Effects of honey to mobilize endogenous stem cells in efforts intestinal and ovarian tissue regeneration in rats with protein energy malnutrition
R. Heru Prasetyo, Erma Safitri
1Departement of Parasitology, Faculty of Medicine, Airlangga University-Surabaya-Indonesia
2Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Airlangga University-Surabaya-Indonesia
Effects of honey to mobilize endogenous stem cells in efforts intestinal and ovarian tissue regeneration in rats with protein energy malnutrition
R. Heru Prasetyo1*, Erma Safitri2
1Departement of Parasitology, Faculty of Medicine, Airlangga University-Surabaya-Indonesia
2Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Airlangga University-Surabaya-Indonesia
ARTICLE INFO Article history:
Received
Received in revised form
Accepted
Available online
Honey
Malnutrition
Stem cells
Intestine
Ovary
Objective: Auto-regeneration of the intestinal and ovarian tissue are experiencing degenerative due to protein energy malnutrition (PEM) through of auto-mobilization, increase of immune response and dif ferentiation of endogenous stem cells. Methods: Female rat model of PEM obtained through fasting meals for 5 d and only to drink, causing malnutrition and damage of the intestinal and ovarian tissue. Furthermore, the therapy of honey with a dose of 30% water (T1) and 50% water (T2) respectively for 5 d and compared with the positive control, were fasted without being given honey (T0+) and negative control, not fasted and without being given honey (T0-). Observations on: auto-mobilization, increased immune response, dif ferentiation of stem cells, and regeneration of the intestinal and ovarian tissue with HE staining. Results: Auto-mobilization of stem cells based on the expression of CD34+ and CD45+, which is a marker of endogenous stem cells (haematophoeietic stem cells/HSCs). Increased immune response is based on Hsp70 expression and PGE2 in intestinal tissue. Diff erentiation of stem cells into progenitor cells that expected based expression of growth dif ferentiation factor-9 (GDF-9) by immunohistochemistry in ovarian tissue. Conclusions: Expression of CD34+and CD45+, which signifi cantly diff erent in treatment 2 (2). Furthermore, increase of immune response (decrease Hsp70 expression and increased PGE2) in intestinal tissue. Increased immune response causes expression of GDF-9 in ovarian tissue. Decreased of Hsp70 expression, increased PGE2 and increased GDF-9 followed the process of regeneration of the intestinal and ovarian tissue.
1. Introduction
Protein energy malnutrition (PEM) has become one of the causes of immune defi ciency. Immune defi ciency conditions PEM cause a decrease in the number of immune cells such as B-IgA+, population of T-CD5+, CD4+ cells, CD8+, CD8β+, TCRβ+, and TCRyö in the lamina propria and intraepithelial villi intestine[1]. PEM can cause macrophage dysfunction[2]. Besides, the condition of protein energy malnutrition is the most common cause of secondary immune defi ciency[3], thus opening incidence of opportunistic infections of intestinal parasites such as Cryptosporidium [4-9]. In the United States and Western Europe, the prevalence of cryptosporidiosis in patients with immune defi ciency was about 10%–20%, and in the developing countries of Africa and Latin America it reached 50% [4].
Until now, malnutrition is still a health problem in Indonesia. The prevalence of protein energy malnutritionin in Indonesia has shown an increase since 2000. Death of nutrient defi ciency in children is more than 50% due to protein energy malnutrition, and the cause of death of nutrient defi ciency in children increased mortality due to diarrheal diseases. The result of the Direktorat Bina Gizi Masyarakat Ministry of Health, East Java was included in the category of ten provinces with the highest protein energy malnutrition cases in 2005. In 2009, East Java occupied the top position of national cases of severe malnutrition. This year, the number of PEM patients under 5 years old in East Java reached 77 500, the fi gure reached 2.5% out of the 3.1 million. Even the number of nutrient defi ciency of children under 5 year is 527 000 children, or 17% of the total children under fi ve year which is much higher[10]. In trial animals who have reached the age of puberty, protein energy malnutrition caused of the degeneration of the testes and ovaries cases so that the animals become infertile[11].
Interest in stem cell therapy today and the next few decades greatly increased sharply. This is because the potential of stem cell is very promising to be used as a treatment of various diseases. Stem cell transplantation provide new hope in the treatment of various diseases including immune defi ciency diseases and infertility due degenerative conditions of the gonads can not be cured through treatment and operative measures[12-15].
However, due to the complexity of the method of isolation, culture in vitro and transplant process with the high cost of transplantationof stem cells, it would require an innovation in an eff ort to automobilize and increase immune response is accompanied by diff erentiation of endogenous stem cells without going through the transplant process. Auto-mobilization and increased immune response accompanied diff erentiation was achieved through the provision of food or beverages derived from natural materials[14]. In this research through the provision of honey, it is expected there will be an auto-mobilization and increased immune response and diff erentiation of the patient’s with degeneration of intestine and ovary[16]. The presence of auto-mobilization and increased immune response and diff erentiation of stem cells is accompanied sourced from the body itself will take place regeneration of lamina propria and epithelial intestinal villi and follicle and corpus luteum of the ovary.
The regeneration can be proven both histopathological and molecular. The histopathologically will occur regeneration of intestinal and ovarian tissue. Molecularly proven through several expressions such as expression of CD34+ and CD45+ of hematopoietic stem cells (HSCs), Hsp70 and PGE2 intestinal tissue and growth diff erentiation factor-9 (GDF-9 ) of the ovary[17].
2. Material and methods
2.1. PEM modeling causes intestinal and ovarian degeneration
This study begins by modeling PEM causes the intestine and ovaries degeneration in female mice were fasted for 5 d without food, only water to drink every 8 h per sonde[10]. Animals used in this study were Wistar strain female rats, aged 10–12 weeks with a weight of 200–250 grams, in a healthy condition characterized by active movement. Mice kept in a plastic cage space per individual in laboratory animals experiments in Veterinary Medicine Faculty Airlangga University with adequate ventilation.
2.2. Treatment
The study were divided into 4 groups, each contained 6 replicates: The control group-(T0-): Rats not fasted and without honey; The control group + (T0+ ): Rats were fasted for 5 d and without honey; The treatment group (T1): Rats were fasted for 5 d, then given a 30% (v/v) honey in the drinking water for the next 5 d; The treatment group (T2): Mice were fasted for 5 d, then given a 50% (v/v) honey in the drinking water for the next 5 d.
2.3. Fluocytometri observation of HSCs mobilisation based on expresion of CD34+ and CD45+
After the rats were treated, further examination of whole blood as a sample is taken via cardiac puncture and inserted into the tube heparin to prevent coagulation. Further observations were done on the expression of CD34+ and CD45+ by fl uocytometri.
Fluocytometri method, starting with the preparation of whole blood centrifugation in a temperature of 4 °C, with a speed of 6 000 r/min for 15 min. Results centrifuging the cell in the form of sludge mixed with cytoperm / cytofi x amount of 2 times the number of cells are obtained. A mixture of cells and cytoperm / cytofi x centrifuged to obtain a supernatant and a pellet. BD then added to wash the pellet amount 4 times the number of cells obtained in the first centrifugation.
Furthermore add lysis buff er amounting to 2 times the amount of the initial cells were obtained. After that add labeled antibody conjugate to each sample, fi ve tubes are prepared and processed in parallel. (1) Single staining with CD34 PE added to the wash tube. (2) Double Staining with CD34 PE and CD45 PerCP and CD44 FITC wash tube. (3) Double Staining with CD34 PE and CD45 PerCP trucount tube. The entire sample was then stored at 4 °C in the dark and analyzed using fl owcytometri for 1 h [17].
2.4. Imunohistochemical (IHC) methodes observation of HSP70, PGE2 and GDF-9
Immunohistochemical observation was performed to determine the expression of HSP70, PGE2 dan GDF-9. Before to IHC methods were made histological preparation, by way of an incision is made transversely intestine and ovarium tissue from paraffin blocks. Further examination was performed by making outward through immunohistochemical techniques using monoclonal antibodies. This is done to determine the expression of HSP70, PGE2and GDF-9. Observations of HSP70, PGE2and GDF-9 were made using a light microscope with a magnifi cation of 200 times and the expression of each variable is indicated by the number of cells with brownish discoloration chromogen in each incision [18].
2.5. Histopatology anatomy observation of ovarium
Regeneration identifi ed of intestine and ovarium tissue through histopathological examination begins with the making of histological preparations. Histological preparations such as the following: Rat intestine and ovarium fi xation in 10% buff er formalin. Subsequently rat intestine and ovarium dehydrated in alcohol solution with a higher concentration, ie from 70%, 80%, 90%, 96% (absolute). Then do the clearing in the intestine and ovarium of rat in xylol solution or chloroform or benzene. Furthermore performed embedding using liquid paraffi n and rat ovarium were put into molds containing liquid paraffi n. Before stained and sectioning performed, an incision using a microtome and mounted on glass objects. Furthermore is done the staining by removing of paraffi n with xylol then put into a solution of alcohol with decreased concentration and then put into stain matter. The last stage after stained is done mounting, put into water or alcohol to remove excess stain. Then put into a solution of alcohol with increasing concentration, and then put into xylol. Preparations then covered with a cover glass and mounted with Canada balsam or anthelan[19].
After making preparations histopathologic like the above examination using a light microscope with a magnifi cation of 200 times. Observations identifi cation of ovarium regeneration is based on the existing histological description
2.6. Statistical analysis
Expression of CD34, CD45, HSP70, PGE2and GDF-9 were statistically analyzed using SPSS 15 for Windows XP with the level of significance 0.05 (P=0.01) and the confidence level of 99% (α=0.01). Steps comparative hypothesis testing is as follows: Test data normality with the Kol mogorof Smirnov test, homogeneity of variance test, Analysis of variants (ANOVA) factorial, Post hoc test (Least Signifi cant Diff erence test) using the Tukey HSD 5%.
3. Results
Data were collected from 24 female rats which were divided into four treatments: negative control group (T0-) is normal intestine and ovary without bee honey treatment; positive control group (T0+)is degenerative intestine and ovary without bee honey treatment; (T1) group is degenerative intestine and ovary + 30% (v/v) honey in drinking water for 5 d; (T2) group is degenerative intestine and ovary + 50% (v/v) honey in drinking water for 5 d. In detail, the results of the study are as follows: The eff ectively of bee honey was based on: mobilization of endogenous stem cells (HSCs), HSP70, PGE2 and GDF-9 expressions and regeneration of intestine and ovarium tissue. Mobilization of HSCs was analyzed by fl ow cytometry based on increased concentration of CD34 and CD45. The analysis showed that: either a negative control group (T0-), positive control group (T0+) or group (T1) showed no mobilization of HSCs, based on the percentage of CD34 and CD45 which are at a percentage of less than 25% (Figure 1A, 1B, and 1C), whereas in the group (T2) showed mobilization of HSCs is based on the percentage of CD34 and CD45 which is located on the percentage of over 70% (Figure 1B). Based on statistical calculations T2 groups was signifi cantly diff erent (P < 0.05) than the other three treatments (T0-, T0+ and T1), whereas among the three treatments no significant difference (P > 0.05) (Table 1).
Furthermore, increased of immune response based on Hsp70 expression, in the normal control group (T0-) was on the score 0.17±-0.78 (Hsp70 expression between 1%–5%). The group of intestine degenerative (T0+) was on the score 2.83±0.45 (Hsp70 expression >.50% ). The group 30% (v/v) honey (T1) was on the score 2.33±0.37 (Hsp70 expression 25%–50%). The group use 50% (v/v) honey (T2) was on the score 0.67±-0.18 (Hsp70 expression between 6%–25%) (Table 1).

Figure 1. Flowcytometric analysis of endogenous stem cells mobilisation (HSCs).
Furthermore, the increased of immune response based on PGE2 expression, in the normal control group (T0-) was on the score 1.00±0.00 (PGE2 expression between 6%–25%). The group of intestine degenerative (T0+) was on the score 0.17±-0.78 (PGE2 expression between 1%–5%). The group 30% (v/v) honey (T1) was on the score 0.33±-0.48 (PGE2 expression between 1%–5%). The group use 50% (v/v) honey (T2) was on the score 2.83±0.45 (PGE2 expression > 50%) (Table 1). Honey treatment in this study proved to increase the immune response and intestinal motility via decreased expression of Hsp70 and increased PGE2 in the intestinal tissue.
Furthermore eff ectiveness of honey is based on GDF-9 expression as a result of the diff erentiation of the progenitor cells. Expression of GDF-9 in the group use 50% (v/v) honey (T2) was on the score 2.00±0.00 (GDF-9 expression between 25%–50%) (Figure 2D). Although the score was below the normal control group (T0-) was on the score 2.83±0.43 (GDF-9 expression > 50%) (Figure 2A), but the percentage was still well above the group 30% honey (T1) was on the score 0.33±-0.48 (GDF-9 expression between 1%–5%) (Figure 2C) and a group of ovarium degenerative (T0+) was not expressed at all 0.00±0.00 (GDF-9 expression 0%) (Figure 2 and Table 1).
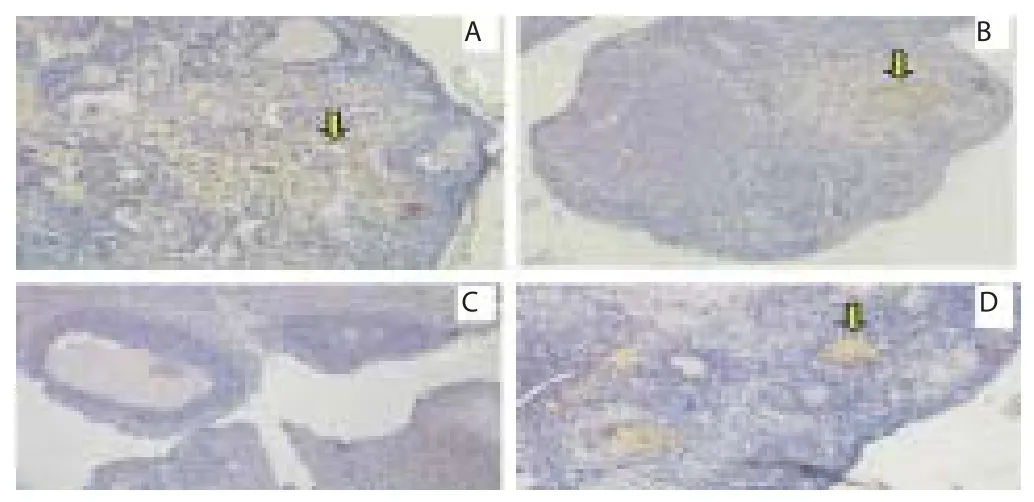
Figure 2. GDF-9 expression in rat ovarian tissue through immunohistochemical methods on several treatments.
In this study, the regeneration of the ovarium can be observed through the method of histopathology with hematoxylin eosin (HE) staining. Microscopic examination showed that the group of 50%honey (T2), leading to the occurrence of ovarium tissue repair. Improvements are identifi ed based on the regeneration of ovarium with growing folicle expression. Overview of these improvements can be compared with a control negative group (T0-) who did not experience ovarium degeneration, which remains in normal condition with growing folicle expression. As for the group of 30% (v/v) honey (T1) didn’t indicate the occurrence of ovarium tissue repair. Not the improvement in the form of ovarium that are no longer intact (broken). Picture of the damage can be compared with control positive of rat (T0+) rat with ovarian degeneration. The group of ovarian degenerative (T0+), ovary congested and hemorrhagi extensive, also visible hemosiderin due to blood cell lysis (brownish yellow color) accompanied by fibrin deposition indicating that chronic congestion has occurred. The group use 30% (v/v) honey (T1), ovary does not regenerate, it appears there is congestion along hemosiderin expression and are still widely hemorrhagi (Figure 3).
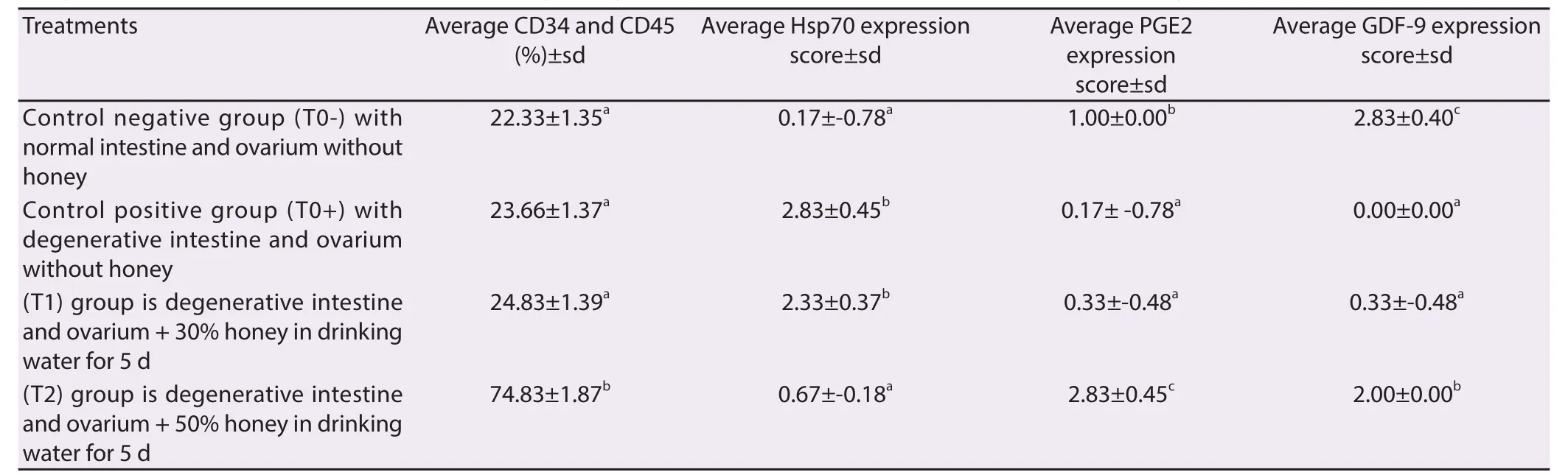
Table 1 Analyse of average percentage of CD34 and CD45 by fl owcytometri, expression score of Hsp70, PGE2 and GDF-9 by immunohistochemical method.

Figure 3. The regeneration of ovarian tissue through the method imunohistopathology anatomy (HP ) with hematoxylin eosin (HE) staining in rat ovarian tissue in a few treatments.
4. Discussion
The present study Showed that giving honey by 50% (v/v) for 5 d in treatment (T2) can used for the treatment of female rat model of PEM. The effectivity of honey was based on: mobilization of endogenous stem cells (HSCs), HSP70, PGE2 and GDF-9 expressions, and regeneration of intestine and ovary tissue.
Mobilization of endogenous stem cells can occur due to undergo induction of stem cells so mobilized toward the defect. The process of mobilization can occur in several ways, one of which is an increase due to the immune response induced infl ammatory reaction due to injury signals (Cytokines, NFkB, Wnt through β catenin) of tissue damage[20]. In this study, injury due to malnutrition signal causes an increase in Hsp70 and decreases PGE so that damage to the intestinal tissue can not be inevitable. Damage to the intestinal tissue is the cause of the disruption of the absorption of food that is needed by all body tissues including the ovary as the primary network of the female reproductive system[21].
This condition was need of repair, especially improvements in intestinal tissue as the tissue is responsible for the absorption of food. PGE2 have long been known to have cytoprotective eff ects on the gastrointestinal epithelium. Their cytoprotective eff ect appears to result from a complex ability to stimulate mucosal mucus and bicarbonate secretion to increase mucosal blood fl ow and particularly in teh stomach, to limit back diff usion of acid into the epithelium[22].
Furthermore, this causes endogenous stem cells that had been induced to be will give suport continual replenishment of gastrointestinal epithelium reside in the middle of gastric pits and within the crypts of the small and large intestine. These stem cells proliferate continually to supply cells that then diff erentiate into absorptive anteroctytes, mucus-secreting goblet cells, enteroendocrine cells and Paneth cells. Except for Paneth migrate up from the crypts to replace cells xtruded from the tips villi. This migration takes 3–6 d[21].
Furthermore, the improvement of the intestine is eventually causes the ovarian tissue repair through GDF-9 expression. GDF-9 which is progenitor cells of germ line stem cells will stimulate ovarian cortex cell proliferation. This is in accordance with the opinion, that honey will cause the stem cells develop rapidly differentiate into cells that are needed as a response of the defect and enhancement of the immune respons[23].
Regeneration of ovarium tissue, such as: intact of ovarium tissue with growing folicle expression is the third identification of the eff ectiveness from the use of honey. In this study, the regeneration of the ovarium can be observed through the method of histopathology anatomy (HPA) with HE staining. Microscopic examination showed that the group of 50% (v/v) honey (T2), leading to the occurrence of ovarium tissue repair. Improvements are identifi ed based on the regeneration of ovarium with growing folicle expression. Overview of these improvements can be compared with a control negative group (T0-) who did not experience ovarium degeneration, which remains in normal condition with growing folicle expression. As for the group of 30% (v/v) honey (T1) does not indicate the occurrence of ovarium tissue repair. Not the improvement in the form of ovarium that are no longer intact (broken). Figure of the damage can be compared with control positive of rat (T0+) rat with ovarium degeneration. The group of ovarium degenerative (T0+), ovary was congested and hemorrhagi extensive, also visible hemosiderin (yellow brown) due to blood cell lysis with fi brin deposition (baby color pink) indicating that chronic congestion has occurred. The group use 30% (v/v) of honey (T1), the ovary does not regenerate, it appears there is congestion along hemosiderin expression and are still widely hemorrhagi.
Treatment of 50% concentration honey for 5 d in female rat with degenerated intestine and ovary can show (i) Mobilization of endogenous stem cells (HSCs), in the form of markers CD34 and CD45 are expressed in blood serum; (ii) Increased immune response in the form of a decrease in the expression of Hsp70 and increased PGE2 by immunohistochemistry in tissue intestine andGDF-9 expression by immunohistochemistry in ovarian tissue; (iii) Regeneration of ovarian tissue, such as: intact of ovarian tissue with growing folicle expression, although there is still little hemorrhagi and congestion but hemosiderin expression and fi brin deposition has not looked back.
Declare of interest statement
We declare that we have no confl ict of interest.
Acknowledgments
The study was supported by funding from the Directorate General of Higher Education (DIKTI) 2015. The National Education Ministry. Republic of Indonesia. The authors are also grateful to LPPM chairman Airlangga University Prof. Dr. Djoko Agus Purwanto, Apt., M.Si.
[1] Vidueiros SM, Fernandez I, Slobodianik N, Roux ME, Pallaro A. Nutrition disorder and immunologic parameters: study of the intestinal villi in growing rats. J Nutr 2008; 24(6): 575-581.
[2] Nahed M, Hassanein A, Hamed MR. Impact of protein malnutrition on susceptibility to experimentally-Induced testicular macrophage dysfunction. J Appl Sci Res 2008; 4(12): 2161-2168.
[3] Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol 2010; 125(2): 195-203.
[4] Costa LB, JohnBull EA, Reeves JT, Sevilleja JE, Freire RS, Hoff man PS, et al. Cryptosporidium-malnutrition interactions: mucosal disruption, cytokines, and TLR signaling in a weaned murine model. J Parasitol 2011; 97(6): 1113-1120.
[5] Costa LB, Noronha FJ, Roche JK, Sevilleja JE, Warren CA, Oriá R, et al. Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of Cryptosporidium infection and malnutrition. J Infect Dis 2012; 205(9): 1464-1471.
[6] Rodrigues RS, Oliveira RA, Li Y, Zaja-Milatovic S, Costa LB, Braga Neto MB, et al. Intestinal epithelial restitution after TcdB challenge and recovery from Clostridium difficile infection in mice with alanyl-glutamine treatment. J Infect Dis 2013; 207(10): 1505-1515.
[7] Castro IC, Oliveira BB, Slowikowski JJ, Coutinho BP, Siqueira FJ, Costa LB, et al. Arginine decreases Cryptosporidium parvum infection in undernourished suckling mice involving nitric oxide synthase and arginase. J Nutr 2012; 28(6): 678-685.
[8] Roche JK, Rojo AL, Costa LB, Smeltz R, Manque P, Woehlbier U, et al. Intranasal vaccination in mice with an attenuated Salmonella enterica Serovar 908htr A expressing Cp15 of Cryptosporidium: impact of malnutrition with preservation of cytokine secretion. J Vaccine 2013; 31(6): 912-918.
[9] Gatei W. Unique cryptosporidium population in HIV-infected person Jamaica. Emerg Infect Dis 2008: 31(3): 208-217.
[10] Prasetyo RH. Perubahan ekspresi CD4, IgA, PGE2 dan Hsp70 mukosa usus Mus musculus Balb C kurang energy protein yang diinfeksi Cryptosporidium. 1st ed. Airlangga University Disertation; 2010: 1-10.
[11] Safitri E, Utama S, Bumi C, Mulyani SWM, Susilowati H, Retnowati E, et al. The role of adaptive MSCs an attempt regeneration of spermatogenesis process using by hypoxia precondition in vitro. J Anim Prod Adv 2013; 3(11): 318-322
[12] Song H, Song BW, Cha MJ, Choi IG, Hwang KC. Expert opinion modifiation of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther 2010; 10(3): 309-319
[13] Halim DH, Murty F, Sandra A, Boediono T, Djuwantono B, dan Setiawan B. Stem Cell Dasar Teori dan Aplikasi Klinis. 1st ed. Penerbit Erlangga Jakarta; 2010: 1-10
[14] Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 2011; 6(8): 11-15.
[15] Volarevic V, Arsenijevic N, Lukic ML, Srojkovic M. Concise review: Mesenchymal stem cells treatment of the complications of diabetes mellitus. Stem Cells 2011; 29(1): 5-10.
[16] Sakri FM. Madu dan Khasiatnya, Suplemen Sehat Tanpa Efek Samping. 1st ed. Diandra Pustaka Indonenesia Yogyakarta; 2012: 11-15.
[17] Macey MG. Flowcytometry, Principle and Aplications. 3rd ed. Human Press; 2007: 1-31
[18] Kumar GL, Rudbeck L. Immunohistochemical Staining Methods. 5th ed. Dako North America, Carpinteria, California; 2009: 11-14.
[19] Eckmann L. Animal models of inflamatory bowel disease, lesson from enteric infections. Ann NY Acad Sci 2007; 1072: 28-38.
[20] Rantam, Ferdiansyah, Nasronudin, Purwati. Stem Cell Exploration. Methods of Isolation and Culture. 1st ed. Surabay: Airlangga University Press; 2009: 20-25.
[21] Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature Rev Mol Cell Biol 2014; 10(15): 19-33.
[22] Flier vdLG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009; 71: 241-260.
[23] Dussaubat C, Brunet JL, Higes M, Colbourne JK, Lopez J, Choi JH, et al. Gut pathology and responses to the microsporidium nosema ceranae in the honey bee apis mellifera. PLoS One 2012; 7(5): 1-12.
ent heading
10.1016/j.apjr.2016.04.008
*Corresponding author: R. Heru Prasetyo, Departement of Parasitology, Faculty of Medicine, Airlangga University-Surabaya-Indonesia.
E-mail: rheru_prasetyo@yahoo.co.id
Foundation project: The study was supported by funding from the Directorate General of Higher Education (DIKTI) 2015.
 Asian Pacific Journal of Reproduction2016年3期
Asian Pacific Journal of Reproduction2016年3期
- Asian Pacific Journal of Reproduction的其它文章
- A rare cause of infertility: A late complication of female genital mutilation
- Corpus callosum agenesis: Role of fetal magnetic resonance imaging
- Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG)
- Establishment, characterization and cryopreservation of Fars native goat fetal fibroblast cell lines
- Somatic embryogenesis and in vitro flowering in Hybanthus enneaspermus (L.) F. Muell.-a rare multipotent herb
- Pollutant exposure in Manila Bay: Effects on the allometry and histological structures of Perna viridis (Linn.)
