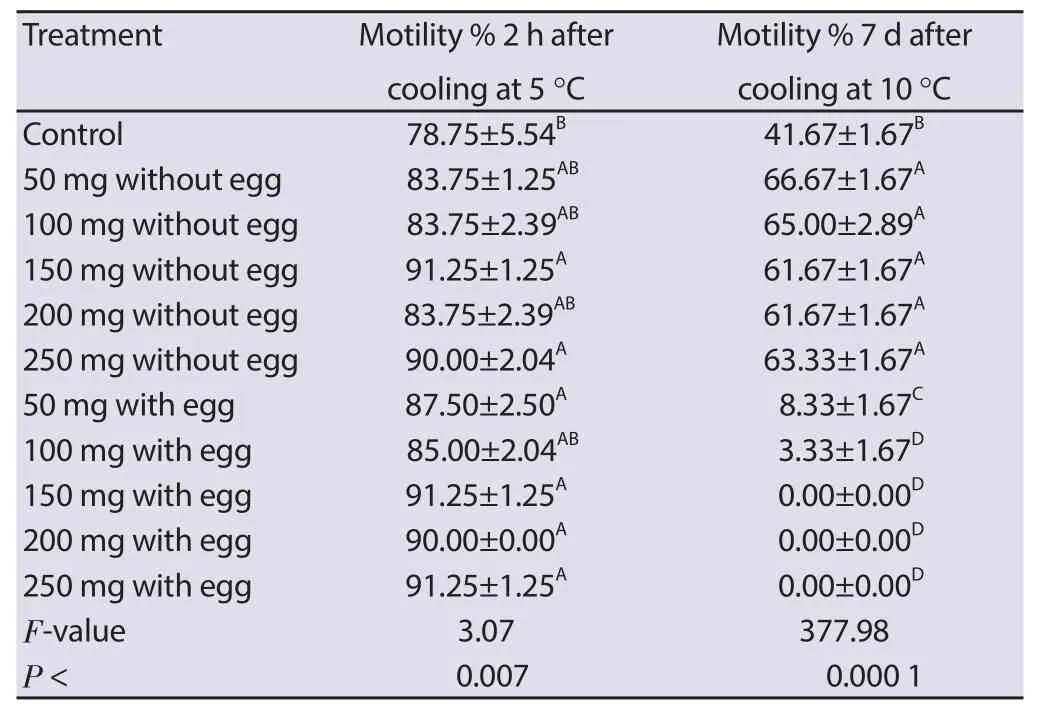
Table 1 Effect of Pollen grain addition to Tris diluent on the motility of buffalo semen after cooling and chilling.
3.2. Effect of different concentrations of TCF enriched with DPPG on post thawing semen characteristics
TPGY 250[(45.00±2.04)%], 150[(43.75±2.39)%] and 200[(38.75±5.15)%] showed that its post-thawing motility was apparently higher than the control TCFY (37.5±4.79). Concerning the intact sperm membrane test (HOST%), TPG 50 (88.33±1.67) and TPG 150 (83.33±1.67) and TPGY 100 (80.61±0.61), 200 (83.99±2.07), and 250 (80.61±0.61) showed higher signifi cant (P < 0.000 1) diff erence than control (74.38±2.37). Regarding the alive sperm percentage, TPGY 150 revealed higher signifi cant (P < 0.000 1) diff erence than the control TCFY (91.94±1.94) and other TPG and TPGY extended semen (Table 2).
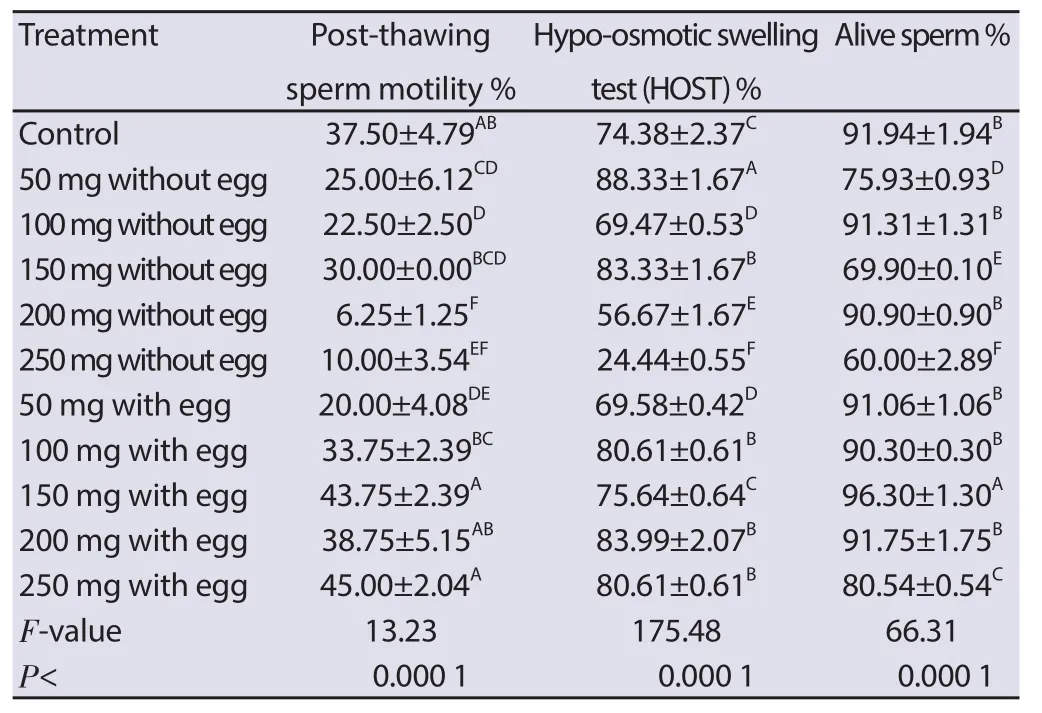
Table 2 Eff ect of Pollen grain addition to Tris diluent on the post-thawing buff alo semen characters.
4. Discussion
Cryopreservation of bovine semen often induce an additional source for reactive oxygen (ROS) attack on sperm due to decreased activities of antioxidant enzymes and the sperm membrane become more susceptible to lipid peroxidation[16] which aff ect the membrane permeability[17]. Natural antioxidants exert a protective effect preserving the metabolic activity and cellular viability of cryopreserved bovine spermatozoa[18]. The results of the present study revealed good sperm motility after 7 d of chilling especially TPG 50, 100, 150, 200 and 250; so, it can be used in artificial insemination (AI) up to 7 d of chilling and pollen can be used as an alternative of egg yolk in chilled buff alo semen to be protected against cold shock. These results were slightly diff ered from those obtained in cattle using the same concentrations and treatments with DPPG where TPGY 150 was the best enriched extender concentration (42.50%) after 7 d of chilling[19]. Post thawing semen characteristics were superior with TPGY 150 and 250 (for sperm motility %) although in cattle bull there were no signifi cant diff erence between TPG 50 (43.75%), TPGY 250 (42.50%) and the control (41.25%)[19], TPGY 100, 200, 250 and TPG 50, 150 (for intact spermtozoal membrane, HOST%) although in cattle bull there was fl uctuations that agreed with these results[19] and TPGY 150 (for alive %) this disagreed with the cattle results[19]. Hence, the present results could be attributed to the interaction between the species and the antioxidant eff ect of DPPG through reduction of ROS level exerted by its high content of flavonoids[11,12,20]. Flavonoids are excellent scavengers of free radicals and the number of hydroxyl group on the phenyl ring seems to enhance the antioxidant capacity of polyphenolic molecule[21,22]. On the other hand, the buff alo sperm may benefi t from some components in the DPPG infusion that may interact with the outer sheath of the sperm to protect itself against cold thence it may survive in low temperature.
The antioxidant eff ect of DPPG is due to its high concentration of vitamin C, B (Thiamine), B2(Riboflavin), nicotinic acid (Niacin) and vitamin A[23,24]. DPPG improved semen preservability through reduction of bacterial growth in the semen extender that used egg yolk as anti-cold shock[25]. In conclusion, the cold aqueous extract of the DPPG, added to the Tris-citrate-fructose extender with and without the addition of egg yolk, proved its good preserving and maintaining capacity of chilled (TPG 50 to TPG 150) and after thawing (TPGY 250 and TPGY 150) fertile buffalo bull sperms which was expressed mainly by the sperm motility.
Declare of interest statement
We declare that we have no confl ict of interest.
Acknowledgments
The authors are greatly indebted to the National Research Center for sponsoring this work through the project entitled: “Conservation of the genetic resources of local male breeds using natural additives for semen extenders”, grant no. 10120801, also thanks are due to the team assistants in the project for their help. Also, thanks are due to Dr. Gharieb A. El- Morsy and all staff of Artificial Insemination Center and to Dr. Abd El-Hamid El-Sokary in the General Organization for their kind assistance during this study.
[1] Sansone G, Nastri MJF, Fabbrocini A. Storage of buffalo (Bubalus bubalis) semen. Anim Reprod Sci 2000; 62: 55-76.
[2] Nunes, JF, Sales FGM. El agua de coco (Cocusnucifera) in natura integral y adicionada con citoquinina, comodiluidor de semen caprino. Rev CiencAnim 1993; 3(3): 273-281.
[3] Nunes JF, Combarnous Y, Oliviera LF, Teixeira MD. Utilição da agua de coco e suasfraçoesativasnaconservaçao in vitro e avaliação in vivo do semen naespeciecaprina. Rev Cienc Anim 1996; 2(1): 32-43.
[4] Vale WG. News on reproductive biotechnology in males. Proc. 5 World Buff alo Congr., Caserta, Italy; 1997; 1: 103-123.
[5] Al-Daraji HJ, Abdul Hassan IA, Ahmed AS, Razuki RH. Improving sperm characteristics during in vitro storage of roosters semen by supplementing semen diluent with tomato juice. Iraqi J Agri Sci 2006; 37(4): 165-171.
[6] Adeyemo OK, Adeyemo OA, Oyeyemi MO, Agbede SA. Eff ect of semen extenders on the motility and viability of stored African Catfi sh (Clarias gariepinus) spermatozoa. J Appl Sci Environ Manag 2007; 11(1): 13-16.
[7] Faleh BH, Sawad AA. Effect of palm pollen grains extracts (Phoenixdactylifera L) on spermatogenic activity of male rabbits. J Al-Basrah Res Palm Dried Dates 2006; 5(1-2): 1-10.
[8] Gu L, Kelm MA, Hammerstone JF, Boecher G, Holden J, Haytowitz D, Prior RL. Screening of foods containing proanthocyanidins and their structural characterization using LCMS/MS and thiolytic degradation. J Agric Food Chem 2003; 51: 7531-7521.
[9] Al-Farasi M, Alasalvar C, Morris A, Baron M, Shahidi F. Compositional and sensory characteristics of three native sun dried date (Phoenix dactylifera L.) varities grown in Oman. J Agric Food Chem 2005; 53: 7586-7591.
[10] Mansouri A, Embarek G, Kokkalou E, Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem 2005; 89: 411-420.
[11] Hassan, WA, El-Kaslan AM, Ehssan NA. Egyptian date palm pollen ameliorates testicular dysfunction induced by cadmium chloride in adult male rats. J Am Sci 2012; 8(4): 659-669.
[12] Bahmanpour S, Talaei T, Vojdani Z, Panjehshahin MR, Poostpasand A, Zareei S, et al. Eff ect of Phoenix dactylifera pollen on sperm parameters and reproductive system of adult male rats. Iran Med Sci J 2006; 31(4): 208-212.
[13] Foote RH, Brockett CC, Kaproth MT. Motility and fertility of bull sperm in whole milk extender containing antioxidants. Anim Reprod Sci 2002; 71: 13-23.
[14] Salisbury GW, VanDemark NL, Lodge JR. Semen evaluation. In: Physiology of Reproduction and Artificial Insemination of Cattle. 2 ed. San Francisco: WH Freeman and Company; 1978: 400-427.
[15] SAS. User’s Guide release 9.2 edition, Cary: SAS Institute Inc.; 2008.
[16] El-Sisy GA, El-Nattat WS, El-Sheshtawy RI. Buffalo semen quality, antioxidants and peroxidation during chilling and cryopreservation. Online J Vet Res 2007; 11(2): 55-61.
[17] Awda BJ, Mackenzie-Bell M, Buhr MM. Reactive oxygen species and boar sperm function. Biol Reprod 2009; 81: 553-561.
[18] Câmara DR, Mello-Pinto MMC, Pinto IC, Brasil OO, Nunes JF, Guerr MMP. Eff ects of reduced glutathione and catalase on the kinematics and membrane functionality of sperm during liquid storage of ram semen. Small Rumin Res 2011; 100: 44-49.
[19] El-Sheshtawy RI, El-Nattat WS, Ali AH, Sabra HA. The effect of Phoenix dactylifera pollen grains tris-infusion on semen preservability of local bull breeds. Global Vet 2014; 13(5): 728-732.
[20] Leja M, Mareczek A, Wyzgolik G, Klepacz-Baniak J, Czekonska K. Antioxidative properties of bee pollen in selected plant species. Food Chem 2007; 100: 237-240.
[21] Wettasinghe M, Shahidi F. Scavenging of reactive-oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chem 2000; 70: 17-26.
[22] LeBlanc BW, Davis OK, Boue S, DeLucca A, Deeby T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem 2009; 115: 1299-1305. [23] Al-Shahib W, Marsahll RJ. The fruit of the date palm: its possible use as the food for the future. Int J Food Sci Nut 1993; 54: 247-259.
[24] Hassan HMM. Chemical composition and nutritional value of palm pollen grains. Glob J Biotech Biochem 2011; 6(1): 1-7.
[25] Aba Al-Khail AA, Ibrahim JH, Waveform KM. A Practical Book in Microbiology. 1st ed. Riyadh: Publisher: majority of the publishing and distribution; 2003.
ent heading
10.1016/j.apjr.2016.04.001
*Corresponding author: Reda I. El-Sheshtawy, Animal Reproduction and AI Dept., Veterinary Research Division, 13 National Research Centre, Dokki 12622, Giza .
Tel: 202 33371635
Fax: 202-37601877
E-mail: rielsheshtawy@gmail.com
Foundation project: The authors are greatly indebted to the National Research Center for sponsoring this work through the project entitled: "Conservation of the genetic resources of local male breeds using natural additives for semen extenders", grant no. 10120801.
 Asian Pacific Journal of Reproduction2016年3期
Asian Pacific Journal of Reproduction2016年3期
- Asian Pacific Journal of Reproduction的其它文章
- A rare cause of infertility: A late complication of female genital mutilation
- Corpus callosum agenesis: Role of fetal magnetic resonance imaging
- Establishment, characterization and cryopreservation of Fars native goat fetal fibroblast cell lines
- Somatic embryogenesis and in vitro flowering in Hybanthus enneaspermus (L.) F. Muell.-a rare multipotent herb
- Pollutant exposure in Manila Bay: Effects on the allometry and histological structures of Perna viridis (Linn.)
- Changes in sperm characteristics of the three main breeds of sheep in Algeria after dietary supplementation
