microRNA在高磷诱导血管平滑肌细胞钙化早期的动态变化
肖 洋,杜瑶瑶,高 成,孔 炜
(北京大学基础医学院生理学与病理生理学系,教育部分子心血管学重点实验室, 北京 100191)
·论著·
microRNA在高磷诱导血管平滑肌细胞钙化早期的动态变化
肖 洋,杜瑶瑶,高 成,孔 炜△
(北京大学基础医学院生理学与病理生理学系,教育部分子心血管学重点实验室, 北京 100191)
目的:观察高磷诱导的血管平滑肌细胞钙化早期microRNA表达变化,分析其可能参与的信号通路途径。方法:采用高磷(无机磷 2.6 mmol/L)刺激大鼠血管平滑肌细胞系A7r5钙化7 d,邻甲酚酞络合酮比色法和考马斯亮蓝法检测细胞内钙含量,RT-PCR法检测平滑肌细胞表型和钙化相关基因的表达变化,茜素红染色观察钙结节。microRNA microarray 法检测高磷刺激后0、3、12 h,680种microRNA表达变化。采用TAM软件分析不同时间点间信号激活情况。结果:高磷诱导的A7r5细胞钙盐含量升高9.6倍(P<0.05),平滑肌细胞表型mRNA(SM-α actin,SM22)下调(P<0.05), 钙化相关mRNA(BMP2、MSX2、Runx2)上调(P<0.05),茜素红染色后可见高磷刺激组有明显钙结节。680种microRNA在3个时间点的表达各不相同,只有6种microRNA分别逐级上调或渐渐下调。26种信号通路被明显激活,其中包括细胞凋亡、分化增殖等已知与钙化相关的信号通路。结论:microRNA参与调节血管钙化是一种动态微调的过程,为血管钙化研究带来新的思路。
血管钙化;肌,平滑,血管;微RNA;信号转导
血管钙化是动脉粥样硬化、慢性肾病以及衰老等常见的临床病理表现,在其发生时血管弹性降低、僵硬化,易形成血栓和出现斑块破裂,血管钙化是心脑血管疾病高发病率和高死亡率的重要因素之一。血管钙化以往被认为是一种被动过程,但近年来研究表明血管钙化是一种复杂并且高度调控的过程[1]。血管平滑肌细胞(vascular smooth muscle cell,VSMC)在血管钙化中起关键作用,其通过分化成成骨样细胞,生成基质小泡进而在血管壁形成钙磷沉积病灶,目前对此过程了解较少。
microRNA是一种内源性表达的非编码小RNA,其大小长约19~23个核苷酸,剪切加工成熟的microRNA与蛋白质复合物结合形成RNA沉默复合体,与靶mRNA进行不同程度的碱基互补配对,降解靶mRNA或抑制靶mRNA的翻译。研究证实 microRNA在机体发育、细胞分化、心血管疾病、肿瘤、病毒感染等各种生理或病理过程发挥重要作用[2]。目前对于 microRNA与血管钙化之间的关系已有初步认识,例如,miR-125b是最早于钙化人冠状动脉平滑肌细胞上被发现显著下调的micro-RNA[3],Wang等[4]观察到终末期肾病患者相比健康人群循环系统中miR-15b明显降低,该研究发现其可能参与了磷代谢过程。本课题组于2012年报道[5],miR-29a/b能够抑制血管平滑肌细胞钙化。以往对于microRNA参与钙化机制的研究,多数学者只关注钙化刺激后某一时间点microRNA的变化,但microRNA参与机体信号调节是一种动态微调的过程,为了能够对钙化机制有更深入的理解,本研究检测给予血管平滑肌细胞钙化刺激(高磷)后0、3、12 h 3个时间点不同microRNA的表达,以探讨在钙化发生早期microRNA的动态变化,并分析其可能参与激活的信号通路。
1 材料与方法
1.1 细胞培养
大鼠胚胎胸主动脉平滑肌细胞系A7r5购自美国 Type Culture Collection (Manassas, VA)公司。A7r5细胞采用含有10%(体积分数)胎牛血清Dulbecco modified Eagle medium (DMEM)培养基,在37 ℃并含有5%(体积分数) CO2的湿润环境中培养。钙化血管平滑肌细胞模型采取给予2.6 mmol/L无机磷刺激7 d的方法,期间每2天换液一次。
1.2 细胞钙沉积定量实验
A7r5细胞给予钙化刺激7 d后,弃掉培养基,用PBS清洗2遍,加入含有6%(体积分数)HCl溶液消化收集细胞,超声破碎3次,每次3 s,间隔3 s。12 000 r/min,离心15 min,取上清。采用邻甲酚酞络合酮比色法测定Ca离子浓度,考马斯亮蓝法测定蛋白质浓度进行标准化,得出细胞内钙含量。
1.3 细胞茜素红染色
A7r5细胞给予钙化刺激7 d后,弃掉培养基,用PBS清洗3遍。加入4%(质量分数)多聚甲醛固定10 min,弃掉多聚甲醛后,PBS清洗3遍,之后加入1%(质量分数)茜素红溶液1 mL,浸染30 min。光镜下采集图像,可见钙化组平滑肌细胞染成红色的钙结节。
1.4 real-time-PCR定量实验
A7r5细胞给予钙化刺激48 h后,Trizol法(Invitrogen)提取RNA,再采用逆转录试剂盒(Promega)逆转录成cDNA,-20℃保存。real-time PCR反应采用美国Mx3000 Multiplex Quantitative PCR System (Stratagene公司, La Jolla, CA)完成。mRNA水平与β-actin进行标准化,引物序列见表1。
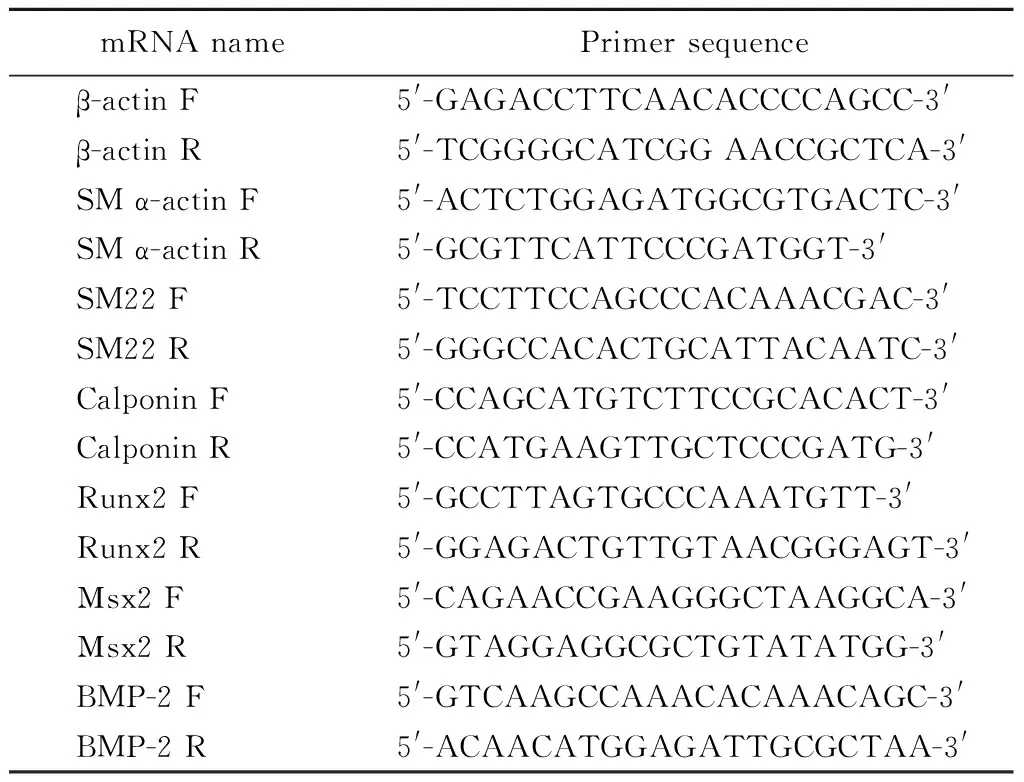
表1 大鼠相关mRNA引物序列Table 1 Rats related mRNA primer sequence
1.5 microRNA microarray实验
A7r5细胞给予2.6 mmol/L无机磷刺激0、3和12 h,用Trizol法(Invitrogen)或miRNeasy minikit (QIAGEN)收集总RNA。采用miRCURYTMHy3TM/Hy5TMPower labeling kit标记,与miRCURYTMLNA Array (v.16.0) (Exiqon, Denmark)杂交,清洗后使用Axon GenePix 4000B microarray scanner扫描芯片。得出原始数据上传到NCBI-Pubmed-GEO dataset数据库,GEO accession: GSE39700。microRNA在3个时间点的倍数变化通过不同的荧光强度来显示,1.5倍以上变化被认为差异有统计学意义。
1.6 microRNA信号通路分析
将上述显著变化的microRNA数据,带入TAM软件进行数据库分析(www.cuilab.cn/tam),得出激活的信号通路数据,P<0.05认为差异有统计学意义,再将P值进行负的常用对数换算(-lgP),作图得出分析结果。
1.7 统计学分析
采用GraphPad Prism 4软件进行分析,正态分布的连续随机变量采用平均值±标准误表示。两组间比较采用t检验,成对数据均采用配对t检验(双侧检验)。
2 结果
2.1 钙化血管平滑肌细胞模型的建立
首先在A7r5细胞上验证钙化模型是否成立。在给予2.6 mmol/L无机磷刺激7 d后,高磷组(Pi)钙沉积相比对照组(Ctrl)显著上调9.6倍[Ctrl (0.23±0.03) mmol/gvs. Pi (2.44±0.34) mmol/g,n=3,P<0.05,图1A],提示钙化模型上钙盐的沉积。采用茜素红染色法观察给予高磷刺激后A7r5细胞钙化情况,镜下可见高磷组(Pi)出现明显红色钙结节(图1B),从组织学角度证实钙盐在平滑肌细胞的沉积。进一步研究该模型上平滑肌细胞表型与钙化相关mRNA的表达变化,通过real-time PCR实验检测发现给予高磷刺激48 h后,平滑肌细胞表型分子骨骼肌-α肌动蛋白 (skeletal muscle-α actin, SM-α actin)、平滑肌22 (smooth muscle 22, SM22)均下调(P<0.05),钙调理蛋白(Calponin)有下降趋势但差异无统计学意义(P=0.131 5, 图 1C~E)。相反骨形态发生蛋白2(bone morphogenetic protein-2, BMP2)、骨细胞的同源 2异型蛋白(msh homeobox 2,MSX2)、Runt相关转录因子2(runt-related transcription factor 2,Runx2)3种钙化相关因子均明显上调(P<0.05,图 1F~H),以上结果证实在给予高磷刺激后,A7r5细胞向成骨样细胞转化,同时证明钙化模型成立。
*P<0.05, compared with the control (Ctrl) group.
图1 高磷刺激7 d后,A7r5细胞内钙含量(A)、茜素红染色情况(B)和平滑肌细胞表型mRNA[SM-α actin(C)、Calponin(D)和SM22(E)],以及钙化相关mRNA[BMP2(F)、MSX2(G)和Runx2(H)]表达变化
Figure 1 A7r5 cell were stimulated by high phosphate for 7d. The cell calcium content (A), alizarin red staining (B), smooth muscle cell type mRNA expression of SM-α actin (C),Calponin (D) and SM22 (E),Calcified related mRNA expression of BMP2 (F), MSX2 (G) and Runx2 (H)
2.2 microRNA microarray实验
采用上述模型,通过microRNA microarray实验研究给予高磷刺激后0、3和12 h这3个时间点microRNA表达谱变化(图 2)。原始数据已上传至NCBI-Pubmed-GEO dataset数据库,GEO accession: GSE39700(http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE39700)。
A, scatterplot of miR expression profiles, left panel, 3 h vs. 0 h, right panel, 12 h vs. 3 h; B, hierarchical clustering for microRNA expression profiles in rat VSMCs with high phosphate for 0, 3 or 12 h. Red indicates high relative expression, and green indicates low relative expression.
图2 给予A7r5细胞高磷刺激后0、3、12 h 3个时间点microRNA表达变化
Figure 2 Microarray profile of time-series change of microRNA in rat VSMCs stimulated with high phosphate for 0, 3, 12 h
实验共检测680种microRNA,并分别比较不同microRNA在3 h与0 h点间以及12 h与3 h点间的变化。以变化超过1.5倍认为差异具有统计学意义,统计分析结果见表 2~5。
2.3 给予高磷刺激后0 h与3 h点间所激活的信号通路
有25种microRNA上调以及14种microRNA下调超过33%(表2~3)。为了探寻0 h与3 h点间不同的信号通路活动情况,对上述39种microRNA进行信号通路数据库分析(图 3)。下调的microRNA所激活的通路包括细胞运动(miR-128、miR-9)、上皮-间质转化(epithelial-mesenchymal transition,EMT,包括miR-542、miR-203、miR-29a)和细胞死亡(miR-128、miR-203、let-7f)等;上调的microRNA所激活的通路包括肿瘤抑制基因(let-7b/e、miR-195、miR-29c 等)、脂肪细胞分化(let-7b/e、miR-378、miR-448)、EMT(let-7b/e、miR-448、miR-29c 等)等。
2.4 给予高磷刺激后3 h与12 h点间所激活的信号通路
3 h与12 h点间分别有48个 microRNA上调超过1.5倍和116个microRNA下调超过33%(表4~5),数据库分析后结果见图 4。下调的microRNA除在前面0 h与3 h两时点间已激活的通路外,还包括细胞凋亡(miR-15b、miR-221/222、miR-29a 等)、免疫炎症反应(miR-25、miR-21、miR-126 等)和骨再生(miR-221/222、let-7b/d、miR-24 等)等共计24条信号通路被激活,令人意外的是在此时间段唯一被上调的microRNA明显激活的信号通路只有脑发育(miR-323、miR-326、miR-9 等)。
2.5 6种逐级变化的microRNA
第2.3和2.4小节展示了在A7r5细胞上给予钙化刺激后信号通路的活动情况,与预期相符,模拟血管钙化发生早期进程,能够帮助理解microRNA参与钙化机制调节的动态微调作用。将上述结果综合分析发现,在3个时间点上,只有3种microRNA[miR-183、 miR-664、 miR-9*(*RNA fragment paired with mature microRNA)]逐级上调以及另外3种microRNA(miR-542-5P、let-7f、miR-29a)渐渐下调(图 5)。
3 讨论
本研究首先在大鼠血管平滑肌细胞系A7r5上验证给予高磷刺激能够促进血管平滑肌细胞向成骨样细胞的转化,确认钙化模型成立,并进一步在该模型上观察680种microRNA在0、3、12 h 3个时间点的变化情况,进行信号通路数据库分析,结果显示,microRNA在此过程中参与多种信号通路途径,包括已知与钙化相关的细胞死亡/凋亡、分化迁移、免疫炎症反应和骨再生等[6],与预期相符。尤为瞩目的是,在这680种microRNA中只有6种是分别逐级上调或下调的,而其他大部分microRNA在3个时间点都呈现各种不同的变化方式,再次证实microRNA参与机体活动是一种动态精细调控的过程。
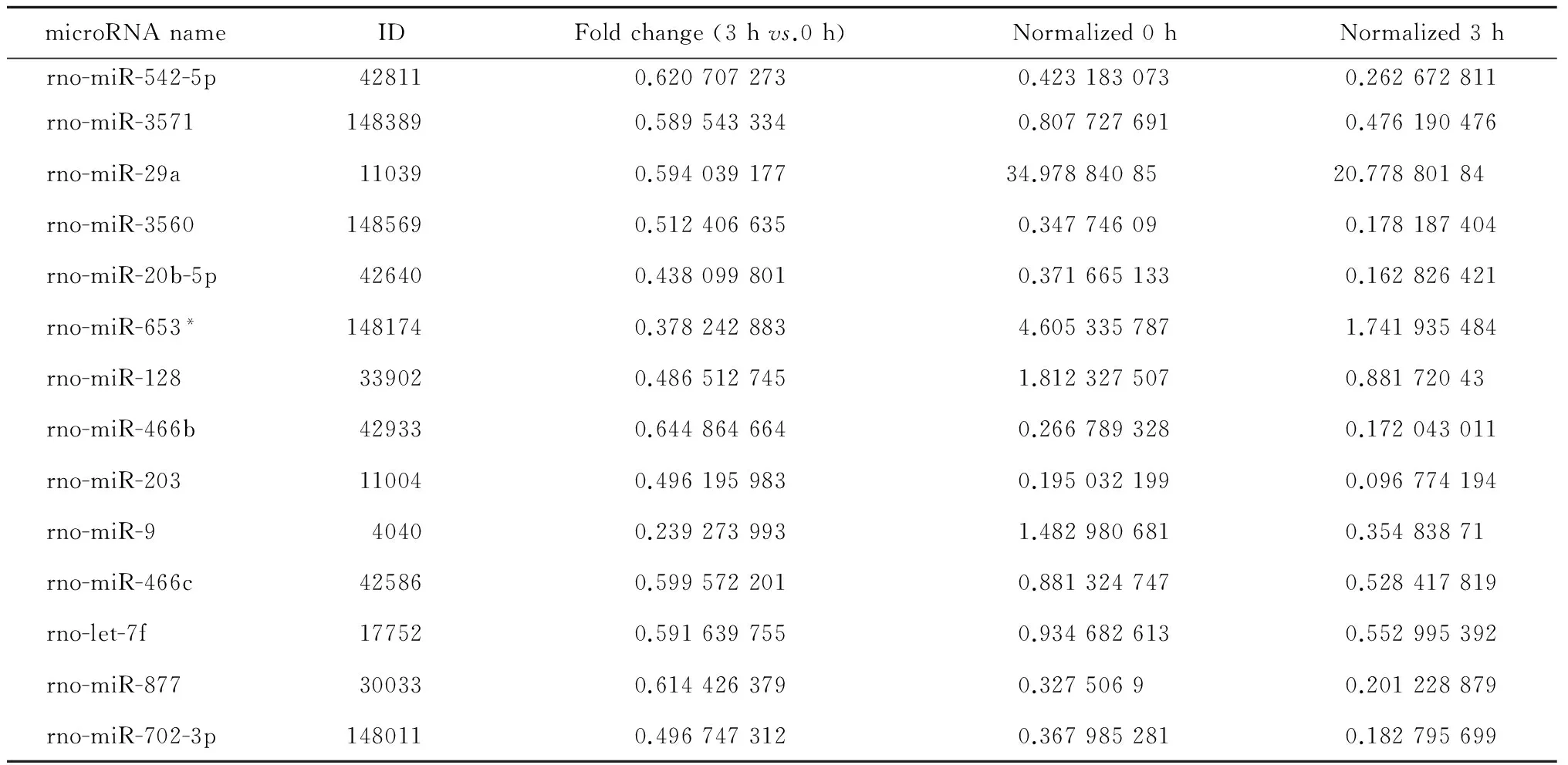
表2 高磷刺激A7r5细胞 0 h与3 h点间下调的microRNATable 2 List of down-regulated microRNA in high-phosphate stimulated A7r5 cell at 3 h vs. 0 h
* RNA fragment paired with mature microRNA. ID, identification.
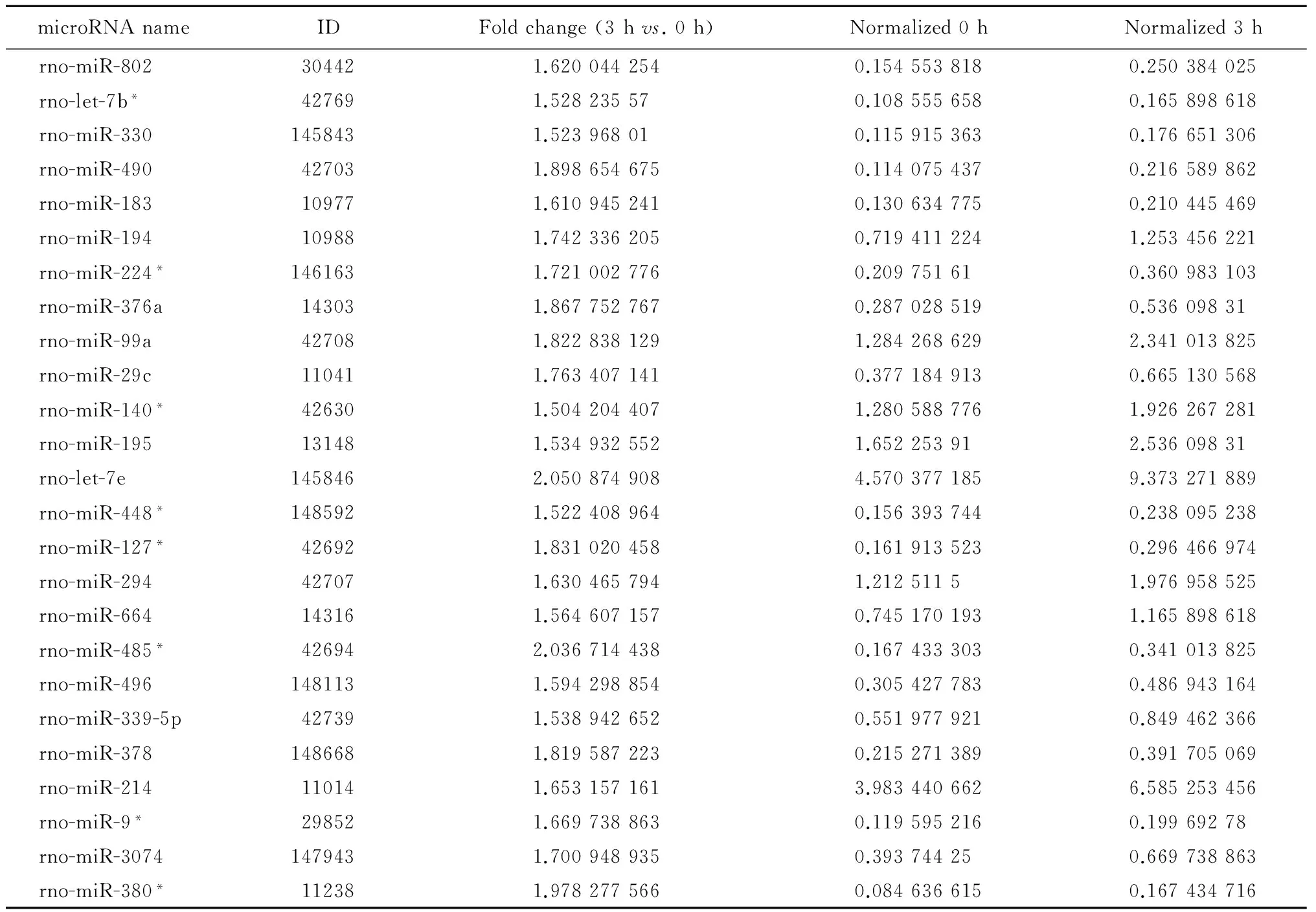
表3 高磷刺激A7r5细胞 0 h与3 h点间上调的microRNATable 3 List of up-regulated microRNA in high-phosphate stimulated A7r5 cell at 3 h vs. 0 h
* RNA fragment paired with mature microRNA. ID, identification.

表4 高磷刺激A7r5细胞 3 h与12 h点间下调的microRNATable 4 List of down-regulated microRNA in high-phosphate stimulated A7r5 cell at 12 h vs. 3 h

续表microRNAnameIDFoldchange(12hvs.3h)Normalized3hNormalized12hrno-miR-338*178250.6156236280.5345622120.329089128rno-miR-503*1481290.5572391540.1827956990.101860921rno-miR-433428530.6325896330.1950844850.123408423rno-miR-107109230.6506696722.5499231951.659157689rno-miR-25426820.2932730832.8387096770.83251714rno-miR-19b109980.4391193630.8832565280.387855044rno-let-7d1459680.4033811534.0937019971.651322233rno-miR-28*1457140.5045587380.5668202760.285994123rno-miR-34b291530.51415416417.921658999.214495593rno-miR-434*112470.585771960.3778801840.221351616rno-miR-99b111840.6277302275.5514592933.484818805rno-miR-495426760.5395163110.5192012290.280117532rno-miR-379110930.5197124541.2211981570.63467189rno-miR-497428470.2928283820.6221198160.182174339rno-miR-195*427230.655321580.2211981570.144955926rno-miR-19a109970.3658138871.7188940090.628795299rno-miR-24-2*429500.524840421.3809523810.724779628rno-miR-32110530.2176143980.4500768050.097943193rno-miR-107*1475360.4567953570.2058371740.094025465rno-miR-1291486450.5435365520.5622119820.305582762rno-miR-871*1481600.5616621580.554531490.311459354rno-miR-743a*1480170.5877558920.3732718890.219392752rno-miR-384-5p428440.27544760.1920122890.052889324rno-miR-142-3p109470.5208202020.6205837170.323212537rno-miR-222*1481330.3126346720.2380952380.074436827rno-miR-3580-3p1485190.5763015140.1597542240.092066601rno-miR-136*425120.595102840.5299539170.315377081rno-miR-99a427080.5480769972.3410138251.283055828rno-let-7i99380.39186082610.832565284.244857982rno-miR-493*111250.5031950660.2841781870.142997062rno-miR-195131480.6225485282.536098311.57884427rno-let-7c1458200.6171944387.0522273434.352595495rno-miR-741-3p1484550.4215341281.8448540710.777668952rno-let-7f177520.2833823050.5529953920.156709109rno-miR-15b172800.42638007915.491551466.605288932rno-miR-16109670.5284715089.781874045.169441724rno-miR-324-3p1457080.5499091570.4132104450.227228208rno-miR-2901482870.2071657640.4254992320.088148874rno-miR-199a-5p295620.56598004326.909370215.2301665rno-miR-376b-3p143040.6560916410.4239631340.278158668rno-miR-34a272170.3898001333.2764976961.277179236rno-let-7a1471620.4462532472.6513056841.183153771rno-miR-211475060.63486876936.0844854122.90891283rno-miR-466d1485940.386560091.3732718890.530852106rno-miR-181b109720.3480601490.6528417820.227228208rno-miR-145426410.5701424423.672811062.094025465rno-miR-93306870.48155174911.861751155.712047013rno-miR-362*424740.6227820420.2642089090.164544564rno-miR-1001459430.4488085240.9078341010.407443683rno-miR-181d1456360.5529614393.3901689711.874632713rno-miR-4941475140.1879272130.4377880180.082272282rno-miR-4961481130.5672115850.4869431640.276199804rno-miR-301a131430.5060331254.6374807992.346718903rno-let-7a-1*/rno-let-7c-2*178880.0535806880.1827956990.009794319rno-miR-674-3p310530.5256396170.6298003070.331047992rno-miR-411174820.6117879670.4930875580.301665034rno-miR-295*1484380.6376101861.8402457761.173359452
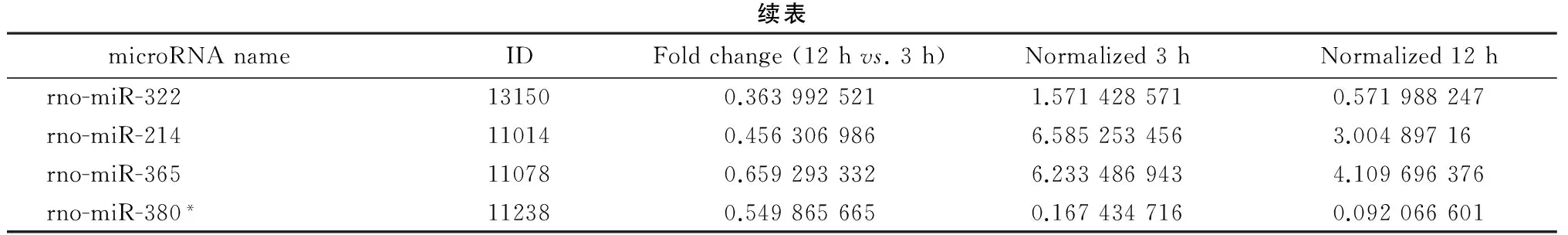
续表microRNAnameIDFoldchange(12hvs.3h)Normalized3hNormalized12hrno-miR-322131500.3639925211.5714285710.571988247rno-miR-214110140.4563069866.5852534563.00489716rno-miR-365110780.6592933326.2334869434.109696376rno-miR-380*112380.5498656650.1674347160.092066601
*RNA fragment paired with mature microRNA. ID, identification.

表5 高磷刺激A7r5细胞 3h与12 h点间上调的microRNATable 5 List of up-regulated microRNA in high-phosphate stimulated A7r5 cell at 12 h vs. 3 h
*RNA fragment paired with mature microRNA. ID, identification.
血管钙化已知是一种高度复杂、多种信号通路参与调控的主动过程,病理性的血管钙化与动脉粥样硬化、糖尿病、心力衰竭这些心血管疾病的预后密切相关。Pasquinelli等[7]最早于2002年在线虫体内发现microRNA(lin-4和let-7)。目前的研究表明microRNA与众多调节途径相关联,包括生物个体发育、病毒防御、组织分化、细胞增殖和凋亡、脂肪代谢、参与原癌基因作用等。近年来发现,microRNA同样参与血管钙化的调控过程,学者们在不同的离体、在体钙化模型上发现miR-125b、miR-204/205、miR-221/222和miR-29a/b等的表达下调[3,5,8-10], 以及miR-223和miR-135a等的上调[11-12]。后续研究发现过表达或敲低miR-204、miR-205、miR-133a和miR-30b/c会分别抑制或促进钙化,其机制是通过直接作用并抑制钙化相关转录因子Runx2的表达而完成[8-9,13-14]。miR-125b是目前被研究较多与血管钙化相关的microRNA,多种钙化模型上均发现miR-125b表达量明显降低,研究证实其可能是通过调控钙化相关信号通路因子SP7(osterix)以及Ets1起到抑制钙化的作用[3,15]。miR-135a*、miR-762、miR-714以及 miR-712*被发现随着钙化发生而表达上调,其可能是以抑制钙离子外排的方式促进血管钙化[12]。miR-223亦有促进钙化的作用,过表达miR-223可以抑制Mef2c和RhoB的表达,提示可能是其作用机制[11]。
A, down-regulated microRNA; B, up-regulated microRNA.
图3 0 h与3 h点间被显著激活的信号通路
Figure 3 Signal pathways were significantly activated between 0 and 3 h
本研究观察到microRNA在给予高磷刺激12 h内,3个时间点上共有26种信号通路被明显激活。部分信号通路早已被报道参与了钙化调控过程:(1)miR-15b、miR-221/222、miR-29a等19种microRNA参与了细胞凋亡过程,Liu等[16]在脊髓损伤模型上观察到miR-15b与促凋亡基因(PTEN、PDCD4、RAS)和抑制凋亡基因Bcl2的表达相关联,Fu等[17]在肿瘤细胞系上敲低miR-221可以上调Bax并下调Bcl2等凋亡调控基因,并且miR-15b、miR-221/222、miR-29a均存在与钙化相关的报道;(2)miR-25、miR-21、miR-126等12种microRNA参与了炎症反应:文献证实miR-25参与了炎症因子TNFα诱导的血管平滑肌细胞增殖作用[18],miR-21亦被发现与TGF-β信号通路及纤维化过程相关联[19];(3)miR-19a、let-7b/d、miR-24等11种microRNA参与了骨再生:Palmieri等[20]在研究P-15促进骨生成的实验中发现有11种microRNA上调,6种下调,其中包括本研究同样发现的miR-19a、let-7d、miR-221,但其变化方式不同。本研究还发现在0 h与3 h点间下调和3 h与12 h点间上调的microRNA,包括miR-323、miR-326、miR-133b等前后共8种,均激活了脑发育信号通路,这一似乎与血管钙化毫无关系的机制。McCartney等[21]发现多种脑发育过程疾病存在血管钙化,其诱因包括营养不良和血管自身主动钙化,提示血管钙化与脑发育存在某种联系,同时验证本研究中的信号通路分析结果,但对此机制的理解需要更深入地研究。
A, down-regulated microRNA; B, up-regulated microRNA.
图4 3 h与12 h点间被显著激活的信号通路
Figure 4 Signal pathways were significantly activated between 3 and 12 h
本研究发现的6种逐级变化的microRNA中,以往工作已证实miR-29a/b可以通过抑制高磷诱导的ADAMTS-7表达的增加,调控血管钙化[5],ADAMTS-7(a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs-7)是一种含Ⅰ型血小板反应蛋白基序和解聚素的金属蛋白酶,其在离体或在体的血管钙化模型明显上调。过表达或敲低ADAMTS-7能够分别加重或减轻钙化,人类GWAS研究同样证实ADAMTS-7是血管钙化和冠心病的致病基因。ADAMTS-7参与钙化调控机制是通过调节一种叫做软骨寡聚基质蛋白(cartilage oligomeric matrix protein,COMP)的降解来完成的,过表达ADAMTS-7可以促进COMP降解。COMP是一种内源性的血管平滑肌细胞钙化抑制因子,其减少或缺失将导致血管平滑肌细胞失去稳态,造成病理性结果。本课题组曾证实miR-29a/b可以直接作用于ADAMTS-7的3′UTR,抑制其表达,与本研究microRNA microarray结果相一致,验证了结果的可信性,同时提示其他5种microRNA是否同样在钙化调控中起作用。
A, miR-183; B, miR-664; C, miR-9; D, miR-542-5P; E, let-7f; F, miR-29a.
图5 芯片结果中6种在3个时间点逐级上调或下调的microRNA
Figure 5 6 miRs level in A7r5 cell stimulated by high phosphate for 0,3 and 12 h by miR chip screening
[1]唐朝枢,齐永芬. 关注血管钙化发病新机制的研究[J]. 中国医学前沿杂志, 2010, 2(3): 5-8.
[2]朱明燕, 莫中成, 曾高峰. 血管平滑肌细胞增殖相关 micro-RNA 的研究进展[J]. 实用医学杂志, 2013, 29(21): 3610-3612.
[3]Goettsch C, Rauner M, Pacyna N, et al. miR-125b regulates calcification of vascular smooth muscle cells[J]. Am J Pathol, 2011, 179(4): 1594-1600.
[4]Wang H, Peng W, Ouyang X, et al. Reduced circulating mi-R-15b is correlated with phosphate metabolism in patients with end-stage renal disease on maintenance hemodialysis[J]. Ren Fail, 2012, 34(6): 685-690.
[5]Du Y, Gao C, Liu Z, et al. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification[J]. Arterioscler Thromb Vasc Biol, 2012, 32(11): 2580-2588.
[6]Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications[J]. Circ Res, 2006, 99(10): 1044-1159.
[7]Pasquinelli AE, Ruvkun G. Control of developmental timing by micrornas and their targets[J]. Annu Rev Cell Dev Biol, 2002, 18: 495-513.
[8]Cui RR, Li SJ, Liu LJ, et al. MicroRNA-204 regulates vascular smooth muscle cell calcificationinvitroandinvivo[J]. Cardiovasc Res, 2012, 96(2): 320-329.
[9]Qiao W, Chen L, Zhang M. MicroRNA-205 regulates the calcification and osteoblastic differentiation of vascular smooth muscle cells[J]. Cell Physiol Biochem, 2014, 33(6): 1945-1953.
[10]Mackenzie NC, Staines KA, Zhu D, et al. miRNA-221 and mi-RNA-222 synergistically function to promote vascular calcification[J].Cell Biochem Funct, 2014, 32(2): 209-216.
[11]Rangrez AY, M’Baya-Moutoula E, Metzinger-Le Meuth V, et al. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: evidence for the involvement of miR-223[J]. PLoS One, 2012, 7(10): e47807.
[12]Gui T, Zhou G, Sun Y, et al. MicroRNAs that target Ca(2+) transporters are involved in vascular smooth muscle cell calcification[J]. Lab Invest, 2012, 92(9): 1250-1259.
[13]Liao XB, Zhang ZY, Yuan K, et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells[J]. Endocrinology, 2013, 154(9): 3344-3352.
[14]Balderman JA, Lee HY, Mahoney CE, et al. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification[J]. J Am Heart Assoc, 2012, 1(6): e003905.
[15]Wen P, Cao H, Fang L, et al. miR-125b/Ets1 axis regulates transdifferentiation and calcification of vascularsmooth muscle cells in a high-phosphate environment[J]. Exp Cell Res, 2014, 322(2): 302-312.
[16]Liu G, Keeler BE, Zhukareva V, et al. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats[J]. Exp Neurol, 2010, 226(1): 200-206.
[17]Fu B, Wang Y, Zhang X, et al. MiR-221-induced PUMA silencing mediates immune evasion of bladder cancer cells[J]. Int J Oncol, 2015, 46(3): 1169-1180.
[18]Qi L, Zhi J, Zhang T, et al. Inhibition of microRNA-25 by tumor necrosis factor α is critical in the modulation of vascular smooth muscle cell proliferation[J]. Mol Med Rep, 2015, 11(6): 4353-4358.
[19]Dattaroy D, Pourhoseini S, Das S, et al. Micro-RNA 21 inhibition of SMAD7 enhances fibrogenesis via leptin-mediated NADPH oxidase in experimental and human nonalcoholic steatohepatitis[J]. Am J Physiol Gastrointest Liver Physiol, 2015, 308(4): G298-312.
[20]Palmieri A, Pezzetti F, Brunelli G, et al. Peptide-15 changes miRNA expression in osteoblast-like cells[J]. Implant Dent, 2008, 17(1): 100-108.
[21]McCartney E, Squier W. Patterns and pathways of calcification in the developing brain[J]. Dev Med Child Neurol, 2014, 56(10): 1009-1015.
(2015-03-17收稿)
(本文编辑:王 蕾)
Dynamic alteration of microRNA in high phosphorus induced calcification of vascular smooth muscle cell
XIAO Yang, DU Yao-yao, GAO Cheng, KONG Wei△
(Department of Physiology and Pathophysiology, Peking University School of Basic Medical Sciences; China and Key Laboratory of Molecular Cardiovascular Science, Ministry of Education, Beijing 100191, China)
Objective:To study the change of microRNA during the early stage of high phosphorus induced vascular smooth muscle cell (VSMC) calcification and its related mechanism.Methods:Theinvitrocalcification model was created through stimulating VSMC cell line A7r5 with high Pi (2.6 mmol/L) for 7 d. The calcification was validated through ocresolphthalein complexone colorimetry to detect the cellular calcium content, real-time PCR to measure the calcification-related gene expression and alizarin red staining to observe the formation of calcium nodules. Based on the cell calcification model, micro-RNA microarray array was applied to screen the profiles of microRNA expression in VSMC following high Pi stimulation for different periods (0, 3 and 12 h). The array data were analyzed by TAM tool to explore the activated signaling pathway.Results: The calcium content of A7r5 cells induced by high Pi was increased 9.6 times high as cells without Pi treatment (P<0.05). VSMC contractile phenotype genes (SM-α actin, SM22) were down-regulated (P<0.05), while calcification-related genes (BMP2, MSX2, Runx2) were up-regulated (P<0.05) in VSMC stimulated by high Pi. The calcium nodules were obviously formed in cells after 7 d high Pi treatment. In microarray experiment, 680 individual microRNAs were detected in high Pi-treated VSMCs at different time points (0, 3 and 12 h). Among these genes, miR-183, miR-664 and miR-9*were increased whereas miR-542-5P, let-7f and miR-29a were decreased in time-dependent manners. Twenty-six kinds of signaling pathways, including cell apoptosis, differentiation and proliferation, were significantly activated. All these activated pathways were associated with calcification. Conclusion:This study implies that microRNA changed in high Pi-induced VSMCs may involve in the process of calcification.
Vascular calcification; Muscle, smooth, vascular; microRNA; Signal transduction
国家基金委重大国际合作项目 (81220108004)、国家重点基础研究发展计划(973计划,2012CB518002)、 国家自然科学基金 (81070243、81121061、91339000)和国家杰出青年基金 (81225002)资助Supported by the Foundation of Major International Cooperation (81220108004),the National Basic Research Program of China (973 Program, 2012CB518002), the National Natural Science Foundation of China(81070243, 81121061, 91339000) and the National Science Fund for Distinguished Young Scholars (81225002)
时间:2016-5-15 13:25:04
http://www.cnki.net/kcms/detail/11.4691.R.20160515.1325.006.html
R331.3
A
1671-167X(2016)05-0756-10
10.3969/j.issn.1671-167X.2016.05.002
△ Corresponding auther’s e-mail, kongw@bjmu.edu.cn

