硼颗粒的包覆机理及工艺研究进展
陈冰虹,刘建忠,梁导伦,周禹男,周俊虎
(浙江大学能源清洁利用国家重点实验室,浙江 杭州310027)
硼颗粒的包覆机理及工艺研究进展
陈冰虹,刘建忠,梁导伦,周禹男,周俊虎
(浙江大学能源清洁利用国家重点实验室,浙江 杭州310027)
阐述了不同包覆材料对硼颗粒的包覆机理,从5个方面总结了硼颗粒包覆材料的选取原则,包括:去除硼颗粒表面氧化膜、提高燃烧温度、降低硼的点火温度、提高表面相容性、催化硼颗粒的氧化反应。总结了沉淀法、表面反应包覆法、高分子吸附聚合法、气相包覆法和机械球磨法等多种硼颗粒包覆工艺的研究状况,分析并比较了不同工艺的作用机理和实际应用效果。介绍了现代硼颗粒表面包覆效果测试技术的特点和应用范围。评述了目前硼颗粒包覆技术的研究现状和不足,并对未来的研究方向进行了展望。附参考文献46篇。
物理化学; 硼颗粒; 包覆机理;富燃料推进剂;金属燃料
引 言
富燃料推进剂是适应固体火箭冲压发动机的良好燃料,添加金属燃料是当前高能贫氧推进剂的一个重要发展方向。硼以其高的质量热值和容积热值被认为是固体贫氧推进剂的最佳燃料[1],但由于硼颗粒的部分固有特性[2],含硼推进剂在实际应用中受到一定的限制。硼在固体富燃料推进剂中应用存在的突出问题包括4个方面:(1)单质硼的熔沸点较高,难以熔化气化,B2O3的沸点也较高,燃烧过程要经历B2O3的气化,进一步增加了硼颗粒点火的困难;(2)硼颗粒点火延滞,燃烧时间长,在发动机燃烧室中存在燃烧不完全现象,能量释放不完全;(3)硼的燃烧效率低,耗氧量大,产生残渣多,无法发挥其高能量热值;(4)硼颗粒表面存在B2O3、H3BO3等杂质,使得硼粒子与推进剂体系不相容[4]。
研究表明[5-9],使用包覆材料对硼颗粒进行包覆是改善硼颗粒点火燃烧特性的较好途径,国内外学者对此也进行了大量研究。本文综述了硼颗粒表面包覆作用机理、包覆工艺流程及包覆效果表征3个方面的研究进展,为提升硼颗粒的实际应用效果提供借鉴。
1 包覆作用机理
在硼颗粒表面包覆材料的选择上,为提升硼颗粒燃烧效果及其与推进剂的相容性[10],目前的研究中涵盖了氧化剂、金属、黏合剂等众多类型的材料。在促进硼颗粒及相关硼基推进剂性能上,各种包覆材料的作用机理主要分为以下几个方面。
1.1 硼颗粒表面除膜
与硼相比,硼颗粒表面形成的B2O3氧化膜具有熔点低(460℃)、沸点高(1860℃)[11]的特性,在燃烧过程中呈液膜态,阻碍内部硼颗粒的进一步燃烧,导致硼颗粒的燃烧效率较低[12]。利用包覆材料与氧化膜的化学反应可实现硼颗粒表面氧化膜的去除[13],从而改善硼颗粒的点火和燃烧性能。该类包覆材料主要包括氟树脂 (Viton A)、LiF、聚偏氟乙烯(PVDF)和三羟甲基丙烷 (TMP)等。
LiF的除膜作用主要通过反应(1)实现[14]。LiF与B2O3发生化学反应,生成气态产物,从而实现硼颗粒表面氧化膜的去除。
(1)
Viton A、PVDF通过分解产生HF并与B2O3发生反应(2)、(3),以达到除膜的目的[15]。
(2)
(3)
TMP作为一种常用交联剂和扩链剂[16],能与硼颗粒表面B2O3发生反应(4),从而促进硼颗粒的点火燃烧。
(4)
Liu等[17]用激光实验方法研究了Viton A、LiF包覆硼颗粒对B/MA/AP/HTPB含硼推进剂燃烧性能的影响。结果表明,用LiF包覆硼颗粒能缩短含硼推进剂的点火延迟时间,而用Viton A包覆则会延长含硼推进剂的点火延迟时间。
Hidetsugu等[18]研究了Viton A包覆对B/KNO3混合物燃烧的影响。结果表明,Viton A包覆层能防止硼颗粒表面吸水,同时降低混合物的撞击感度与点火温度。
陈涛等[19]研究了LiF包覆对推进剂一次、二次燃烧过程中能量释放特性的影响。结果表明,高温(高于1353℃)下,LiF通过吸热反应消耗了硼颗粒表面的B2O3氧化膜,加速B/O反应,使得LiF包覆的硼颗粒在599℃发生快速氧化反应。硼的燃烧效率从65.48%提高到81.57%,其对应推进剂一次能量释放效率和二次能量释放效率得到明显提高。
此外,在生产硼颗粒过程中,用B4C包覆硼颗粒能有效避免新生硼颗粒表面生成氧化膜,并防止硼颗粒的凝聚[20]。
1.2 提高燃烧温度
研究表明[21],通过在硼颗粒表面包覆一些分解放热明显的材料,可有效提高硼颗粒周围温度,有利于B2O3氧化膜的气化蒸发,从而促进硼颗粒的燃烧。常用的此类包覆材料包括氧化剂和叠氮化物两大类,如AP、高氯酸钾(KP)、聚叠氮缩水甘油醚(GAP)、叠氮化钠(NaN3)等。
李疏芬等[22]研究了AP、KP包覆层对硼基推进剂燃烧的影响。结果表明,使用AP、KP对硼颗粒表面进行包覆有利于提高其反应活性,使推进剂的燃面及火焰温度提高200℃以上,硼颗粒的转化率和燃烧效率得到明显提高;此外,氧化剂分解得到的新生态氧[O]吸附积累在硼颗粒表面,还可以提高硼颗粒表面的氧含量,对新生态[O]的渗透扩散有利。
Shyu等[23]研究了GAP包覆硼颗粒及其对含硼推进剂燃烧性能的影响。对比了GAP包覆硼颗粒与纯硼颗粒的点火燃烧性能。结果表明,GAP包覆能缩短硼颗粒的点火延迟时间,但在低氧浓度下该效应不明显。GAP包覆硼颗粒组成的硼基推进剂燃速更快,燃烧更剧烈和完全。
利用NaN3在400℃以上剧烈分解所释放的热量加热硼颗粒也可以达到提高燃烧温度的效果[14]。此外,其分解产物Na3N可进一步在O2中燃烧产生Na2O,该物质能与B2O3作用,降低硼颗粒表面的黏稠性,有利于O2向硼内部扩散。但由于 NaN3不含氧,不会产生新生态的[O]。研究表明,用其包覆硼颗粒没有用氧化剂包覆的燃烧剧烈。
1.3 降低硼的点火温度
硼的点火温度较高,点火困难。在硼颗粒表面包覆可燃金属,可有效防止硼颗粒表面生成低温氧化层。此外,部分金属能与硼反应生成燃点较低的金属硼化物,可以降低硼的燃点,促进硼的点火和燃烧[24]。目前,用于硼颗粒表面包覆研究的金属主要包括Ti、Zr、Mg等。
美国航空化学研究试验公司[25-26]对硼颗粒表面包覆进行了深入的研究。他们分别利用金属Ti和金属Zr对硼颗粒进行表面包覆。结果表明,在温度为2200K,压力400kPa条件下,当Ti的涂层占整个硼颗粒总质量的9%~17%时,直径为2μm的包覆硼颗粒在0.09ms内点燃,比未经包覆的硼要快得多。金属Zr包覆硼颗粒也能改善硼的点火燃烧性能,但所消耗的金属量较多,为硼总质量分数的20%~30%。
Pace等[27]研究了金属镁包覆层对硼颗粒点火燃烧性能的影响。镁除通过氧化放热提高硼颗粒表面温度外,还可与硼反应生成MgB2,从而降低点火温度。结果表明,当金属镁包覆度不超过30%时,随着金属镁含量的提高,硼颗粒的燃速增加。镁包覆层对燃速的提高在压强低于0.55MPa情况下较为明显,当压强高于0.55MPa时,影响则较弱。当压强为0.35MPa时,含镁包覆硼颗粒(B/Mg/HTPB质量比为8∶2∶90)的推进剂燃速为含未包覆硼颗粒(B/Mg/HTPB质量比10∶0∶90)的推进剂的1.25倍。
1.4 提高表面相容性
研究表明[28],在含硼推进剂中,硼颗粒表面存在的杂质(B2O3、H3BO3等)会与HTPB的羟基反应生成硼酸酯,从而引起凝胶化反应,导致硼颗粒与黏合剂不相容,严重影响了硼的工艺性能。为了解决该问题,可在硼颗粒表面包覆相容性较强的材料,从而改善硼颗粒表面的相容性。
张教强等[29]研究了HTPB对硼颗粒的表面包覆。结果表明,利用HTPB与硼颗粒表面酸性物质反应,能在硼颗粒表面形成均匀包覆层,有利于提高硼颗粒表面的规整性,并有效避免制药过程中硼颗粒表面杂质与黏合剂的反应,有利于改善硼基推进剂的制备工艺。
异氰酸酯类固化剂与硼颗粒表面的硼酸(H3BO3)会发生反应(见式(5)),对固化体系产生干扰。赵孝彬等[13]用异氰酸酯类固化剂对硼颗粒表面进行包覆,以消除硼与固化体系之间的副反应。发现以TDI对硼颗粒进行表面包覆处理后,能在GAP/AN体系中获得致密无孔的药柱。
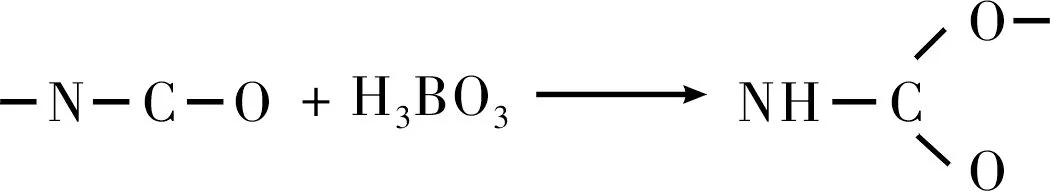
(5)
TMP除了前述的除膜作用外,还能与硼颗粒表面的H3BO3发生反应(见式(6)),从而有利于解决硼颗粒与推进剂体系的不相容问题,减弱硼颗粒的吸湿性[13]。
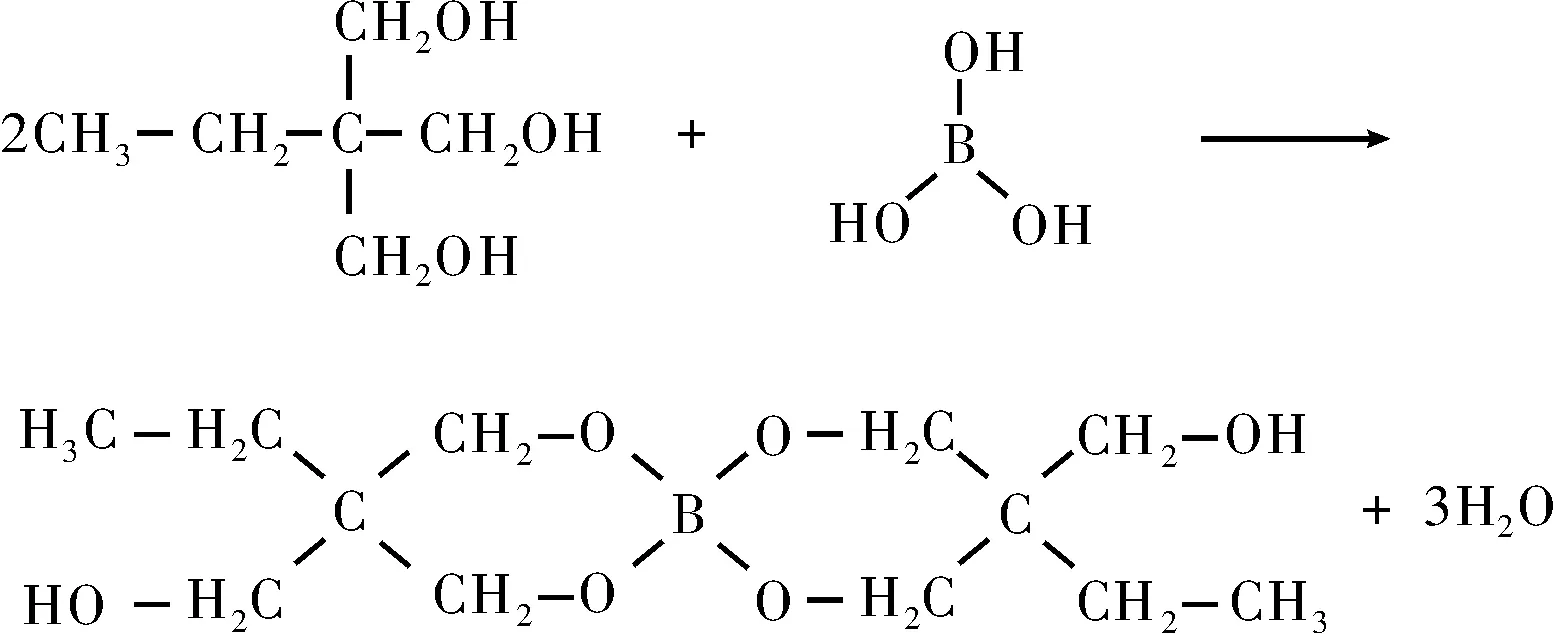
(6)
从上述研究来看,为解决硼颗粒表面杂质所导致的与推进剂体系不相容的问题,大多采用推进剂体系成分对硼颗粒进行包覆,除去表面杂质,从而改变硼颗粒的表面特性,为后续推进剂的制备工艺奠定良好的基础。
1.5 催化硼颗粒氧化反应
催化剂能降低化学反应所需的活化能,促进反应的进行。使用适当的催化剂对硼颗粒进行包覆,是促进硼颗粒氧化的可行途径。
Xi等[30]利用CO2激光点火燃烧试验系统(图 1)研究了7种金属氧化物作为催化剂对硼颗粒点火燃烧特性的影响。结果发现,Bi2O3的催化效果最佳,能使硼颗粒的点火温度降低约15.2%,并缩短硼颗粒的点火燃烧时间。其催化原理如式(7)所示:颗粒氧化过程中,Bi2O3向B-B2O3表面扩散,与B发生反应,促进硼颗粒的氧化。之后,Bi向颗粒表面扩散,被空气中的氧气重新氧化为Bi2O3。
(7)

图1 CO2激光点火燃烧试验系统Fig.1 CO2 laser ignition and combustion test system
Dreizin等[31]提出一种硼点火模型并进行了实验验证。他们提出,在硼颗粒点火过程中,氧在B2O3氧化层中的溶解度不断上升,当其达到溶解极限时,硼颗粒进入燃烧阶段。实验中所观察到的点火延迟时间与氧达到B-O溶解饱和所需时间相等。因此,提高点火阶段硼颗粒的摄氧量可有效缩短点火延迟时间。
根据该模型,目前研究中通过在硼颗粒表面包覆稀土催化剂CeO2,可携带氧气并提高硼颗粒在点火阶段的摄氧量,能够促进硼颗粒的点火,并可在一定程度上催化硼颗粒周围碳氢化合物的反应,从而帮助硼颗粒的燃烧。CeO2的催化作用主要来源于其+3与+4两个价态之间的转化,该转化在低于硼颗粒点火温度下就能完成。进一步研究表明,与其他催化剂相比,Ce表面的高活性氧具有更高的迁移率,故在还原环境下CeO2能转化为CeO2-x(0 ≤x≤ 0.5)[32-33]。
Karmakar等[34]研究了稀土元素催化剂对硼颗粒点火特性的影响。实验中选取CeO2及REOm-41(包含CeO2、La2O3和Gd2O3,其中Ce与La摩尔比为3∶1,Ce与Gd摩尔比为80∶1)纳米颗粒作为包覆材料制备包覆硼颗粒。结果发现,当稀土包覆层质量分数为20%时,能有效缩短硼颗粒的点火延迟时间,当质量分数大于20%时,则会限制硼颗粒中氧的扩散,反而不利于硼颗粒的点火燃烧。从上述研究可以发现,CeO2作为催化剂负载在硼颗粒上,能有效促进硼颗粒的点火燃烧。
为了提升硼颗粒的不同性能及属性,以上研究中选取的包覆材料,涵盖氧化剂、黏合剂、高热值金属、叠氮化物、催化剂等多种类型,其在提高硼颗粒点火燃烧特性与相容性上发挥了不同的作用,主要分为以下几个方面:(1)能通过化学反应去除硼颗粒表面氧化膜;(2)分解会放出大量的热,有利于提高燃烧温度,加快B2O3的气化;(3)反应活性较高,有利于降低硼的点火温度;(4)改变硼颗粒表面性能,提高其与推进剂的相容性;(5)能有效加快硼颗粒的反应速率。
从目前的研究来看,对硼颗粒的包覆材料类型众多,大多是针对硼颗粒单一特性尤其是燃烧特性方面的提升。为实现从多方面提升硼颗粒的性能,提出了高能黏合剂GAP等包覆材料类型。而以具有不同效果的多种材料共同包覆硼颗粒,以提升其综合性能的思路则由于包覆剂比例不宜过大的限制未有相应的实践。随着包覆手段与包覆工艺的发展,可实现更小的包覆层厚度,从而使得多种材料共同包覆成为未来硼颗粒包覆中可行的发展方向。此外,基于研究手段所限,目前大部分对包覆硼颗粒及其推进剂点火燃烧性能的测试条件与实际应用条件仍存在一定的距离,影响了对实际应用的指导作用。
2 包覆工艺
针对不同的硼颗粒表面包覆材料,其所适宜的包覆条件与包覆方法有所不同。为此,国内外学者根据实际应用需求,对包覆工艺进行研究和改进,以获得包覆更为均匀的硼颗粒。
2.1 沉积法(重结晶法)
沉积法是硼颗粒表面包覆中常用的工艺,又称为重结晶法。实验中,把硼粉与包覆剂按一定比例加入溶剂中(常用溶剂有二氯甲烷、二甲基亚砜、四氯化碳等,具体根据包覆剂选择),在一定温度下充分搅拌,使硼颗粒的表面与包覆剂充分接触,蒸发溶剂即可得到包覆的硼颗粒。沉积法的实施中,溶剂的种类、蒸发速率、包覆量等因素对包覆效果都有着明显的影响,国内外针对各影响因素进行了相关的研究,并对沉积法进行了相应的改进。张教强等[35]利用沉积法在硼颗粒表面包覆AP的过程中发现,采用甲醇或丙酮作为溶剂,硼粉干燥后不易板结,操作较为简易。合适的蒸发速率有利于包覆剂完整、均匀地沉积在硼颗粒表面(溶剂的最佳蒸发速率为10 g/h)。此外,在包覆前先对硼颗粒进行硅烷偶联剂预处理有利于实现更均匀的包覆,表面直接反应16 h以上,可达到最佳的包覆效果。
席剑飞等[36-37]针对沉积法可能导致的包覆不均匀问题进一步提出了改进的双溶剂法。选择两种合适的溶剂(如溶剂1和溶剂2),应满足:(1)包覆材料能溶于溶剂1但不溶于溶剂2;(2)溶剂1可溶于溶剂2;(3)溶剂1的沸点小于溶剂2。通过实验研究给出了常用包覆剂及对应的溶剂,结果如表1所示[36]。首先,用溶剂1来溶解包覆剂制成溶液,然后将溶液分散于溶剂2中,蒸发溶剂过程中,溶剂1由于沸点较低先蒸出,随后溶剂2蒸出,获得包覆硼颗粒。双溶剂的引入使硼粉在整个包覆过程中都可以悬浮分散在溶剂中,从而令包覆更均匀。
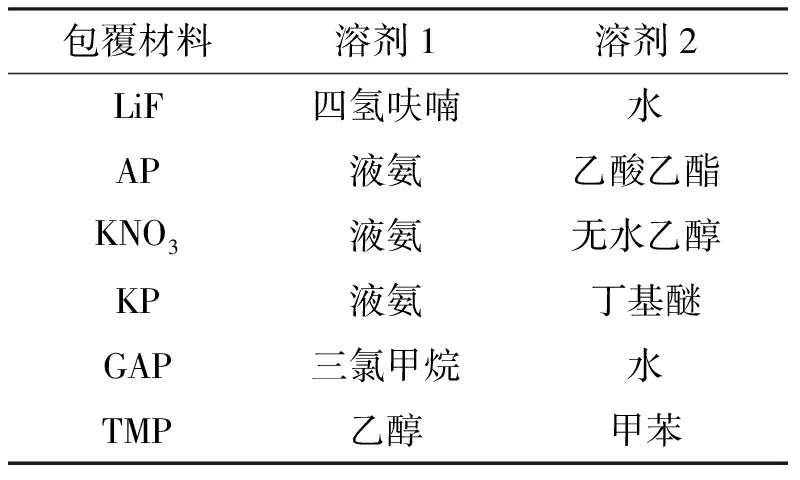
表1 硼颗粒双溶剂包覆法中常用材料对应溶剂
2.2 表面反应包覆法
对于一些难以溶解的盐类,直接利用沉积法进行包覆存在一定的困难,获得的包覆颗粒均匀度不佳。为此,可通过化学反应在硼颗粒表面直接生成包覆剂并附着在硼颗粒表面,从而实现包覆。
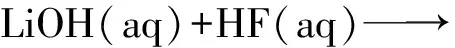
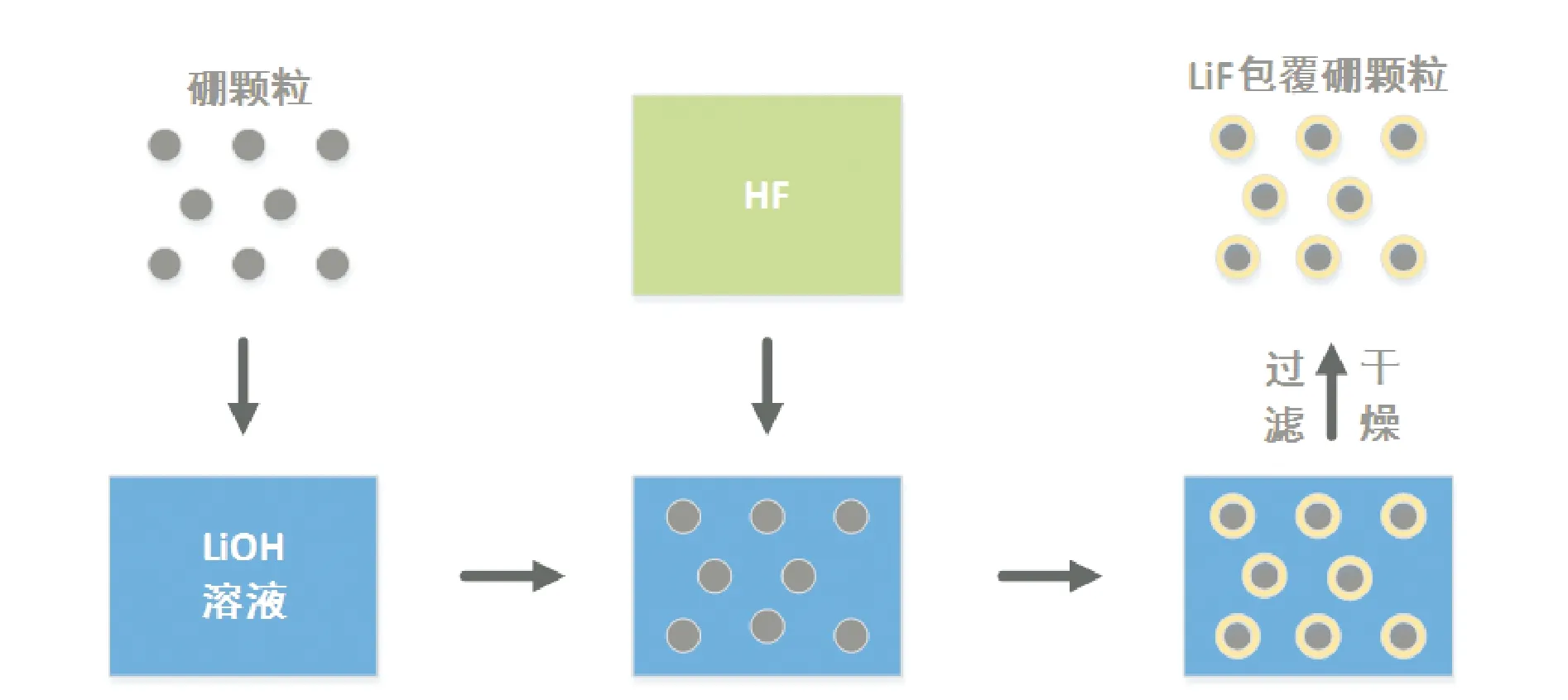
图2 LiF包覆硼颗粒工艺流程Fig.2 Process flow of coating boron particles with LiF
庞维强等[39]在用LiF对硼颗粒进行表面包覆过程中发现,搅拌速率会影响基体在改性体系中分散的均匀性,当搅拌速率为750 r/min左右时,包覆效果最好。
张教强等[40]研究了LiOH浓度对超细硼粉包覆效果的影响。结果表明,LiOH浓度对包覆层的致密性有一定的影响,LiOH浓度越高,超细硼粉的包覆层越致密,包覆效果越佳。
该方法在操作上较为简单,与沉积法类似,但其可通过化学反应实现难溶盐的表面包覆,针对性较强。
2.3 高分子吸附聚合法
对于HTPB、聚对苯二酸丁二酯(PBT)等高分子包覆材料,其对硼颗粒的包覆存在着有机材料与无机材料的相容性问题,是包覆工艺中所需要重点关注的问题。目前,常用的高分子包覆方法主要分为两种:高分子在表面直接吸附和单体在颗粒表面聚合。其中,高分子直接吸附法即直接把硼颗粒作为吸附核心分散到溶有高分子的有机溶液中,蒸发溶剂后可得到包覆高分子的硼颗粒。而单体表面聚合法则是使聚合反应发生在硼颗粒表面从而实现包覆。
张教强等[29]采用表面聚合及直接吸附法研究了HTPB对硼颗粒的表面包覆。其中,表面聚合包覆过程中所采用的条件为:以苯为溶剂,于80℃经14h的酯化反应后,以甲苯二异氰酸酯(TDI)为固化剂,于70~80℃进行固化处理。对比发现,在表面直接吸附包覆过程中,包覆量不易控制,常导致硼颗粒结块,不利于固化反应的进行;而采用表面聚合包覆法时,过滤后硼颗粒分散较好,有利于后期的固化反应。故表面聚合包覆法更有利于HTPB在硼颗粒表面的包覆。
张琼方[38]研究了不同因素对PBT包覆硼颗粒的影响。结果发现,最佳的包覆条件为以四氢吠喃为溶剂,在包覆前先对硼粉进行硅烷偶联剂的预处理,然后采用表面直接反应的方法,反应进行16h以上,固化6~8h,真空干燥。
高分子吸附聚合法可通过控制包覆物质的用量、包覆时间等较好地控制包覆效果,但核层颗粒与包覆层须具有较好的相容性。常需加入提高相容性的介质,如具有某些功能基团的高分子,来提高包覆质量[41]。
2.4 气相包覆法
气相法是直接利用气体或通过各种手段将壳层物质转变成气体,使之在气态下发生物理或化学变化而实现颗粒表面包覆的方法[42]。
W. Felder等[43]用连续扩散流法制备镁或铝包覆的硼颗粒。制备过程中,硼颗粒的气溶胶以共轴环形路径被注入到一个金属蒸气-Ar的混合气流中,混合后金属蒸气沉积在硼颗粒表面,从而完成包覆。操作条件由所需包覆层的厚度、硼颗粒的密度及包覆金属蒸气的均相成核间的平衡来决定。
美国航空化学研究试验公司[25-26]提出了一种金属钛包覆硼颗粒的方法,其原理是基于钠与四氯化钛和三氯化硼的放热反应(式(7)和式(8))。
(7)
(8)
包覆过程中,将过量的Na和BCl3加入到反应器(图3)中,并进行充分的搅拌混合,反应(7)生成的硼颗粒悬浮在NaCl气体和过量的Na气体中。这些生成物通过超音速喷管发生膨胀,在喷管处压入TiCl4气体,过量的Na与TiCl4反应生成Ti气体(反应(8)),气态的Ti遇到硼颗粒后冷凝并包覆在硼颗粒表面。
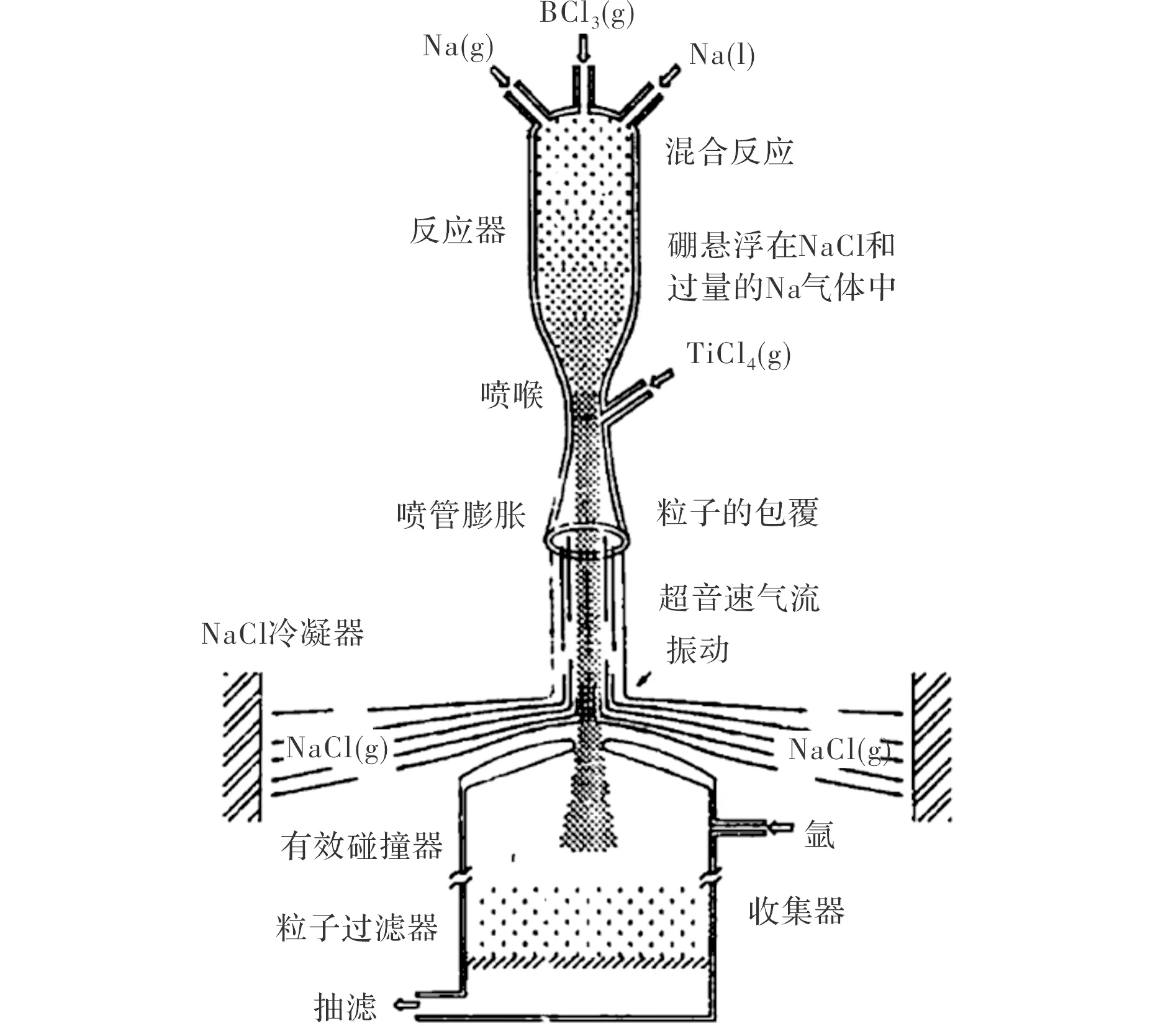
图3 金属Ti包覆硼颗粒的反应器[25-26]Fig 3 Reactor for coating boron particle production[25-26]
该法所获得的包覆硼颗粒中混有NaCl气体,可通过图4中所示的超音速有效碰撞收集器去除,获得纯净的包覆硼颗粒。
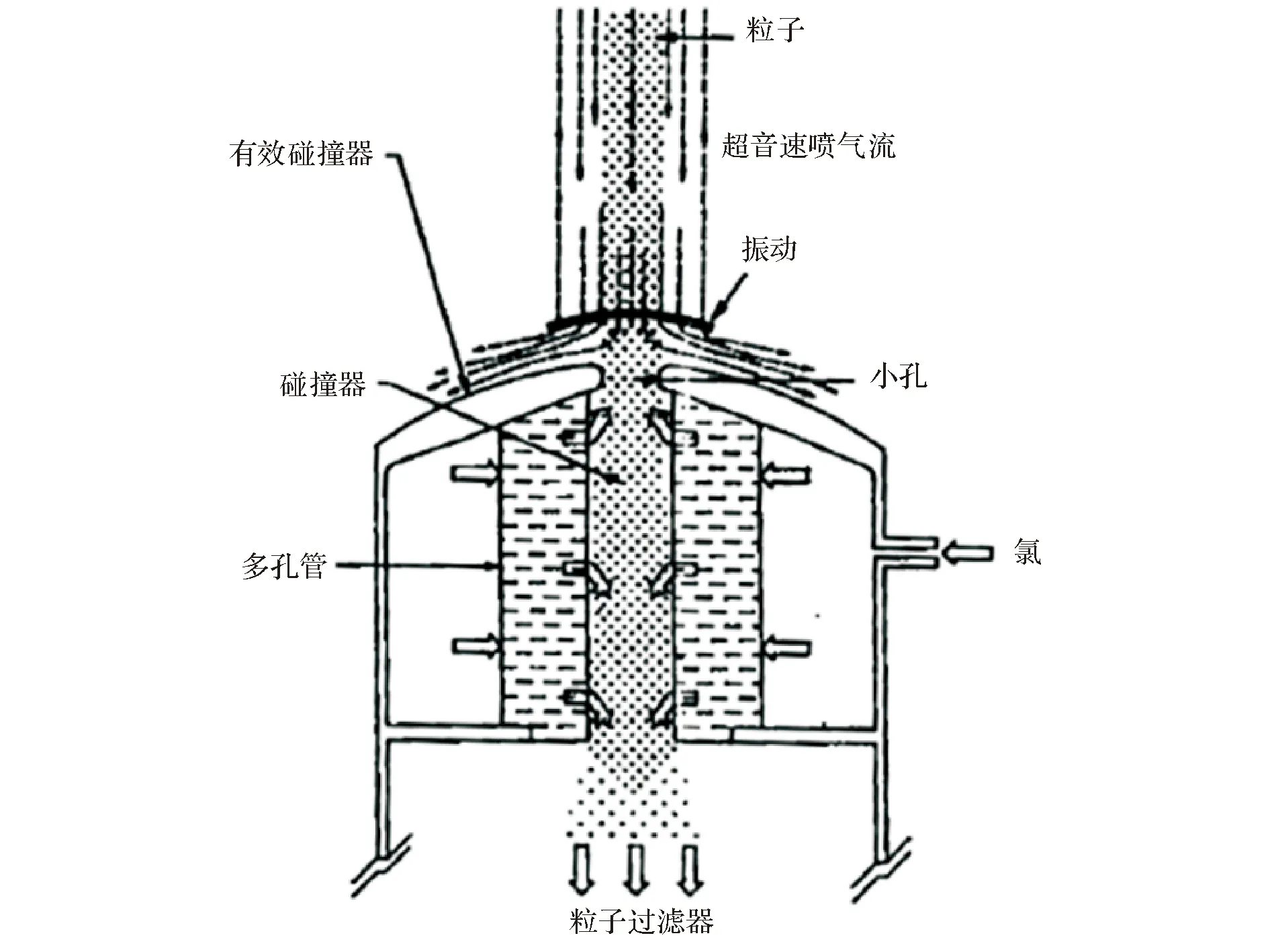
图4 超音速有效碰撞收集器简图[25-26] Fig 4 Diagrammatic sketch of supersonic effective collision trap[25-26]
气相包覆法所获得的包覆硼颗粒纯度高,收集简单、效率高,但对设备要求高、投入大。
2.5 机械球磨法
Van Devener等[44]利用球磨法制备表面包覆CeO2催化剂的纳米级硼颗粒,首次获得未氧化的、稳定的、燃料相容性高的纳米级包覆硼颗粒,该法也可用于B4C的包覆。实验中,首先将2g硼颗粒、0.1g CeO2用160g研磨球研磨30min,使两种材料充分接触反应,块状CeO2附着在硼颗粒表面。然后在N2气氛下打开研磨器,加入15mL己烷和1mL十八烯酸,湿法研磨2.5h。此法所获得的包覆硼颗粒表面性能佳,在碳氢化合物中的分散性较好,有利于其在推进剂体系中的使用,但由于结合力不强,包覆效果不如其他方法。
综合以上包覆工艺,由于不同的工艺特点,所适用的范围也不同。其中,沉淀法可精确控制各组分的含量,且设备操作较为简单,有利于规模化生产,目前在颗粒包覆方面使用较为广泛,其可能产生的包覆不均匀问题也随着工艺的发展得到了相应的改善,是一种效果良好、成本较低的工艺手段。
目前,包覆方法研究中对包覆过程中的参数控制研究较为深入,而对包覆机理的认识则尚待加强。此外,基于工艺简单化的考虑,在实际过程中通常采用单一的包覆方法,要实现硼颗粒多层包覆存在一定的困难。在未来的发展中,应深入了解包覆工艺的包覆机理,分析不同包覆手段之间的兼容性,为实现硼颗粒的多层包覆、提升硼颗粒实际应用效果奠定基础。
3 包覆效果表征
不同的包覆工艺及操作流程等对包覆效果有着明显的影响,包括包覆材料在硼颗粒表面分布的均匀性、包覆层厚度等。目前,随着实验仪器的不断发展,对包覆效果的评估方法也变得越来越多样化。
Liu等[17]采用场发射扫描电镜(SEM)对包覆硼颗粒表面进行观测,并利用能谱分析(EDS)对表面元素进行分析。结果发现,经LiF包覆后金属颗粒燃烧后团聚现象大大减少。张教强等[35]采用傅里叶红外光谱(FT-IR)、原子力显微镜(AFM)等手段分析了AP包覆硼颗粒效果。发现包覆后硼颗粒表面规整,而且外表面存在较明显的包覆层。
敖文等[45]采用X射线衍射仪(XRD)、马尔文、氮吸附仪、ICP光谱仪、透射电镜(TEM)等对硼颗粒物相、颗粒分布、比表面积、表面元素、微观形态等情况进行分析,获取了硼颗粒的大量特性参数。
李疏芬等[46]利用红外光谱(IR)、核磁共振(NMR)、XRD及X光电子能谱仪(XPS)等对硼颗粒表面包覆层进行检测,并利用酸度计与黏度计测量了包覆硼颗粒的表面酸度及包覆硼颗粒加入HTPB体系的黏度变化。
目前,有关硼颗粒表面包覆情况的表征方法多样,所采用的仪器种类繁多。总的来看,相关表征手段可分为直接表征与间接表征两类,如表 2所示。

表2 包覆硼颗粒的表征手段
采用直接表征手段,可通过对直观图像的观察分析,获取硼颗粒包覆后的表面形貌,并通过对比包覆前后的图像,对包覆表面的光滑度、均匀性、致密性等进行分析。目前,直接表征手段主要采用场发射扫描电镜(SEM)、透射电镜(TEM)、原子力显微镜(AFM)等微观显微镜观察手段进行。
采用间接表征手段,则通过对颗粒固有性质变化情况的检测,获取硼颗粒包覆后的粒径变化、元素分布、物相变化、孔隙结构等性质的变化,从而对包覆材料的分布均匀程度、分子结合情况等进行分析,结合直接表征手段对包覆效果进行评估。
4 结 语
综述了硼颗粒表面包覆作用机理、包覆工艺及包覆效果表征方面的研究进展。从促进硼颗粒燃烧效果角度出发,提出了去除硼颗粒表面氧化膜、提高燃烧温度、降低硼的点火温度、提高表面相容性、催化硼颗粒氧化反应等5个方面作为硼颗粒表面包覆材料的选取方向。由此针对不同包覆材料的作用机理,选取合适的包覆工艺与操作条件,实现包覆材料在硼颗粒表面的均匀分布等展开全面的评述,并分析了现代测试技术对硼颗粒包覆效果的表征方法及可行性,从而指导包覆工作的进行。
目前,在硼颗粒包覆方面,国内外研究所涉及的材料类型较为广泛,取得了较好的成果。在包覆剂的选择上,不同包覆材料对硼颗粒的点火燃烧有着不同的促进作用。为提升硼颗粒在含硼推进剂中的实用效果,建议:
(1)在未来的研究中应综合多种促进方式,通过寻找兼具多种促进效果的包覆材料、探究多层包覆工艺等方式实现硼颗粒的包覆改良;
(2)在包覆工艺方面,应根据包覆材料类型、工艺流程的可操作性、投资成本和实际应用效果等进行综合考虑;
(3)此外,硼颗粒的表面包覆最终是为提升硼颗粒在含硼推进剂中的实用效果,应更多地关注硼颗粒在不同推进剂成分影响下的点火燃烧效果,以实际效果为依据,改良包覆配方及工艺,不断提升包覆效果,解决硼颗粒在固体推进剂应用中存在的问题。
[1] Liu D, Xia Z, Huang L, et al. Boron particle combustion in solid rocket ramjets[J]. Journal of Aerospace Engineering, 2014, 28(4):04014112.
[2] Young G, Roberts C W, Stoltz C A. Ignition and combustion enhancement of boron with polytetrafluoroethylene[J]. Journal of Propulsion & Power, 2015, 31(1):386-392.
[3] Liang D, Liu J, Xiao J, et al. Energy release properties of amorphous boron and boron-based propellant primary combustion products[J]. Acta Astronautica, 2015, 112: 182-191.
[4] Hussmann B, Pfitzner M. Extended combustion model for single boron particles-Part I: theory[J]. Combustion and Flame, 2010, 157(4): 803-821.
[5] Rosenband V, Natan B, Gany A. Ignition of boron particles coated by a thin Titanium film[J]. Journal of Propulsion and Power, 1995, 11(6): 1125-1131.
[6] Liu T K, Shyu I M, Hsia Y S. Effect of fluorinated graphite on combustion of boron and boron-based fuel-rich propellants[J]. Journal of Propulsion and Power, 1996, 12(1): 26-33.
[7] 谢中元, 周霖, 王浩, 等. 高氯酸铵包覆层对硼粉燃烧性能的影响[J]. 兵工学报, 2014, 35(2): 194-199.
XIE Zhong-yuan, ZHOU Lin, WANG Hao, et al. Combustion performance of boron coated with AP[J]. Acta Armamentarii, 2014, 35(2): 194-199.
[8] 席剑飞. 硼颗粒点火燃烧促进方法研究[D]. 杭州:浙江大学, 2015.
XI Jian-fei. Research of boron particle ignition combustion promoting method[D].Hangzhou: Zhejiang University, 2015.
[9] 陈冰虹, 刘建忠. 氧化剂包覆硼颗粒对硼基推进剂点火燃烧特性的影响[J].含能材料, 2016,24(8):774-780.
CHEN Bing-hong, LIU Jian-zhong. Effect of oxidant coating boron particle on the ignition and combustion characteristics of boron-based propellant[J]. Chinese Journal of Energetic Materials, 2016,24(8):774-780.
[10] Trowbridge J C, Breazeal J D. Coating of boron particles: US, 4915753[P]. 1990-4-10.
[11] 周俊虎,刘建忠. 硼的点火与燃烧[M]. 北京:科学出版社, 2015.
ZHOU Jun-hu, LIU Jian-zhong. Ignition and Combustion of Boron[M]. Beijing: Science Press, 2015.
[12] Fang Chuan-bo. Study of ignition process of boron particle agglomeration[J]. Acta Aeronautica Et Astronautica Sinica, 2012.
[13] 赵孝彬, 张小平. 硼粒子包覆工艺及对硼的表面和燃烧特性的影响[J]. 固体火箭技术, 1998, 21(1): 35-38.
ZHAO Xiao-bin, ZHANG Xiao-ping. Process of coating boron particles and effect on characteristics of surface and combustion[J]. Journal of Solid Rocket Technology, 1998, 21(1): 35-38.
[14] 张琼方, 张教强. 硼粒子表面包覆的研究进展[J]. 含能材料, 2004(5): 314-317.
ZHANG Qiong-fang, ZHANG Jiao-qiang. Research on surface coating of boron particles[J]. Chinese Journal of Energetic Materials, 2004(5): 314-317.
[15] 李疏芬.含硼推进剂燃烧性能改善[J]. 固体火箭技术,1995,8(2): 39-43.
LI Shu-fen. Improvement of combustion performance of boron-based solid propellants[J]. Journal of Solid Rocket Technology, 1995,8(2): 39-43.
[16] 庞维强, 樊学忠, 胥会祥, 等. 含团聚硼富燃料推进剂表-界面性能研究[J]. 固体火箭技术, 2013, 36(4):521-525.
PANG Wei-qiang, FAN Xue-zhong, XU Hui-xiang, et al. Surface and interfacial properties of fuel rich propellant with agglomerated boron particles[J]. Journal of Solid Rocket Technology, 2013, 36(4):521-525.
[17] Liu T K, Luh S P, Perng H C. Effect of boron particle surface coating on combustion of solid propellants for ducted rockets[J]. Propellants, Explosives, Pyrotechnics, 1991, 16(4): 156-166.
[18] Hidetsugu N, Osamu N, Miyako A. Effect of coating on the reactivity of boron powder[J]. Kayaku Gakkaishi, 2001, 62(1): 8-15.
[19] 陈涛, 张先瑞, 王园园, 等. LiF包覆对硼粉热氧化特性的影响[J]. 含能材料, 2013, 21(1): 57-60.
CHEN Tao, ZHANG Xian-rui, WANG Yuan-yuan, et al. Effect of LiF coating on the thermal oxidation characteristics for boron powder[J]. Chinese Journal of Energetic Materials, 2013, 21(1): 57-60.
[20] LI Chen-fang. Studies to increase the combustion efficiency of boron fuel[J]. Journal of Propulsion Technology, 1994: 653-661.
[21] Li S C, Williams F A. Ignition and combustion of boron in wet and dry atmospheres[J]. Symposium on Combustion, 1991, 23(1):1147-1154.
[22] 李疏芬, 金荣超. 提高含硼固体燃料燃烧性能的研究[J]. 推进技术, 1997, 18(5): 100-105.
LI Shu-fen, JIN Rong-chao. The studes of improving the combustion performance of fuel rich propellant containing boron[J]. Journal of Propulsion Technology, 1997, 18(5): 100-105.
[23] Shyu M, Liu T K. Combustion characteristics of GAP-coated boron particles and the fuel-rich solid propellant[J]. Combustion and Flame, 1995, 100(4): 634-644.
[24] King M K. A review of studies of boron ignition and combustion phenomena at Atlantic Research Corporation over the past decade[J]. International Journal of Energetic Materials and Chemical Propulsion,1991,2(1-6):1-80.
[25] Calcote H F, Gill R J, Berman C H, et al. Production and coating of pure boron powders[R].Princeton NJ:Aerochem Research Labs Inc, 1990.
[26] King M K, Komar J. Fuel-Solid propellant boron combustion[R].Alecandria VA:Atlantic Research Corp, 1986.
[27] Pace K K, Jarymowycz T A, Yang V. Effect of magnesium-coated boron particles on burning characteristics of solid fuels in high-speed crossflows[J]. International Journal of Energetic Materials and Chemical Propulsion, 1991,2(1-6):332-347.
[28] 郑剑,汪爱华,庞爱民. 含硼HTPB富燃料推进剂工艺恶化机理研究[J]. 推进技术,2003,24(3):282-284,288.
ZHENG Jian, WANG Ai-hua, PANG Ai-min. Mechanism of the deteriorated processability in boron-fuel-rich HTPB propellants[J]. Journal of Propulsion Technology, 2003, 24(3): 282-284,288.
[29] 张教强, 庞维强, 苏力宏, 等. 超细硼粉的 HTPB 包覆[J]. 化工进展, 2008, 26(11): 1641-1644.
ZHANG Jiao-qiang, PANG Wei-qiang, SU Li-hong, et al. Research on the coating of superfine boron particles with HTPB[J]. Chemical Industry & Engineering Progress, 2008, 26(11):1641-1644.
[30] Xi J, Liu J, Wang Y, et al. Metal oxides as catalysts for boron oxidation[J]. Journal of Propulsion and Power, 2013, 30(1): 47-53.
[31] Dreizin E L, Calcote H F. A new mechanism of boron ignition: through the formation of a saturated BO solution[J]. Chemical and Physical Processes in Combustion, 1995: 333-336.
[32] Perrichon V, Laachir A, Bergeret G, et al. Reduction of cerias with different textures by hydrogen and their reoxidation by oxygen[J]. Journal of the Chemical Society, Faraday Transactions, 1994, 90(5): 773-781.
[33] Ricken M, Nölting J, Riess I. Specific heat and phase diagram of nonstoichiometric ceria (CeO2-x)[J]. Journal of Solid State Chemistry, 1984, 54(1): 89-99.
[34] Karmakar S, Wang N, Acharya S, et al. Effects of rare-earth oxide catalysts on the ignition and combustion characteristics of boron nanoparticles[J]. Combustion and Flame, 2013, 160(12): 3004-3014.
[35] 张教强, 庞维强, 张琼方, 等. AP 包覆超细硼粉的改进方法[J]. 含能材料, 2007, 15(4): 382-386.
ZHANG Jiao-qiang, PANG Wei-qiang, ZHANG Qiong-fang, et al. Improvement for AP coating superfine boron powder[J]. Chinese Journal of Energetic Materials, 2007, 15(4):382-386.
[36] 席剑飞, 刘建忠, 胡友瑞, 等. 双溶剂法硼包覆工艺及其工艺参数研究[J]. 推进技术, 2013, 43(7): 984-990.
XI Jian-fei, LIU Jian-zhong, HU You-rui, et al. A study for coating boron particles with two-solvent method and its process parameters[J]. Journal of Propulsion Technology, 2013, 34(7): 984-990.
[37] 刘建忠, 周俊虎, 张彦威, 等. 一种高效的硼粒子包覆方法: CN,103044175A[P]. 2013.
LIU Jian-zhong, ZHOU Jun-hu, ZHANG Yan-wei, et al. A effective method of coating boron particles: CN,103044175A[P].2013.
[38] 张琼方. 超细硼粉的表面包覆研究[D]. 西安:西北工业大学, 2005.
ZHANG Qiong-fang. Surface coating of superfine boron powder[D]. Xi′an:Northwestern Polytechnical University, 2005.
[39] 庞维强, 张教强, 张琼方, 等. 硼粉的包覆及含包覆硼推进剂燃烧残渣成分分析[J]. 固体火箭技术, 2006, 29(3): 204-207.
PANG Wei-qiang,ZHANG Jiao-qiang,ZHANG Qiong-fang,et al.Coating of boron particles and combustion residue analysis of boron-based solid propellants[J]. Journal of Solid Rocket Technology, 2006, 29(3): 204-207.
[40] 张教强,张琼方,国际英,等. 超细硼粉的氟化锂包覆[J]. 火炸药学报, 2005, 28(3): 8-11.
ZHANG Jiao-qiang, ZHANG Qiong-fang, GUO Ji-ying, et al. Surface coating of superfine boron particles with lithium fluoride[J]. Chinese Journal of Explosives & Propellants(Huozhayao Xuebao), 2005,28(3): 8-11.
[41] 程花蕾, 杜红亮, 周万城,等. 纳米颗粒包覆方法的研究进展及其应用[J]. 材料导报, 2012,26(15):130-136.
CHENG Hua-lei, DU Hong-liang, ZHOU Wang-cheng, et al. Research progress and application on the coating method of nano-powders[J]. Materials Review, 2012,26(15):130-136.
[42] 肖勇, 吴孟强, 袁颖,等. 无机微/纳米粒子表面包覆改性技术[J]. 电子元件与材料, 2011, 30(9):66-70.
XIAO Yong, WU Meng-qiang, YUAN Ying, et al. Research on the surface coating technologies of inorganic micro/nano-particles[J]. Electronic Components & Materials, 2011, 30(9):66-70.
[43] Felder W, Gill R J, Baker D, et al. Coated boron particles by a diffusion flow method[C]∥Chemical and Physical Processes in Combustion. 1990:70/1-10/4.
[44] Van Devener B, Perez J P L, Jankovich J, et al. Oxide-free, catalyst-coated, fuel-soluble, air-stable boron nanopowder as combined combustion catalyst and high energy density fuel[J]. Energy & Fuels, 2009, 23(12): 6111-6120.
[45] 敖文. 硼颗粒点火燃烧机理研究[D]. 杭州:浙江大学, 2014.
AO Wen. Study on ignition and combustion mechanism of boron particles[D]. Hangzhou:Zhejiang University, 2014.
[46] 李疏芬, 金荣超, 郭敬为. 硼粒子的表面包覆及其性能分析[J]. 含能材料, 1996(3):102-108.
LI Shu-fen, JIN Rong-chao, GUO Jing-wei. Surface coating of boron powder and its effect[J]. Chinese Journal of Energetic Materials, 1996(3):102-108.
DOI:10.14077/j.issn.1007-7812.2016.05.003
Abstract:Several mixtures, based on urea derivatives and some inorganic oxidants, including also alumina, were studied by means of ballistic mortar techniques with TNT as the reference standard. The detonation pressure(P), detonation velocity(D), detonation energy(Q), and volume of gaseous product at standard temperature and pressure (STP), V, were calculated using EXPLO5 V6.3 thermochemical code. The performance of the mixtures studied was discussed in relation to their thermal reactivity, determined by means of differential thermal analysis (DTA). It is shown that the presence of hydrogen peroxide in the form of its complex with urea (i.e. as UHP) has a positive influence on the explosive strength of the corresponding mixtures which is linked to the hydroxy-radical formation in the mixtures during their initiation reaction. These radicals might initiate (at least partially) powdered aluminum into oxidation in the CJ plane of the detonation wave. Mixtures containing UHP and magnesium are dangerous because of potential auto-ignition.
Keywords:ballistic mortar;TNT;DTA; peroxides; perchlorates; nitrates; urea
Received date:2016-06-01; Revised date:2016-06-17
Biography:Ahmed K.HUSSEIN(1984-),male,MSC.,research field:Energetic materials.E-mail:ahmed92eqypt@gmail.com
Introduction
Mixtures of compounds based on ammonium nitrate (AN) and urea (U) are used as liquid nitrogen fertilizers, referred to as UAN[1]with melting points between -18℃ and -5℃ (depending on the water content) and as intermolecular castable industrial explosives, commonly known as Carbatols[2-3], with relatively high density, detonation velocity and resistance to initiation[2]. Another subject of practical interest concerning mixtures based on urea nitrate (UN) is their use for criminal purposes[4-5], unfortunately increasingly commonplace[6-8]. The globally commercially available urea hydrogen peroxide (UHP)[9]has, in light of its explosive risk, only been described recently[10]; its behavior when mixed with some inorganic salts has not been studied until now, and thus this paper focuses on it in comparison with several previously studied other mixtures with peroxides[11-13]and/or ammonium nitrate content.
1 Experimental
1.1 Materials
Among the substances used, i.e. ammonium nitrate (AN), sodium nitrate (SN), urea (U), urea hydrogen peroxide complex (UHP), ammonium perchlorate (AP), the urea hydrogen peroxide complex was not studied in any more detail from the point of view of its use in explosive compositions. UHP has a density of 1.4 g/cm3, and is a white crystalline substance that decomposes on melting at 80-90℃. A kinetic study of UHP′s thermal decomposition gave an activation energy of 113kJ/mol with frequency factor of 10-13s-1[14]. Its heat of formation is 65.1kJ/mol and its experimental detonation velocity was 3470-3600 m/s[10]. Aluminum (Al) quality AP-4[2]was used with specific surface area of grains 1000-1100 cm2/g and particle size of 20-22 μm. In the one case of an attempt using magnesium flake for fireworks, the particle size ranged from 100 to 500 μm.
1.2 Preparation of mixture
A weighed amount of urea (U) and/or UHP was mixed mechanically with ammonium nitrate (AN), ammonium perchlorate (AP) and sodium nitrate (SN). The actual mass fraction are shown in Table 1. These mixtures were formed so as to have an oxygen balance (OB) close to zero thus generating gaseous products with a high level of power and low toxicity. Four mixtures based on triacetone triperoxide (3,3,6,6,9,9-hexamethyl-1,2,4,5,7,8-hexoxonane, TATP) with AN and different mass fractions of water (4.2%, 9.9%, 15.5% and 24.5%, respectively) have previously been studied[8,11-12]and compared with the mixtures studied here. Data for urea nitrate (UN) and a mixture of fuel oil with ammonium nitrate (ANFO) were also taken from recent papers[8].
1.3 Calculations of detonation characteristics
The theoretical detonation characteristics (i.e. detonation velocity, detonation energy and volume of gaseous products) of the mixtures tested were calculated using the EXPLO5 thermochemical code, version V6.3. The calculation of detonation parameters is based on the chemical equilibrium steady-state ideal detonation model. The state of gaseous detonation products is described by the EXP-6 equation of state[15-16], based on intermolecular potentials and fundamental statistical mechanical theories for the calculation of the thermodynamic properties of a classical fluid of molecules interacting with a central pair potential are available today. In our calculations we used the WCA/Ree perturbation theory to generate excess thermodynamic data of a pure fluid with an EXP-6 potential, and by interpolating them with a Chebyshev polynomial. The method, i.e. EOS, is described by Byers Brown[17]. The detonation velocity, detonation energy and amount of gaseous detonation products calculated by EXPLO5 V6.3 are summarized in Table 2.
It is very likely that some of the explosive mixtures tested have non-ideal detonation behavior, particularly those containing aluminum and ammonium nitrate. However, in this study we calculated detonation properties assuming an ideal detonation model for all the mixtures (such calculation gives the theoretically maximum detonation properties, i.e. properties at infinite explosive charge diameter), except for aluminum-containing mixtures for which we carried out calculations in two different ways-one assuming Al completely reacts at the CJ state (this gives higher D, P, Q, T values) and the second assuming Al does not react, i.e. remains as solid Al.
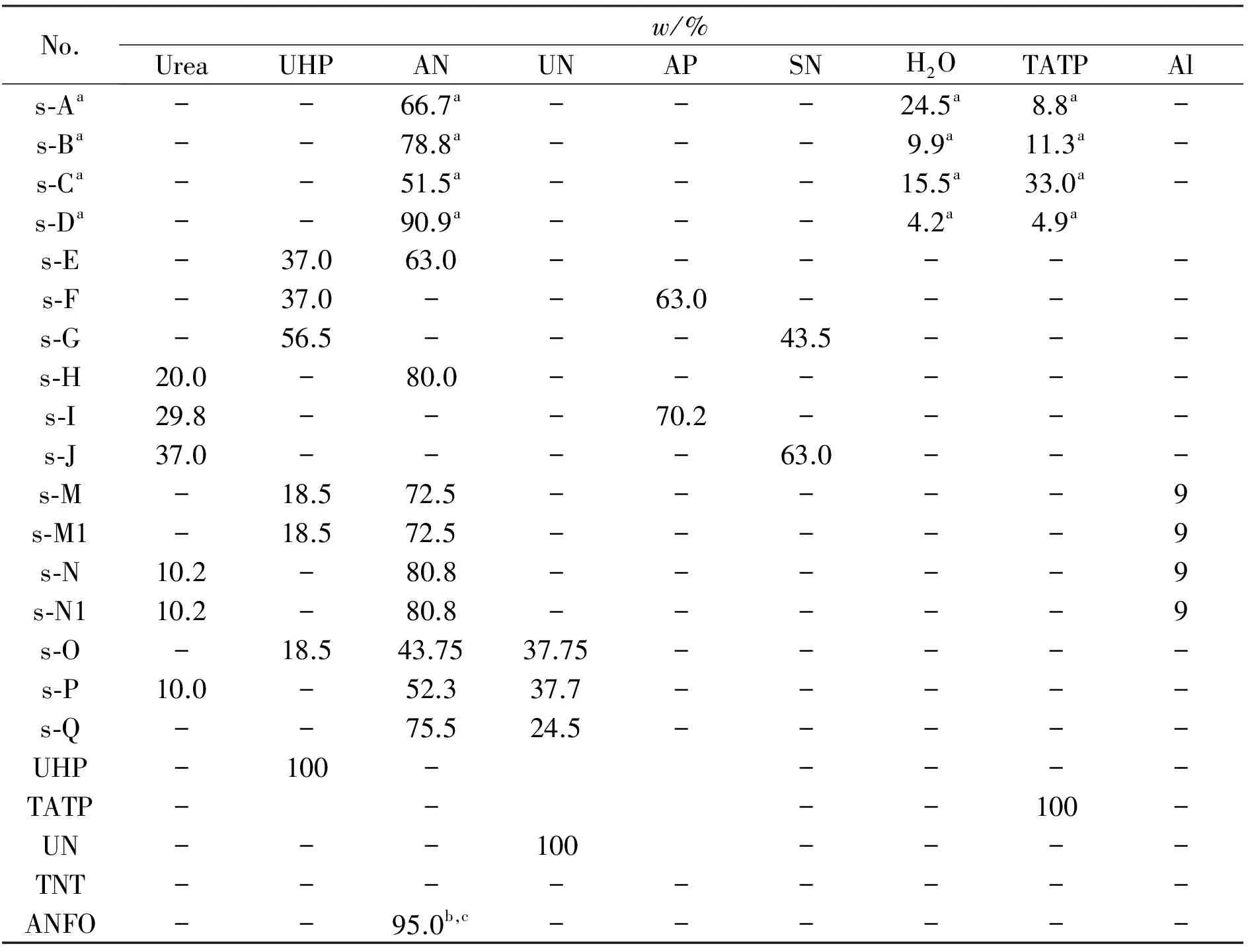
Table 1 Formulations of energetic mixtures
Notes:(a) taken from Ref.[1];(b) taken from Ref.[8];(c) this mixture contains 5% by wt.fuel oil;Data for mistures N and M were calculated taking aluminum as an inert admixture,for mixtures N1 and M1 this aluminum has reacted fully in the CJ point.
1.4 Relative explosive strength measurement
A ballistic mortar test was used for the determination of the relative explosive strength of the samples studied, using TNT as the reference[18-19]. This substitutes for the Trauzl test in the lead block, which was used in the past but which had certain disadvantages such as high cost, the use of toxic lead and the rupture of a lead block[20]. A fixed amount of an explosive (10g) was wrapped in polypropylene foil and inserted into the mortar enclosed by a steel projectile and initiated using a non-electric detonator (No.8). For each measurement, a part of the non-electric detonator was inserted in the sample and was fired by a match. Three measurements were made for each sample and the mean values are reported in Table 2. The determination is based on measuring the swing angle of the pendulum and by comparing the measurement with a calibration curve for the standard TNT explosive at different masses. The explosive strength of the explosive tested was thus expressed relative to TNT (relative explosive strength, RS as % TNT) and compared with previously studied TATP samples from Refs.[8,11]. UHP and the mixture of urea with sodium nitrate (J) gave practically zero swing angles; therefore, their RS values, in the conditions used, were taken as to be equal to zero. However, both these mixtures are energetic materials which, in specific conditions, can succumb to explosive decomposition (for UHP see Ref. 10, and the J mixture after adding hydrogen peroxide (HP)-see mixture G).
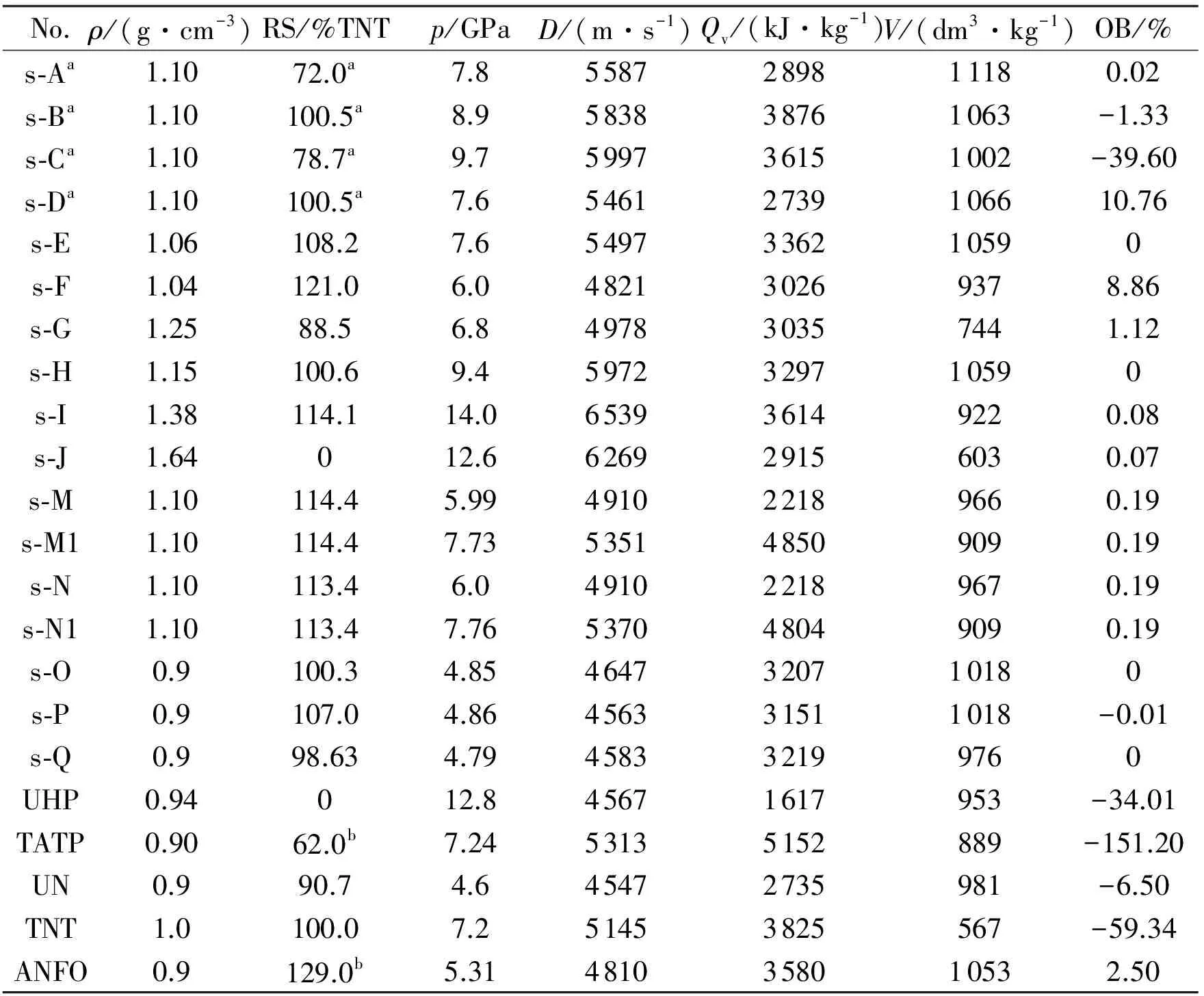
Table 2 Characteristics of energetic mixtures
Notes:(a) taken from Ref[11];(b) taken from Ref[8];Datas for mixtures N and M were calculated taking aluminum as an inert admixture, for mixtures N1 and M1 this aluminum has reacted fully in the CJ point.
1.5 Differential thermal analysis (DTA)
Due to the heterogeneity of the mixtures studied, the DSC and TGA techniques, which use samples of only a few mg, were unsuitable for a study of their thermal reactivity. Therefore, a DTA 550 Ex apparatus was used for differential thermal analysis[21]of the explosives under study. The measurements were carried out at atmospheric pressure, with the tested sample in direct contact with the air. The sample (0.05 g) was placed in a test tube made of Simax glass, 5 mm in diameter and 50 mm long. The reference standard was 0.05 g aluminum oxide. Different linear heating rates of 5, 10 and 15℃/min were used. The output of these measurements was evaluated by the Kissinger relationship (1)[22]
(1)
whereφis the linear heating rate andTis the peak temperature of the exothermic decomposition. The thermal reactivity, expressed as theEaR-1slopes, and its regression value from this relationship (in a similar sense to that in references[23-25]), are summarized in Table 3. Because the mixture J did not decompose with liberation of heat (see Fig.1), its exothermic thermal reactivity was taken as being zero.
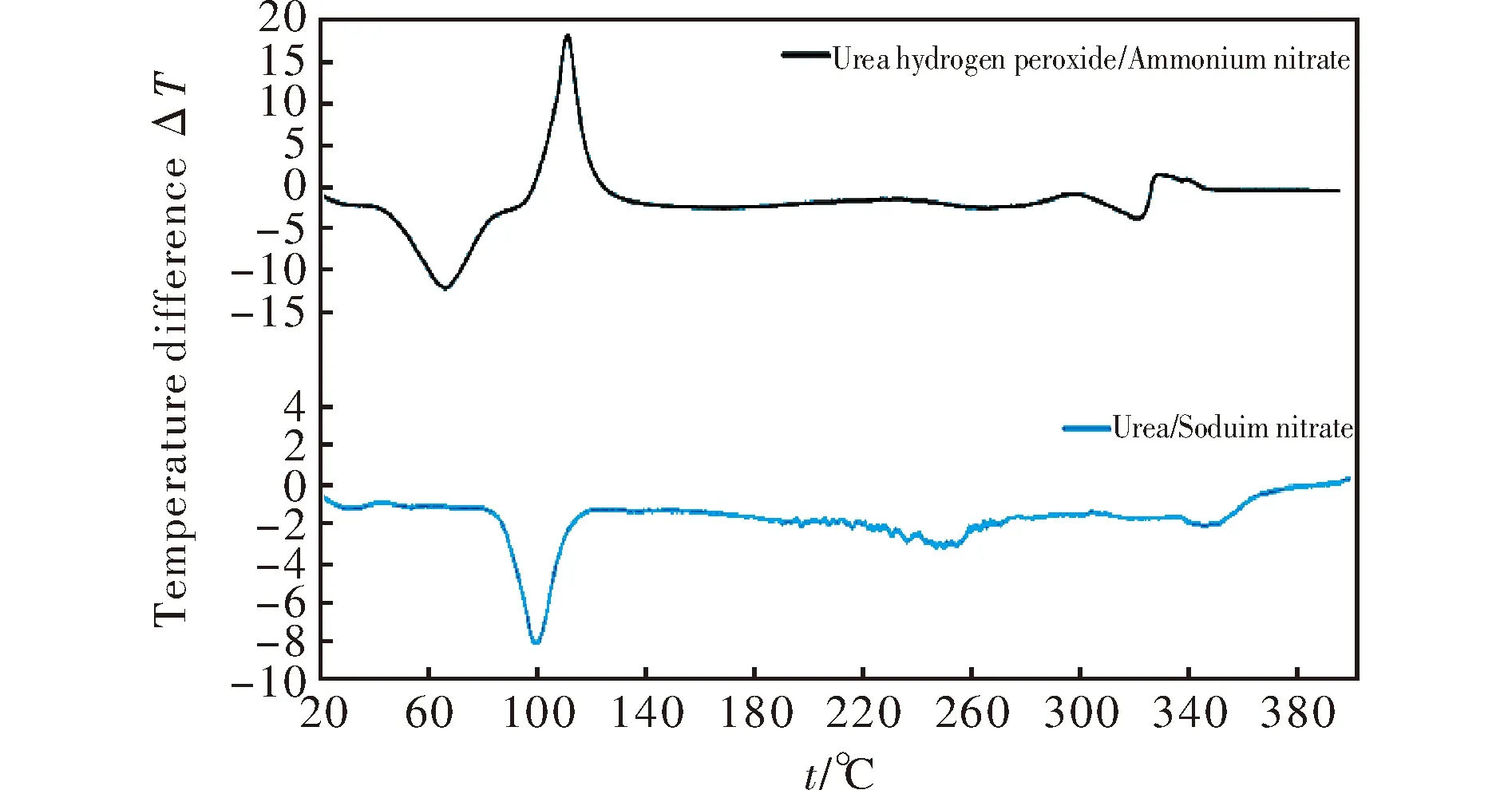
Fig.1 DTA records of mixture of UHP with ammonium nitrate (upper curve) and mixture of urea with sodium nitrate (lower curve)
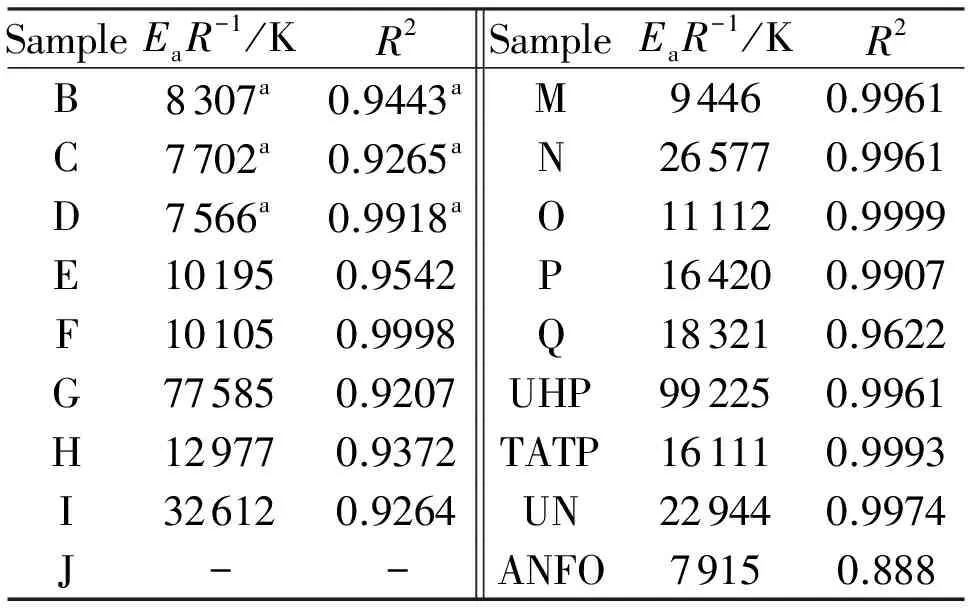
SampleEaR-1/KR2SampleEaR-1/KR2B8307a0.9443aM94460.9961C7702a0.9265aN265770.9961D7566a0.9918aO111120.9999E101950.9542P164200.9907F101050.9998Q183210.9622G775850.9207UHP992250.9961H129770.9372TATP161110.9993I326120.9264UN229440.9974J--ANFO79150.888
Note: (a) Taken from Ref[12]
2 Results and Discussion
2.1 Explosive strength versus detonation pressure
In PBX explosives, the relative explosive strength (RS) is directly proportional to the productρD2[16], whereρD2is representative of detonation pressure. The RS values were also determined by ballistic mortar measurements[18](more about influence of the sample quality on outputs of measurements see in Ref.[18]). However, in the mixtures studied, only lines I, II and III in Fig.2 correspond to this relationship. The data for TNT (ρ= 1.0 g/cm3) are situated on line III. Hydrogen peroxide (HP) is included in appropriate mixtures through the presence of UHP (UHP contains 36.1% by wt. of HP). Thermal decomposition of hydrogen peroxide, i.e. homolysis of the peroxide HO-OH bond, has been identified as the dominant chain-branching reaction[23]that controls any given charge ignition. This fact has a positive effect on the performance of the corresponding mixtures (see RS of the mixture s-E versus s-H), whereas comparison of theirpvalues shows the reverse effect.
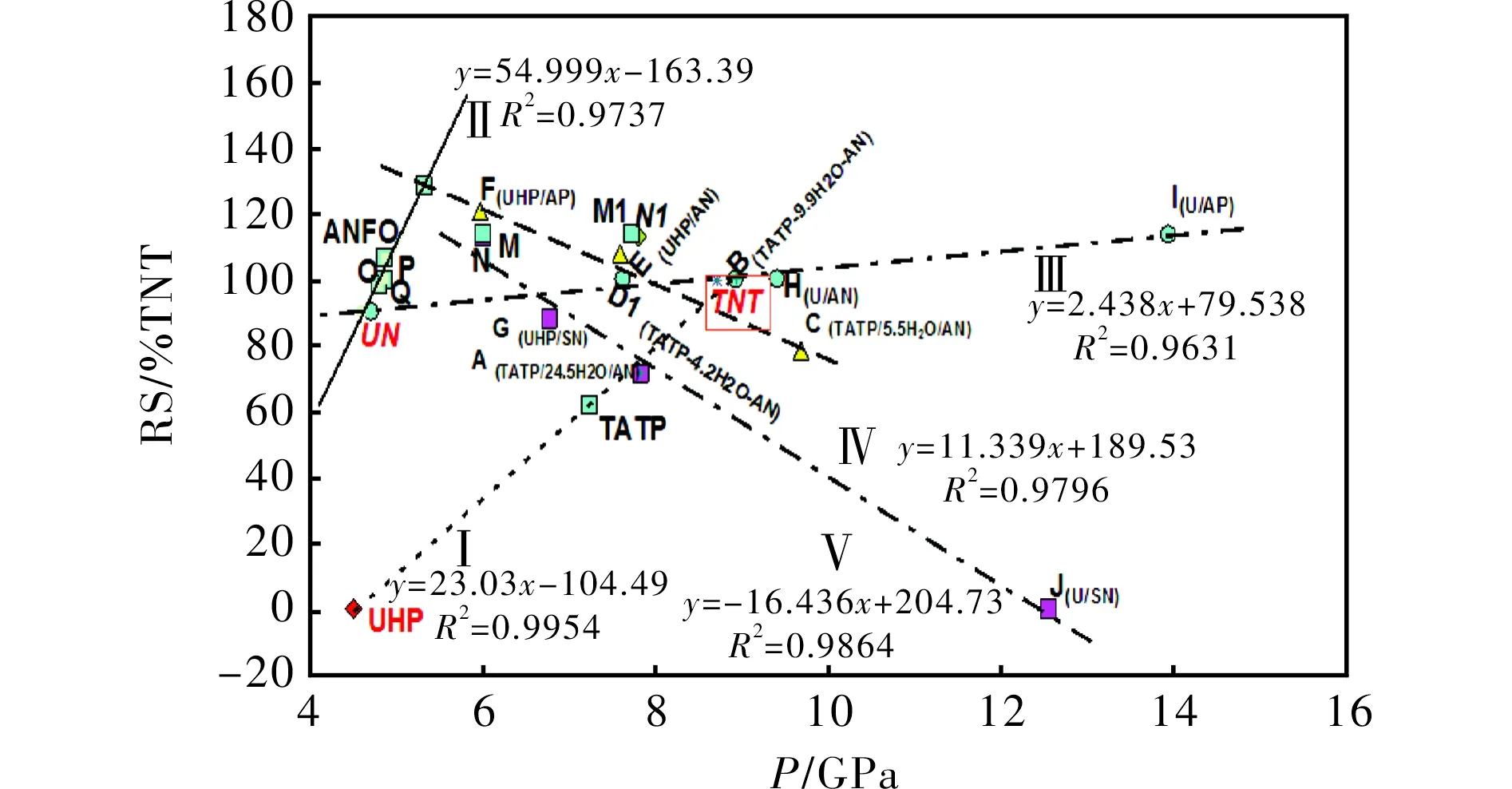
Fig.2 Relationship between experimental relative explosive strength and calculated detonation pressure of the mixtures studied
The reactive radicals (OH-radicals and also radicals derived from oxo-chlorine intermediates of decomposition in the mixture F) might have a strong influence on the reverse slope of line IV in the case of absence of cooling water admixture in samples s-F and s-E, i.e. their decomposition velocity in a ballistics mortar chamber could have been higher than in the case of other mixtures. Mixture s-C is already "cooled" by the water content and the negative oxygen balance (-39.6%). The theory about the influence of very reactive radicals (in this case·OH) may be evidenced by the comparison of the positions in Fig.2 of the mixture pair s-F and s-I, but mainly by the s-G and s-J pair. The difference in densities of the pair s-E and s-H, and thus also in their calculated detonation parameters, may well be removed due to the use of the ballistic mortar for testing their performance (for a discussion about influences in these measurements, see Ref.[16]). The influence of cooling admixtures mentioned above is very well demonstrated by the W/O emulsion explosives fortified by admixtures of high explosives[21]; such admixtures cause a relatively strong increase in the detonation velocity (D) of the fortified W/O explosives and thus the values of the productρD2, while their relative explosive strength is roughly the same[23]due to the presence of water.
Line V is influenced by solid particles in the decomposition products and, in the case of mixture A, by a relatively high water content. As for the aluminized mixtures s-N and s-M, the points N1 and M1 in Fig.2 correspond with the assumption that aluminum completely reacts in the CJ point, and the points N and M correspond with unreacted solid Al (aluminum, depending on the type of explosive used, either behaves as an inert substance or participates in the chemical reactions proceeding inside the detonation wave[27]). It seems that the data for the s-N and s-M mixtures correlate logically with line V.
2.2 Thermal reactivity versus detonation pressure
From our study of the relationship between thermal reactivity and performance of energetic materials[23-25,29,30]it was found that there exists a simple relationship between a slope of the Kissinger relation (1),EaR-1, and the productρD2(from experimental data)[25]or detonation pressure,p. Its version for the mixtures studied in this paper is represented by Fig.3. Here the trends, corresponding to linesCandD, are in agreement with analogous trends in PBXs[25]. The different positions of these lines in a grid system of Fig.3 is caused by the fact that urea can substantially increase the thermal stability of AN (lineCin Fig.3 corresponds to this)[30,31]. Also, with a content of 50% by wt. of urea, its mixture with AN can still produce a steady detonation in the UN gap test[31]. Urea cannot reduce the ability to propagate a detonation[31].
LineAin Fig. 3 mainly groups mixtures based on UHP; here again mixture M with aluminum has two possible positions in a grid system-if we took Al as being inert, the corresponding point belongs in the group around lineA. However, taking Al as a reactant in the CJ plane, the corresponding point M1 is close to lineD; it might be related to the participation of this metal powder in redox-reactions in this plane. A registered auto-ignition of the mixture with Mg could provide evidence for this theory; in this light, the analogous mixture M, in which aluminum was substituted by magnesium, self-ignited 5 hours after its preparation.
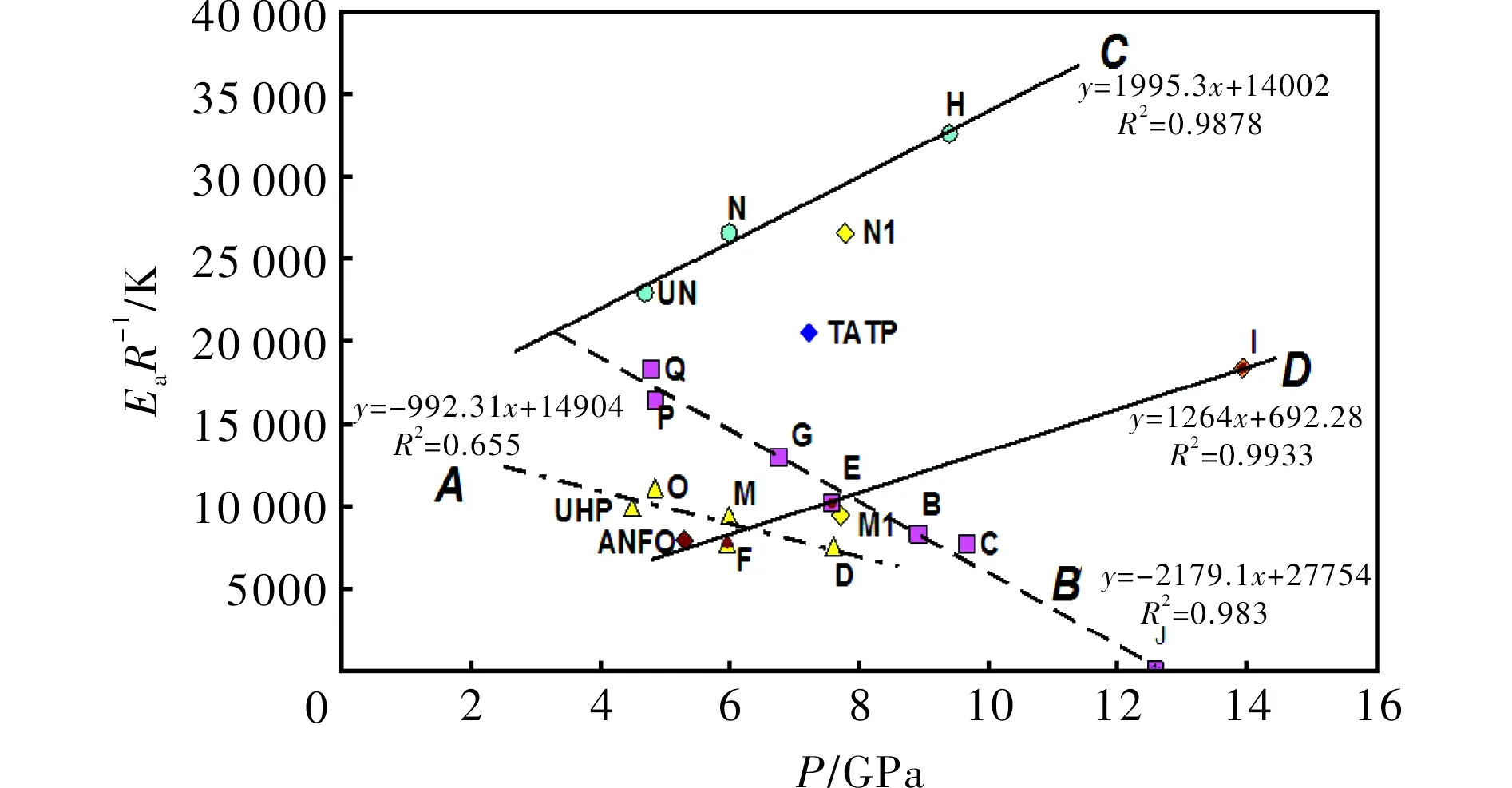
Fig.3 Relationship between thermal reactivity, expressed as the slopes of the Kissinger relationship (1), and calculated detonation pressure (see in Table 1) of the energetic materials studied
In both theAandBlines it is logical that decreasing activation energies correlates with an increase in performance of the corresponding mixtures which correspond to a general relationship between sensitivity and performance[29]. Data for TATP should belong to a group of lineBbut it has a higherEaR-1value in comparison with its mixtures with the "acidic" AN. Almost all mixtures, inherent to these lines, contain peroxides which are their most reactive component. As has already been mentioned, the starting reaction for their initiation should be homolysis of the peroxide O-O bond[26,32]. On the other hand, the most reactive component of the mixtures belonging to linesCandDis ammonium nitrate (eventually urea nitrate) which in its solid state is dissociated according to the following equilibrium[33]
(2)
With subsequent decomposition through nitration of ammonia according to the following equation[33]
H2O+H3O++N2O
(3)
The primary step in the decomposition of UN is its dissociation into urea and nitric acid with subsequent formation of isocyanuric acid from urea[34]. Dissociation into ammonia and perchloric acid is the first step in the decomposition of AP, with subsequent decomposition of the perchloric acid[33]. The difference in the primary fission steps in initiation (ionic versus radical) of the mixtures studied should be the main reason for their division into groups belonging to linesAandB, on the one hand, and to linesCandD, on the other. This difference should be also a reason for the different slopes of the first pair of these lines in comparison with the second one.
3 Conclusions
From ballistic mortar measurements and the theoretical calculation of explosive mixtures based on urea derivatives with some inorganic oxidants. It can be concluded that it is possible to prepare explosive mixtures based on urea with an explosive strength better than TNT. Their performance is seen to be positively influenced by the presence of hydrogen peroxide in their composition, with a distinct advantage being shown by its complex with urea (UHP). This influence is notable namely in the use of sodium nitrate as an oxidant in these mixtures. The chemical nature of this effect lies in the reactive hydroxy-radical formation as the first intermediate in the given mixture′s initiation. On the basis of the relationship between thermal reactivity and performance, it is possible to split the mixture into those with the ionic primary fission and those with the radical one in their initiation.
Theoretical calculation is done assuming an ideal detonation model, so the calculated detonation properties of the mixtures correspond to the theoretical maximum that can be obtained at infinite diameter of explosive charge. In accordance with the current knowledge on burning of metal powders in the reaction zone, it can be assumed that the calculation carried out assuming aluminum does not react at the CJ point gives more realistic results for those mixtures without peroxide content that were studied. However, the OH-radicals, generated during the initiation of mixtures with peroxide content, might initiate (at least partially) powdered aluminum into oxidation in CJ plane of the detonation wave. Mixtures containing both UHP and magnesium are dangerous because of potential auto-ignition.
Acknowledgement
This study was supported by means of the financial resources of Students Grant Projects No. SGSFCHT_2016002 of the Faculty of Chemical Technology at the University of Pardubice.
[1] Raczkowski Ch W, Kissel D E, Vigil M F,et al. Fertilizers placement to maximize nitrogen use by fescue [J]. Journal of Plant Nutrition, 2016, 39(4):581-587.
[2] Dubnov L V, Bakharevich N S, Romanov V I. Promyshlennye vzryvchatye veschestva (Industrial Explosives) [M].Moscow:Izdat. Nedra,1988.
[3] Litovka O B, Kozak G D, Chugreeva E Z,et al.Cast porius charges on a base of ammonium nitrate/Urea eutectic[J]. Central European Journal of Energetic Materials, 2008, 5(2):57-66.
[4] Yin H, Wang G, Du F, et al. Study on non-TNT rock explosive based on nitrates of urea and ammonia [J]. Chinese Journal of Energetic Materials, 1994, 2(4):12-19.
[5] Zhou R, Cao D, Wang J, et al. Relation between formulation and performance of explosives without TNT [J]. Applied chemical Industry, 2006, 35(8):615-617.
[6] Phillips S A, Lowe A, Marshall M,et al. Physical and chemical evidence remaining after the explosion of large improvised bombs. Part 1: firings of ammonium nitrate/sugar and urea nitrate [J]. Journal of Forensic Sciences A, 2000, 45(2):324-332.
[7] Tsippy T, Rinat R, Nitay L,et al. Urea nitrate, an exeptionally easy-to-make improvised explosive: studies towards trace characterization [J]. Analytical and Bioanalytical Chemistry, 2009, 396 (2):421-428.
[8] Matyas R, Selesovsky J. Power of TATP based explosives [J]. Journal of Hazardous Materials, 2009, 165:95-99.
[9] Chemical Trading Guide "Guiechem", http://www.
guidechem.com/cas-124/124-43-6.html, Aug.2016.
[10] Matyas R, Selesovsky J, Pelikan V, et al. Hazardous aspects of urea peroxide adduct [J]. Propellants, Explosives, Pyrotechnics,2016-DOI:10.1002/prep.201600101.
[11] Zeman S, Trzciński W A, Matyáš R. Some properties of explosive mixtures containing peroxides. Part I.Relative performance and ddtonation of mixtures with triacetone triperoxide [J]. Journal of Hazardous Materials, 2008, 154:192-198.
[12] Zeman S, Bartei C. Some properties of explosive mixtures containing peroxides. Part II. relationships between detonation parameters and thermal reactivity of the mixtures with triacetone triperoxide [J]. Journal of Hazardous Materials, 2008, 154:199-203.
[13] Matyáš R, Zeman S, Trzciński W A,et al. Detonation performance of TATP/AN based explosives [J]. Propellants, Explosives, Pyrotechnics, 2008, 33:296-300.
[14] Ball M, Steven M, The thermal decomposition of solid urea hydrogen peroxide [J]. Thermochimica Acta, 1995, 261:95-106.
[15] Fried L E , Howard W H, Souers P C. Exp-6: a new equation of state library for high pressure thermochemistry [C]∥12th International Detonation Symposium. San Diego:Naval Surface Weapons Center,2002.
[16] Victorov S.B. The effect of Al2O3phase transitions on detonation properties of aluminized explosives [C]∥12th International Detonation Symposium. San Diego:Naval Surface Weapons Center,2002.
[17] Byers Brown W. Analytical representation of the excess thermodynamic equation of state for classical fluid mixtures of molecules interacting with a-exponential-six pair potentials up to high densities [J]. Journal of Chemical Physics,1987, 87 (1):566-577.
[18] Elbeih A, Jungova M, Zeman S, et al. Explosive strength and impact sensitivity of several PBXs based on attractive cyclic nitramines [J]. Propellants, Explosives, Pyrotechnics, 2012, 37:329-334.
[19] Establishing More Detailed Conditions for Allowing Explosives, Explosive Objects and Aids into Use, and their Testing,No. 246/1996[R]. Czech Mining Authority,1966:3200-3208.
[20] Suceska M. Test Methods for Explosives [M].New York:Springer-Verlag,1995.
[21] Krupka M, Devices and equipment for testing of energetic materials [C]∥New Trends Res. Energ. Mater., Proc. Semin. Pardubice:[s.n.],2001:222-227.
[22] Kissinger H E. Reaction kinetics in differential thermal analysis [J]. Analytical Chemistry, 1957, 29(11):1702-1706.
[23] Zeman S, Kohlíěek P, Maranda M. A study of chemical micromechanism governing detonation initiation of condensed explosive mixtures by means of differential thermal analysis [J].Thermochimica Acta, 2002, 398:185-194.
[24] Nemec O, Jungova M, Zeman S. Modification of W/O emulsions by demilitarized composition B [J]. Propellants, Explosives, Pyrotechnics, 2013, 38(1):142-146
[25] Zeman S, Yan Q L, Elbeih A. Recent advances in the study of the initiation of energetic materials using the characteristics of their thermal decomposition Part II. Using simple differential thermal analysis [J]. Central European Journal of Energetic Materials, 2014, 11 (3): 395-404.
[26] Hong Z, Farooq A, Barbour E.A, et al. Hydrogen peroxide decomposition Rate: A Shock tube study using tunable laser absorption of H2O near 2.5 μm [J]. Journal of Physical Chemistry A, 2009, 113(46):12919-12925.
[27] Maranda A, Trzcinski W A, Cudzilo S,et al. Detonation problems of non-ideal explosives containing powdered aluminum [C]∥International Pyrotechnics Seminar.Tsukuba:[s.n.], 1997:509-517.
[28] Zeman S. Sensitivities of High Energy Compounds [M]. Heidelberg:Springer,2007:195-271.
[29] Zeman S, Jungova M. Sensitivity and Performance of Energetic Materials [J]. Propellants, Explosives, Pyrotechnics, 2016, 41(3):426-451.
[30] Oxley J C, Smith J L, Rogers E,et al. Ammonium nitrate: thermal stability and explosibility modifiers [J]. Thermochimica Acta, 2002, 384:23-45.[31] Tan L, Xia L H, Wu Q J,et al. Effect of urea on detonation characteristics and thermal stability of ammonium nitrate [J]. Journal of Loss Prevention in the Process Industries,2015, 38:169-175.
[32] Talouba I B, Balland L, Mouhab N,et al. Kinetic parameter estimation for decomposition of organic peroxides by means of DSC measurements [J]. Journal of Loss Prevention in the Process Industries, 2011, 24:391-396.
[33] Manelis G B, Nazin G M, Rubtsov Yu I,et al. Thermal decomposition and combustion of explosives and propellants [M].New York: Taylor & Francis, 2003.
[34] Désiletsa S, Brousseaua P, Chamberlanda D,et al. Degradation mechanism and thermal stability of urea nitrate below the melting point [J].Thermochimica Acta, 2011, 521:76-183.
DOI:10.14077/j.issn.1007-7812.2016.05.004
Abstract:Six furazano-[3,4-d]-pyridazine-based derivatives as main compounds in solid composite propellants have been investigated. It was shown that the use of some furazano-[3,4-d]-pyridazine-based derivatives as main compounds in solid composite propellants can considerably increase ballistic parameters compared with HMX if the compounds under consideration contain difluoramine groups. And the use of the compounds under consideration may be successful only in the presence of an active binder and 10%-30% of AP or ADN as additional oxidizers.
Keywords:solid composite propellant; furazano-[3,4-d]-pyridazine-based derivative; energetic specific impulse
CLC number:TJ55;TQ560 Document Code:A Article ID:1007-7812(2016)05-0028-07
Received date:2016-07-25; Revised date:2016-08-20
Foundation:Ministry of Education and Science of the Russian Federation (14.613.21.0043)
Biography:LEMPERT David B.(1946-), male, Ph.D, Professor. Researcher field: Aerospace propulsion. E-mail:lempert@icp.ac.ru
Introduction
The search of new energetic materials is an important topic worldwide[1]. In recent years, much attention is riveted on N-heterocycles, because they have high densities and high standard enthalpies of formation. High-enthalpy propellants require a bit or no aluminum in the formulation, because the energy contained in high-enthalpy N-heterocyclic ring is often enough to warm up the gaseous combustion products to high temperatures (3500K and even higher)[2].
Currently underway is not only an experimental search for new N-heterocycles, but also a lot of investigations on the theoretical search for new high-energy compounds of this class[3], that is rather natural, since such studies facilitate a targeted search of new compounds[4].
In this study, the estimations of properties (densities, standard enthalpies of formation, detonation parameters) of new compounds, some furazano-[3,4-d]-pyridazine-based derivatives, that are not obtained yet, have been carried out.
1 Statement of the problem and research
methodology
Ief(1)=Isp+100·(ρ-1.9);
Ief(2)=Isp+50·(ρ-1.8);
Ief(3)=Isp+25·(ρ-1.7) ;
We have considered the propellant formulations containing about 19 volume percents of AB, because at lower volume percentage it is almost impossible to create a formulation having satisfactory rheologic properties of uncured mass and physico-mechanical properties of the cured propellant. Aluminum mass fraction was varied from 0 to 18 %.
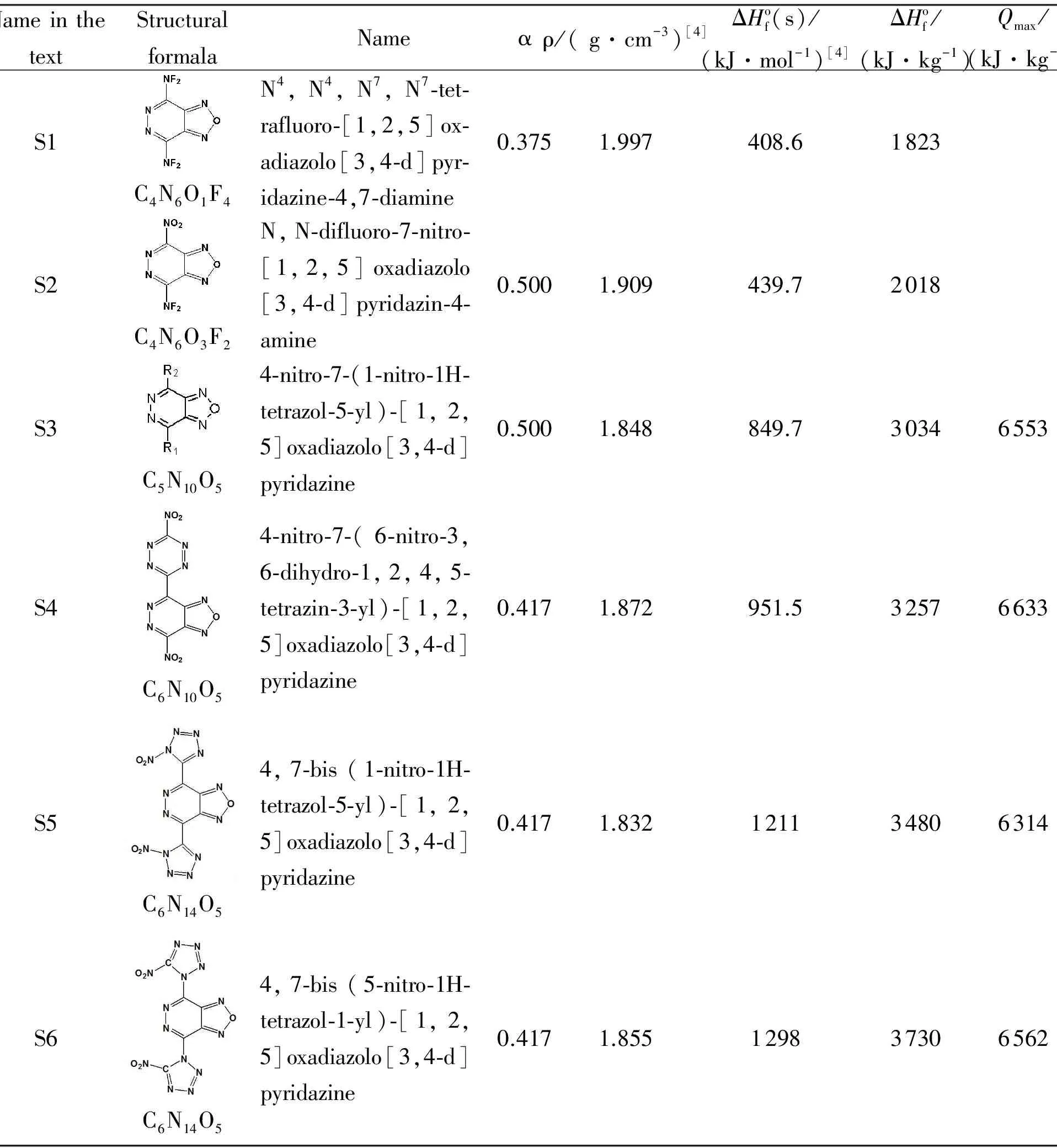
Table 1 Properties of hypotetic furazano-[3,4-d]-pyridazine-based derivatives, that were used at calculations.
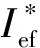
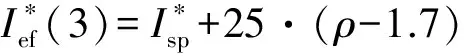
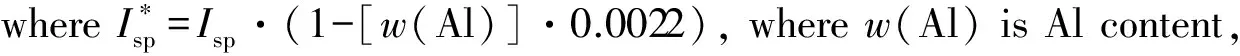
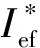
2 Results and discussion
2.1 Formulations with AP as additional inorganic oxidizers
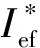
2.1.1 Formulations with S1 + Al + AP + AB (19% volume fraction) (Fig.1)
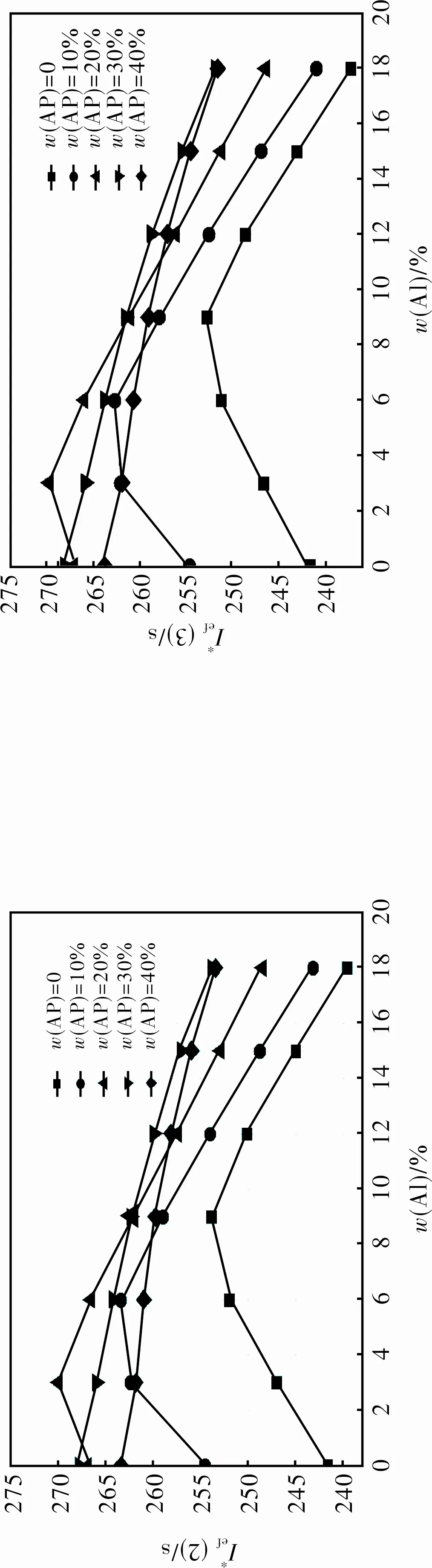
Fig.
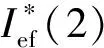
the introduction of Al to the formulations still more reduces these values.
2.1.2 Formulations with S2 + Al + AP + AB (19% volume fraction) (Fig.2)
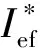
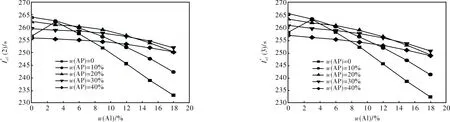
Fig.
2.1.3 Formulations with S3 + Al + AP + AB (19% volume fraction) (Fig.3)
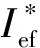
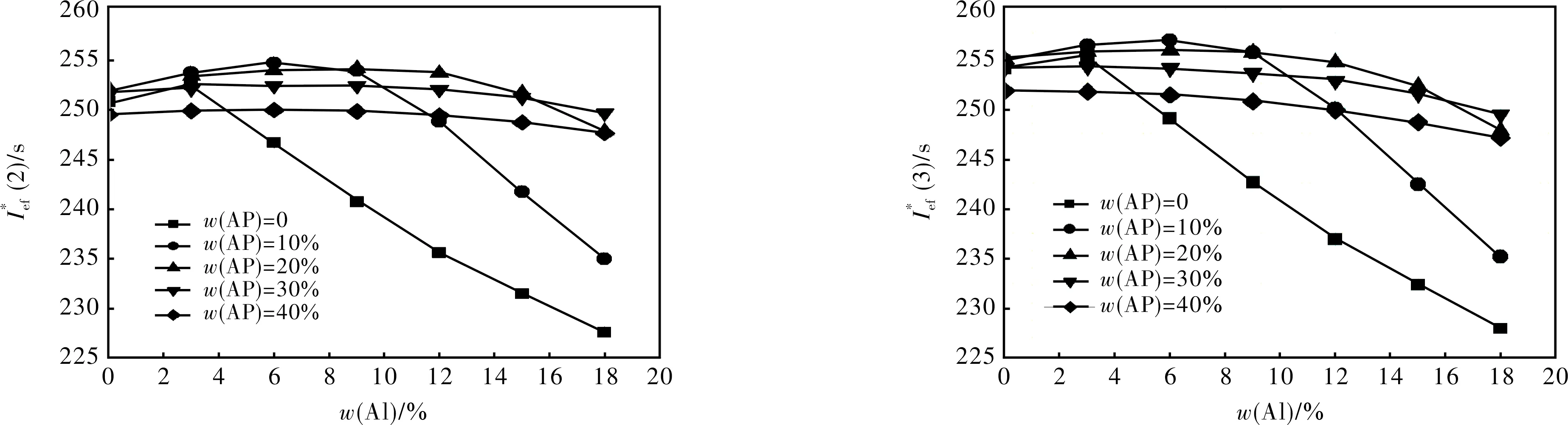
Fig.
2.1.4 Formulations with S4 + Al + AP + AB (19% volume fraction) (Fig.4)
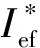
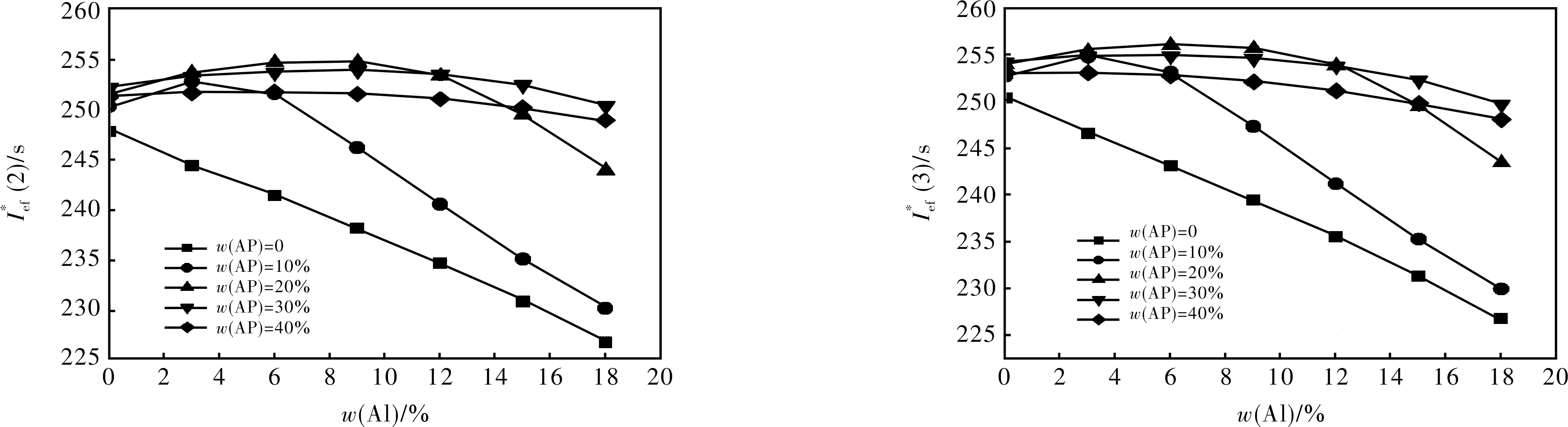
Fig.
2.1.5 Formulations with S5 + Al + AP + AB (19% volume fraction) (Fig.5)
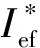
Fig.
2.1.6 Formulations with S6 + Al + AP + AB (19% volume fraction) (Fig.6)
Fig.
2.1.7 Formulations with HMX + Al + AP + AB (19% volume fraction)
Fig.
However, at HMX content higher than 50%-60% compositions with Al, HMX and active binders become dangerous and one always uses a bit of AP to provide the necessary combustion law and to reduce the risk of combustion to detonation transfer. So, a real estimation of energetic characteristics of SCP based on HMX has be compared with compo-
sitions containing at least 10% AP.
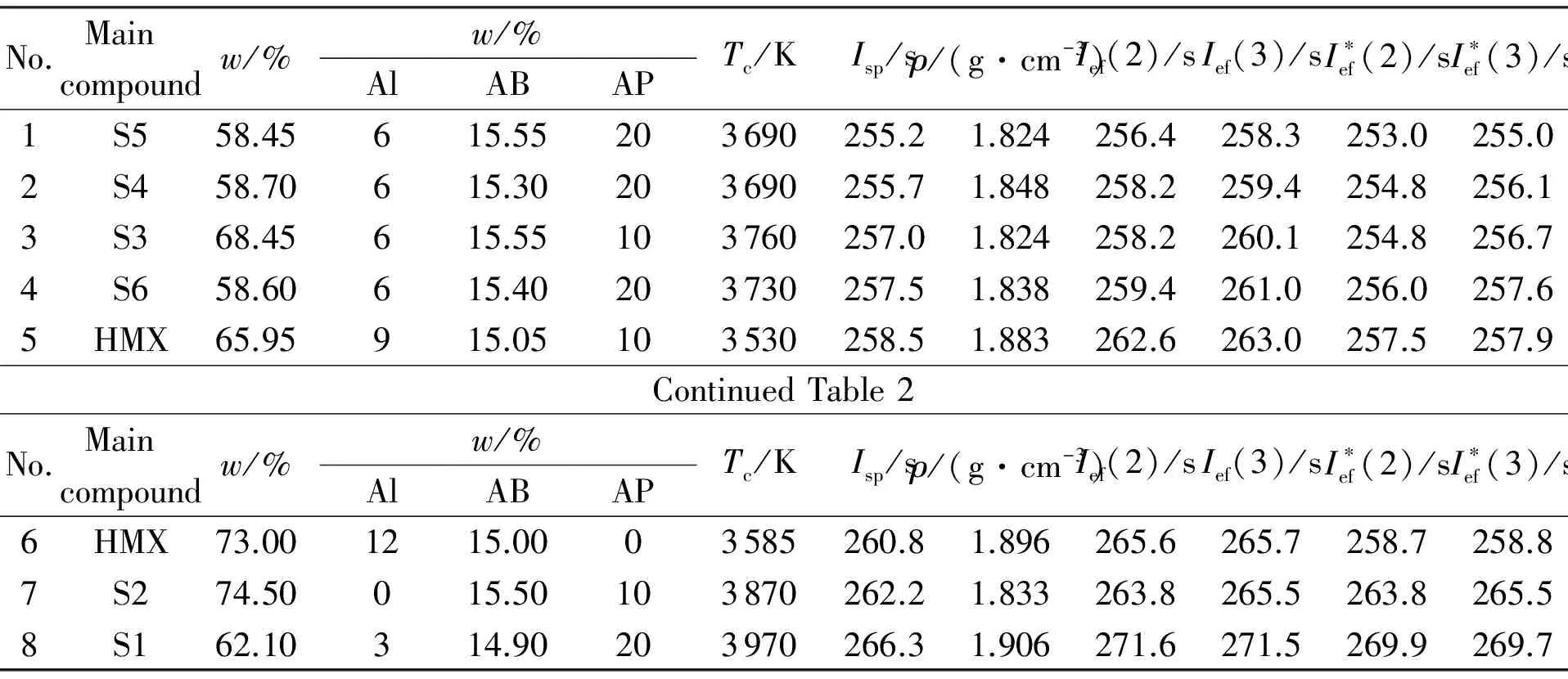
Table 2 Ballistic parameters of the optimal formulations at optimal content of Al and AP
2.2 Formulations with ADN as additional inorganic oxidizers
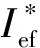
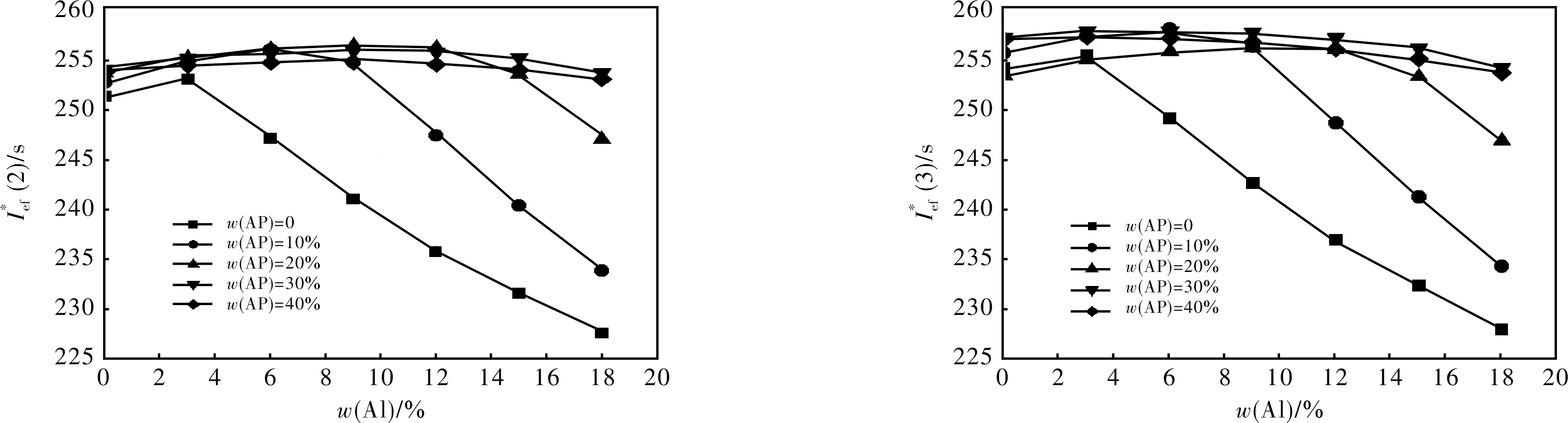
Fig.
Main w/%No.compoundw/%AlABAPTc/KIsp/sρ/(g·cm-3)Ief(2)/sIef(3)/sI∗ef(2)/sI∗ef(3)/s1S545.3915.7303755259.61.810260.1262.4255.0257.22S355.4915.6203820260.11.822261.1263.1256.0257.93S445.5915.5303760260.11.828261.5263.3256.4258.24HMX73.01215.003585260.81.895265.6265.7258.7258.85HMX65.8915.2103520259.81.867263.2264.0258.1258.96S658.3615.7203735260.41.808260.9263.1257.4259.77S274.4015.6103890263.41.818264.3266.3264.3266.38S164.8015.2203915267.81.860270.8271.8270.8271.8
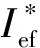
3 Conclusions
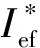
(2)The use of the compounds under consideration may be successful only in the presence of an active binder and 10%-30% of AP or ADN as additional oxidizers.
Acknowledge
The investigation was supported by the Russian Ministry of Education and Science accordingly the agreement No.14.613.21.0043 from 10.11.2015, the unique identifier RFMEFI61315X0043.
References:
[1] SHU Yuan-jie, WU Zong-kai, LIU Ning, et al. Crystal control and cocrystal formation: important route of modification research of energetic materials[J]. Chinese Journal of Explosives & Propellants(Huozhayao Xuebao), 2015,38(5):1-9.
[2] LEMPERT David, NECHIPORENKO Gelii, MANELIS George. Influence of heat release value and gaseous combustion products content on energetic parameters of solid composite propellants[C]∥Theory and Practice of Energetic Materials (VOL.VIII) Proceedings of the 2009 International Autumn Seminar on Propellants, Explosives and Pyrotechnics.Beijing:China Ordnance Society,2009:22-25.
[3] WU Qiong, ZHU Wei-hua, XIAO He-ming. Structural transformation and absorption properties of crystalline 7-amino-6-nitrobenzodifuroxzan under high pressure[J]. The Journal of Physical Chemistry C, 2013, 117:16830-16839.
[4] WANG Ke, SHU Yuan-jie, LIU Ning, et al.Computational investigation on performance and structure of six novel furazano-[3,4-d]-pyridazine-based derivatives[C]∥19th New Trends in Research of Energetic Materials.Pardubice:University of Pardubice,2016, 302-309.
[5] Pepekin V I, Korsunskii B L, Denisaev A A. Initiation of solid explosives by mechanical impact. Combustion[J]. Explosion and Shock Waves, 2008, 44(5): 586-590.
[6] Lempert D, Nechiporenko G, Manelis G. Energetic characteristics of solid composite propellants and ways for energy increasing[J].Central European Journal of Energetic Materials, 2006,3(4): 73-87.
[7] LEMPERT David B, DALINGER Igor L, SHU Yuan-jie, et al. Estimation of the ballistic effectiveness of 3,4- and 3,5-dinitro-1-(trinitromethyl)-1H-pyrazoles as oxidizers for solid composite propellants[J]. Chinese Journal of Explosives & Propellant(Huozhayao Xuebao), 2016, .39(2):16-21.
[8] Trusov B. Program system TERRA for simulation phase and thermal chemical equilibrium [C]∥14th Symposia on Chemical Thermodynamics. St-Petersburg, Russia, 2002, 483-484.
[9] Pavlovets G, Tsutsuran V, Physicochemical properties of powders and propellants[M]. [S.l.]:Russian Ministry of Defense Publishing House, 2009.
[10] Nechiporenko G N, Lempert D B. An analysis of erergy potentialities of composite solid propellants containing beryllium or beryllium hydride as an energetic component [J]. Chemical Physics Reports, 1998, 17(10):1927-1947.
[11] Lempert D B, Dorofeenko E M. Optimal compositions of metal-free energetic compositions with variation in the oxidizer concentration and the ratio of nitro and difluoroamine groups[J]. Combustion, Explosion, and Shock Waves, 2014, 50 (4), 447-453.
Research Progress in Coating Mechanism and Technology of Boron Particles
CHEN Bing-hong, LIU Jian-zhong, LIANG Dao-lun, ZHOU Yu-nan, ZHOU Jun-hu
(State Key Laboratory of Clean Energy Utilization, Zhejiang University, Hangzhou 310027, China)
The coating mechanism of different coating materials for boron particle was described. The principle for selecting the coating materials of boron particle was summarized from five aspects, which includes elaborated removing oxide layer, enhancing combustion temperature, reducing ignition temperature, improving surface compatibility and stimulating the oxidation of boron particles. A variety of the research status of boron particle coating technologies, such as precipitation and surface chemical reaction, macromolecule absorption polymerization, gas phase coating and ball milling was summarized and the actual application results of different processes were analyzed and compared. A variety of modern technologies to test the coating effect of boron particle were introduced. It also reviews the current status of research and insufficience of boron particle coating technology and prospects for the future direction of research.With 46
.
physical chemistry; boron particle; coating mechanism; fuel-rich propellant; metal fuel
Relative Explosive Strength of Some Explosive Mixtures Containing Urea and/or Peroxides
Ahmed K. HUSSEIN1, Svatopluk ZEMAN1, Muhamed SUCESKA2, Marcela JUNGOVA1
(1. Institute of Energetic Materials, Faculty of Chemical Technology, University of Pardubice, Czech Republic;2. Brodarski Institute-Marine Research and Special Technologies, Zagreb, Croatia)
TJ55;TQ560 Document Code:A Article ID:1007-7812(2016)05-0022-06
Energetic Opportunities of Solid Composite Propellants Containing Some Hypothetic Furazano-[3,4-d]-pyridazine-based Derivatives
LEMPERT David B.1,DOROFEENKO Ekaterina M.1,SHU Yuan-jie2,JIANG Wei-dong3,WU Zong-kai2,WANG Ke2,LIU Xiao-qiang3
(1. Institute of Problems of Chemical Physics, Russian Academy of Sciences (IPCP RAS), Moscow 142432, Russia;2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China;3. School of Chemistry and Environmental Engineering, Sichuan University of Science and Engineering, Zigong Sichuan 643000, China)
10.14077/j.issn.1007-7812.2016.05.002
2016-08-12;
2016-08-27
国家自然科学基金(No.51106135)
陈冰虹(1993-),女,博士,从事金属燃料研究。E-mail:3110101575@zju.edu.cn
刘建忠(1965-),男,教授,从事能源转换及利用研究。E-mail:jzliu@zju.edu.cn
TJ55;O64
A
1007-7812(2016)05-0013-09

