Phase transition,electrical and optical switching properties in the well-crystallized VO2(A) nanorods
JIN Cheng,XIONG Kuangwei,ZHANG Hui,JIN Shaowei
(School of Physics and Materials Science,Anhui University,Hefei 230601,China)
Phase transition,electrical and optical switching properties in the well-crystallized VO2(A) nanorods
JIN Cheng,XIONG Kuangwei,ZHANG Hui,JIN Shaowei*
(School of Physics and Materials Science,Anhui University,Hefei 230601,China)
Well-crystallized VO2(A) nanorods were facilely prepared by one-step hydrothermal approach in V2O5-H2C2O4-H2O system.Structure and size of as-obtained products were examined by X-ray diffraction (XRD),scanning and transmission electron microscopies(TEM).DSC curves showed the phase transition of VO2(A) nanorods was 167.8 ℃ upon heating.Varying-temperature XRD patterns showed that the structural transition of the VO2(A) product occurred between 160 ℃ and 180 ℃ on the heating.The magnetic susceptibility exhibited a sudden increase at about 450 K with increasing temperature.The resistance of the VO2(A) sample was measured by four probe method,the hysteresis showed that the electronic phase transition of VO2(A) was strongly first order in nature.The hopping activation energy was calculated based on Arrhenius plot,which was 0.39 eV for the low-temperature VO2(AL),and 0.37 eV for the high-temperature VO2(AH) respectively.Variable-temperature infrared spectra revealed that the VO2(A) nanorods had good optical switching character in the infrared light region,which was concerned with the reversible structure transition of the VO2(A).The research results indicated the VO2(A) nanostructures could be applied in the infrared light switching device.
vanadium oxides;VO2(A) nanorods;hydrothermal synthesis;electrical property;optical property
0 Introduction
Amongst the transition metal compounds,vanadium oxides are the most widely studied materials in recent years as the variety of vanadium-oxygen system inclusive the series of multiple vanadium valencies provide intriguing study within both the theoretical structural and structure-property correlation[1-3].Among them,vanadium dioxides (VO2) has attracted a special interest for researchers because of its polymorphic configurations with multipurpose applications.It is reported that VO2has at least nine polymorphs (except for hydrate) both in the stable and metastable forms,among which the rutile VO2(R)[4],monoclinic VO2(M)[5]and triclinic VO2(T)[6]are similar in structure,other VO2phases designated as tetragonal VO2(A)[7],monoclinic VO2(B)[8],tetragonal VO2(C)[9],monoclinic VO2(D)[10],paramontroseite VO2[11]and VO2with a BCC (body-centered cubic) structure[12].
All of the VO2polymorphs,the VO2(M),VO2(R),VO2(B) and VO2(A) phases are all based on oxygen BCC lattice having the vanadium ions in the octahedral sites,the M,R phases are different than the A,B phases in the light of the mutual orientation of the fourfold axis of the oxygen octahedral.The M,R phases themselves undergo an insulator/metal transition at 340 K,with a many thousand-fold increase in its conductivity[5].This transition has ascribed to paring of vanadium ions in the low temperature monoclinic(M) phase;V4+-V4+ions dimerizing along the c-axis leads to a shift of the π*band away from the Fermi level and removal of the d‖band degeneracy[13].By contrast,the metastable phase VO2(A) also displays a similar reversible phase transition at a temperature about 435 K,has been rarely reported because of the harsh growth conditions.The VO2(A) was first prepared by the hydrothermal reaction of a V2O4-V2O5-H2O system[14],the crystal structure of VO2(A) had not been clarified until 1998.Subsequently,based on the crystal structure,phase transition mechanism and electrical properties of VO2(A) have been studied[15-16],and a crystallographic slip mechanism was proposed to explain the transformation from VO2(B) to VO2(A)[17].The recent interest has also focused on the hydrothermal preparation of VO2(A),the attaching and recrystallization mechanism has been proposed for explaining the formation of VO2(A) nanorods[18],and the structure transition,electrical conductivity and the optical transmittance property in infrared region (IR) were studied limited on the VO2(A) nanostructures[19-20].
In present paper,the well-crystallized VO2(A) nanorods were successfully prepared by one-step hydrothermal method at 230 ℃ for 24 h under a 1∶1.5 molar ratio of V2O5to oxalic acid.To clarify the structure phase transition,electrical and magnetic properties,and optical switching properties in the VO2(A) nanorods,the varying-temperature XRD,the temperature dependence of susceptibility and resistivity,as well as the infrared spectra were performed for the VO2(A) nanorods.Our results indicated that the phase transition from the low-temperature VO2(AL) phase to the high-temperature VO2(AH) phase is about 167.8 ℃ on heating step,the electronic phase transition is strongly first order in nature for the VO2(A) nanorods.The hopping activation energy of the VO2(A) was also calculated based on Arrhenius plot.A reversible optical switching was observed in infrared light region from 680 cm-1to 660 cm-1for the VO2(A) nanorods.
1 Experimental section
1.1 Sample preparation
All chemical reagents used in the experiments,including vanadium pentoxide (V2O5) and oxalic acid di-hydrate (H2C2O4·2H2O) were of analytical grade without further purification.In a typical hydrothermal process,300 mg of V2O5and 312 mg of H2C2O4·2H2O powders were dispersed into 20 mL of the distilled water under magnetic stirring to produce a clear,blue solution.After,20 mL mixed solution was transferred into a Teflon-lined autoclave (50 mL) with stainless steel shell (the filling ratio is 40%),which was sealed and sustained at 230 ℃ holding for 24 h,then cooled to room temperature naturally.The final precipitates were collected by filtering,washed with distilled water and ethanol alternately,and then dried in air at 80 ℃ for 10 h.
1.2 Characterization and measurements
The morphologies and sizes of the resulted product were observed by a field-emission scanning electron microscopy (FESEM,S-4800) at an acceleration voltage of 10 kV,and a transmission electron microscope (TEM,JEOL-2010,JAP) operated at 200 kV.The crystal structure of the as-obtained product was examined by X-ray diffraction (XRD) with Philips X’Pert diffractometer (Cu Kα,λ=1.540 6 Å),the operating voltage and current were kept at 40 kV and 30 mA.In order to study the phase transition of the VO2(A),we carried out the differential scanning calorimetry (DSC,Q2000) measurements under nitrogen atmosphere over a temperature range from 20 ℃ to 200 ℃ along heating/cooling cycles.The magnetic susceptibility of the VO2(A) nanorods was measured from 300 K to 560 K under the heating/cooling cycle using a quantum design physical measurement system (PPMS).The temperature dependent of the resistivity was carried out on the as-pressed pellet of the VO2(A) product in argon ambient.To press a pellet [diameter(Φ)1.35 cm1 mm],the mass of 500 mg VO2(A) nanopowders was applied,four electric contacts were established using silver epoxy.The optical transmittance properties of the VO2(A) products were measured by Fourier transform infrared spectroscopy (FT-IR,Nicolet 8700) at various temperatures with an adapted heating controlled cell and over a range from 400 cm-1to 4 000 cm-1with a resolution of 4 cm-1.
2 Results and discussion
2.1 Structure and morphological
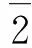
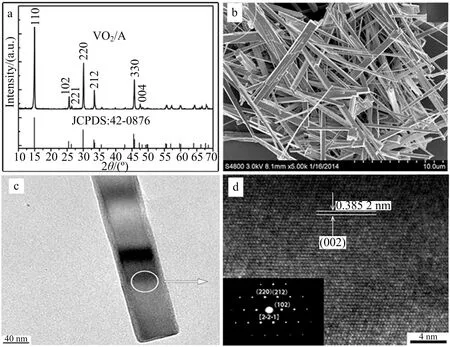
Fig.1 XRD pattern(a),overview SEM image (b) of VO2(A) nanorods that were obtained by hydrothermal reaction at 230 ℃ for 24 h under a molar ratio (1∶1.5) of V2O5 to oxalic acid,TEM image (c) for a single VO2(A)nanorod,high-resolution TEM image (d) of the circular area in Fig.1c
2.2 Phase transition
By means of the driving force of increasing temperature,the first-order phase transition from the primitive tetragonal VO2(A) to the high-temperature body-centered tetragonal VO2(AH) phase usually involves a substantial entropy component.For the VO2(A),the phase transition is due to slight deviation of the V4+-V4+bond length on heating step,which is similar to that change from rutile VO2(R) to monoclinic VO2(M)[7,15].Fig.2a shows the XRD patterns of the as-obtained VO2(A) product,it can be seen that the shape of the XRD patterns has almost no change with temperature except the slight shift of certain peaks and disappearance of (211) and (311) diffraction peaks above the transition temperature (>160 ℃),indicating that the crystal structure does not change drastically but the lattice spacing along the c-axis is halved through the transition[16].As shown in Fig.2b,the lattice parameters of the a-axis and c-axis show a non-linear increase and decrease,respectively with the increasing temperature.The abrupt change of lattice parameters above the critical temperature (>160 ℃) is expected in the VO2(A) nanorods,indicating a structure transition occurs from a low-temperature primitive tetragonal VO2(AL) to a high-temperature body-centered tetragonal VO2(AH) phase[16].
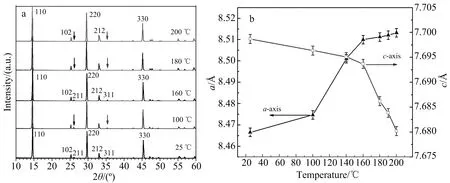
Fig.2 Variable-temperature XRD patterns (a) of VO2(A) nanorods,the disappearance peaks of (211) and (311)were marked by arrows,temperature dependence (b) of the lattice parameters of the VO2(A) nanorods
Fig.3 shows the differential scanning calorimetry (DSC) curves for the as-prepared VO2(A) nanorods.In the DSC scan,the thermal behavior of the VO2(A) sample was characterized when it was heated (from 20 ℃ to 200 ℃) and then cooled at 10 ℃·min-1.During the heating step,a single endothermic peak was observed at 167.8 ℃,this can be assigned to conversion of the primitive tetragonal VO2(A) into body-centered tetragonal VO2(AH).On cooling,a wide exothermic peak was recorded at 134 ℃,which is ascribed to the transformation of the VO2(AH) into the VO2(AL) form.A similar results was also reported in the VO2(A) nanobelts,where solid-solid transition from VO2(AL) to VO2(AH) occurred at fast heating/cooling rates .In this work,the endothermic peak on heating occurred at 167.8 ℃ which is higher than that datum (162 ℃) of Oka’s report[16].The endothermic peak of 167.8 ℃ in the DSC curve is close to that value of the Ref.[21],the exothermic peak is about 34 ℃ lower than the endothermic peak.This higher transition temperature (167.8 ℃) and a wide hysteresis loop in DSC curve could be considered to be due to the nonstoichiometry in the VO2(A) nanorods and/or the scaling to nanoscale dimensions,as described in VO2(M) nanostructures[21].

Fig.3 DSC curves of the VO2(A) nanorods on the heating and cooling cycle
To further study the phase transition of the VO2(A) nanorods,the susceptibility as a function of temperature was also recorded when it was heated (from 300 K to 560 K) and then cooled (from 560 K to 300 K) under a 10 000 Oe field,as shown in Fig.4.The magnetic susceptibility on heating showed a sudden increasing at about 450 K.On the cooling it gradually decreased to the starting value and showed a large hysteresis.The magnetic hysteresis is closely concerned with the reversible structural transition of the VO2(A).The susceptibility change on heating showed a striking resemblance to the case of the VO2(R)[22].The decrease of susceptibility with reducing temperature can be ascribed to the dimerization of the V—V pairs which leads to formation of the non-magnetic V4+-V4+bonding in low temperature phase of VO2(A)[16,23].
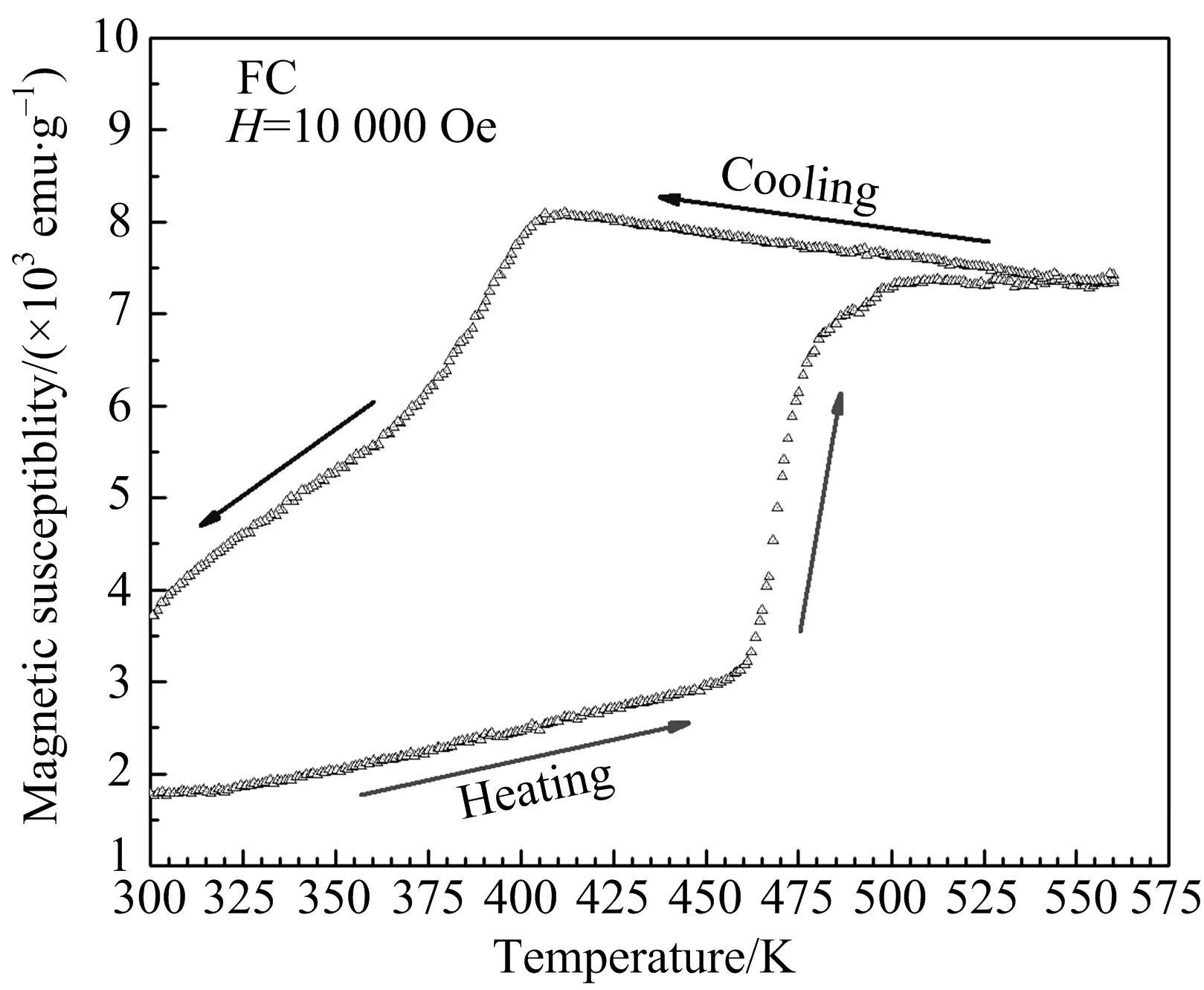
Fig.4 Susceptibility as a function of temperature was measured for the VO2(A) nanorods under a 10 000 Oe field over the temperature range of 300 K to 560 K
2.3 Electrical and optical switching features
To study electrical property of the VO2(A),we carried out the resistance measurement on the as-pressed pellet of the VO2(A) powders using the silver epoxy as four contacts (Fig.5a).The temperature dependent resistivity showed a semiconducting behavior over the temperature range of 293—483 K.As shown in Fig.5a,the pronounced hysteresis is observed,indicating that the electronic transition of the VO2(A) is strongly first order in nature.The electrical resistivity seems to rather high because it measured on an as-pressed pellet of the VO2(A) powders not sintered.The resistivity curve on heating exhibited a clear deflection at the transition temperature (Tc1=430 K),indicating that high-temperature phase (HTP) becomes more conductive than low-temperature phase (LTP) of the VO2(A).Also several tens-fold decrease of the resistivity is observed through the phase transition on the heating cycle,it is dissimilar to that of the rutile VO2(R)[5].To illustrate the electrical transition,the differential of logarithm of the resistivity (logρ )for the pressed VO2(A) sample was also plotted,as an inset in Fig.5a.The insets in Fig.5a correspond to the differential of logρ on the heating (right top) and cooling (left below).It is also noted that the differential of logρ on the heating (right inset of Fig.5a) displays a periodic jump around the transition temperature of Tc1,but it is not occurred upon the cooling cycle (left inset).A plausible reason for this difference in the VO2(A) may be twinning domains which are induced on the LTP to HTP transition but disappears on the reverse transition[16].
To understand the electrical conductivity of the VO2(A),direct current (DC) conductivity as a function of inverse temperature was depicted in Fig.5b.Mott had proposed an optical phonon assisted hopping model of the small polar to explain conductivity of the transition metal oxides[24-25],where the small polar hopping induced conductivity at high temperature (T>180 K) is given by
(1)
or
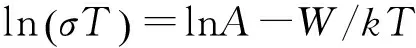
(2)
where σ is the polar hopping induced conductivity,ν0is longitudinal optical phonon frequency,α is the rate of wave-function decay,R is the average hopping distance,c is the fraction of sites occupied by electrons or polarons,A is the fixed numerical calculated by constant above and W is the activation energy (i.e.energy barrier) which hinder the electrons in order to hop to nearest neighbour site.Fig.5b displays the plot of ln(T/R) versus reciprocal temperature on the heating cycle.According to the slope of the linear fitting curve in high temperature range (293—483 K),we evaluated the activation energy W of the VO2(A) nanorods is 0.39 eV for the low-temperature VO2(AL) and 0.37 eV for the high-temperature phase VO2(AH) ,respectively.The energy of 0.39 eV is larger than the reported energy of 0.28 eV in the individual nanobelt of the VO2(A)[26].This disparity in the activation energy is considered to be caused by the different measurement ways (the pressed powders vs.an individual nanobelt).Above results indicated that the electrons (or polarons) hopping to the nearest neighbour site are more easy for the high-temperature VO2(AH) form,while the VO2(AH) phase is more conductive than the VO2(AL).

Fig.5 A plot (a) of resistivity versus temperature for the VO2(A) sample during the heating and then cooling,DC conductivity (b) as a function of T-1 on the heating cycle
Contrast with the rutile VO2(R),the optical properties of the VO2(A) nanostructures in infrared region (IR) are limited reported.In our work,a series of infrared spectra of the VO2(A) product with various temperatures were shown in Fig.6.The infrared spectra exhibited the existence of varying vibrations of V—O bonds.The 935 cm-1band observed for the VO2(A) is attributed to the stretching of the short V=O bonds[27].The vibration band at 600 cm-1to 550 cm-1can be described as the delocalization of the electrons involving in the V4+-V4+bonds between VO6octahedral[16].The bands at 675 cm-1and 428 cm-1are attributed to the stretching vibrations of V—O—V bonds gradually disappear with increasing temperature[28],which is a directly evidence for the occurrence of the first order phase transition in the VO2(A) nanorods.The infrared spectra in Fig.6b displays a clear process of the phase transition of the VO2(A) before and after the temperature of phase transiting (Tc),indicating the phase transition occurred at 170 ℃ in heating cycle,this is consistent with the results of DSC and XRD.The VO2(A) product has optical switching property at absorption bands from 680 cm-1to 660 cm-1,suggesting the VO2(A) can be applied in many optical devices,such as infrared light switching device,optical data storage and so on.In Fig.6c,two IR curves below Tc(one is from the heating process,the other is from cooling) are basically coincided,revealing the phase transition of VO2(A) nanorods is reversibility.

Fig.6 Variable-temperature infrared spectra of the VO2(A) nanoeods:all of IR curves (a) with various temperatures,typical IR curves (b) to reveal the phase transition of the VO2(A) before and after Tc,three IR curves (c)
3 Conclusion
The well-crystallized VO2(A) nanorods were facilely prepared via hydrothermal approach based on the reduction of V2O5by oxalic acid.An endothermic peak at 167.8 ℃,a wide exothermic peak at 134 ℃ was detected in the DSC curve of the VO2(A) nanorods.An expanded a-axis and contracted c-axis was evidenced with increasing temperature.The susceptibility on heating displayed a sudden increasing at 450 K,the decrease of susceptibility with reducing temperature is ascribed to the dimerization of V—Vpairs which lead to formation of the non-magnetic V4+-V4+bonding in low-temperature phase VO2(AL).The resistivity showed a pronounced hysteresis,indicating that the electronic transition of VO2(A) is strongly first-order in nature.The hopping activation energy for the low- and high-temperature phase of VO2(A) is evaluated to be 0.39 eV and 0.37 eV,respectively.The infrared spectra results suggested the VO2(A) nanostructures could be employed as the infrared light switching materials.
[1] PARK J H,COY J M,KASIRGA T S,et al.Measurement of a solid-state triple point at the metal-insulator transition in VO2[J].Nature,2013,500:431-434.
[2] ROZANSKA X,FORTRIE R,SAUER J.Size-dependent catalytic activity of supported vanadium oxide species:oxidative dehydrogenation of propane[J].J Am Chem Soc,2014,136 :7751-7761.
[3] WEI J,JI H,GUO W,et al.Hydrogen stabilization of metallic vanadium dioxide in single-crystal nanobeams[J].Nat Nanotechnol,2012,7:357-362.
[4] EYERT V,HOCK K H.Electronic structure of V2O5:role of octahedral deformations[J].Phys Rev B,1998,57:12727-12737.
[5] MORIN F J.Oxides which show a metal-to-insulator transition at the Neel temperature[J].Phys Rev Lett,1959 ,3 (1):34-36.
[6] MATSUISHI T.On the phase transformation of VO2[J].Jpn J Appl Phys,1967,6:1060-1071.
[7] YAO T,OKA Y,YAMAMOTO N.Powder X-ray crystal structure of VO2(A)[J].J Solid State Chem,1990,86:116-124.
[9] HAGRMAN D,ZUBIETA J,WARREN C J ,et al.A new polymorph of VO2prepared by soft chemical methods[J].J Solid State Chem,1998,138:178-182.
[10] LIU L,CAO F,YAO T,et al.New-phase VO2micro/nanostructures:investigation of phase transformation and magnetic property[J].New J Chem,2012,36:619-625.
[11] WU C,ZHU Z P ,WANG W,et al.Synthetic paramontroseite VO2with good aqueous lithium-ion battery performance[J].Chem Commun,2008,39:3891-3893.
[12] WANG Y Q,ZHANG Z J,ZHU Y,et al.Nanostructured VO2photocatalysts for hydrogen production[J].ACS Nano,2008,2:1492-1496.
[13] ZYLBERSZTEJN A,MOTT N F.Metal-insulator transition in vanadium dioxide[J].Phys Rev B,1975,11:4383-4386.
[15] YAO T,OKA Y,YAMAMOTO N.A structural study of the high-temperature phase of VO2(A)[J].J Solid State Chem,1994,112:196-198.
[16] OKA Y,SATO S,YAO T,et al.Crystal structures and transition mechanism of VO2(A)[J].J Solid State Chem,1998,141:594-598.
[17] GALY J.A proposal for VO2(B)⟹VO2(A) phase transition:a simple crystallographic slip[J].J Solid State Chem,1999,148:224-228.
[18] WEI N,JIN C,XIONG K W,et al.Hydrothermal synthesis,growth mechanism and optical property of VO2(A) nanorods[J].Journal of Anhui University (Natural Science Edition),2016,40 (1):42-49.
[19] ZHONG Y L,ZHANG Y F,LIU X,et al.Synthesis of VO2(A) nanostructures by a hydrothermal method and their transition to VO2(M)[J].Adv Mater Res,2011,295-297:368-372.
[20] LIU P C,ZHU K J,GAO Y F,et al.Ultra-long VO2(A) nanorods using the high-temperature mixing method under hydrothermal conditions:synthesis,evolution and thermochromic properties[J].CrystEngComm,2013,15:2753-2760.
[21] JI S D,ZHANG F,JIN P.Selective formation of VO2(A) or VO2(R) polymorph by controlling the hydrothermal pressure[J].J Solid State Chem,2011,184:2285-2292.
[22] TAKAHASHI K,YASUOKA H,UEDA Y,et al.NMR Studies of VO2and V1-xWxO2[J].J Phys Soc Jpn,1983,52:3953-3959.
[23] WHITTAKER L,JAYE C,FU Z,et al.Depressed phase transition in solution-grown VO2nanostructures[J].J Am Chem Soc,2009,131:8884-8894.
[24] LI L,FANG X,ZHAI T,et al.Electrical transport and high-performance photoconductivity in individual ZrS2nanobelts[J].Adv Mater,2010,22:4151-4156.
[25] PARK J,LEE E,LEE K W,et al.Electrical transport and quasipersistent photocurrent in vanadium oxide nanowire networks[J].Appl Phys Lett,2006,89:183114.
[26] LI M,KONG F Y,LI L,et al.Synthesis,field-emission and electric properties of metastable phase VO2(A) ultra-long nanobelts[J].Dalton Trans,2011,40:10961-10965.
[27] VALMALETTE J C,GAVARRI J R.High efficiency thermochromic VO2(R) resulting from the irreversible transformation of VO2(B)[J].Mater Sci Eng B,1998,54:168-173.
[28] HOU J W,ZHANG J W,WANG Z P,et al.The phase transition of W-doped VO2nanoparticles synthesized by an improved thermolysis method[J].J Nanosci Nanotechnol,2013,13:1543-1548.
(责任编辑 郑小虎)
良好结晶VO2(A)纳米杆的相变、电学和光转变特性
金 诚,熊狂炜,张 惠,金绍维*
(安徽大学 物理与材料科学学院,安徽 合肥 230601)
在VO2-草酸体系中,利用一步水热合成法制备结晶良好的VO2(A)纳米杆.成品的结构和尺寸分别通过X射线衍射(XRD)、扫描电镜(SEM)和透射电子显微镜(TEM)表征.差示扫描量热(DSC)曲线显示在加热过程中VO2的相转变温度为167.8 ℃.变温X射线衍射(XRD)图谱显示加热时VO2(A)在160~180 ℃发生相变.温度升高到450 K时,磁化率突然增加.使用4探针法测量VO2(A)样品的电阻率,滞后现象显示VO2(A)的相变为1级相变.根据阿仑尼乌斯曲线,得出低温VO2(AL)和高温VO2(AH)的活化能分别为0.39 eV和0.37 eV.变温红外光谱显示VO2(A)纳米杆在红外区域具有良好的光学转换特性,此特性与VO2(A)的可逆结构转变有关.研究结果表明VO2(A)纳米材料可应用于红外开关装置.
钒氧化物;VO2(A)纳米杆;水热合成;电学性质;光学性质
10.3969/j.issn.1000-2162.2016.06.008
Received date:2016-03-11
Supported by the National Science Foundation of China(11174001,51402002)
Author’s brief:JIN Cheng(1990-),male,born in Anqing of Anhui Province,master degree candidate of Anhui University;*JIN Shaowei (corresponding author),professor of Anhui University,doctoral student supervisor,E-mail:jinsw@mail.ustc.edu.cn.
O614.51 Document code:A Article ID:1000-2162(2016)06-0037-09

