Transplantation of neural progenitor cells differentiated from adipose tissue-derived stem cells for treatment of sciatic nerve injury
Shasha Dong, Na Liu, Yang Hu (✉), Ping Zhang, Chao Pan, Youping Zhang, Yingxin Tang, Zhouping Tang (✉)
Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China§These authors contributed equally to this work.
Transplantation of neural progenitor cells differentiated from adipose tissue-derived stem cells for treatment of sciatic nerve injury
Shasha Dong§, Na Liu§, Yang Hu (✉), Ping Zhang, Chao Pan, Youping Zhang, Yingxin Tang, Zhouping Tang (✉)
Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
§These authors contributed equally to this work.
ARTICLE INFO
Received: 20 May 2016
Revised: 27 May 2016
Accepted: 30 May 2016
© The authors 2016. This article
is published with open access
at www.TNCjournal.com
adipose tissue-derived stem cells;
sciatic nerve injury;
cell transplantation;
lentivirus carrying GFP
Objectives:Currently, the clinical repair of sciatic nerve injury remains difficult. Previous studies have confirmed that transplantation of adipose tissue-derived stem cells promotes nerve regeneration and restoration at peripheral nerve injury sites.
Methods: In this study, adipose tissue-derived stem cells were induced to differentiate into neural progenitor cells, transfected with a green fluorescent protein-containing lentivirus, and then transplanted into the lesions of rats with sciatic nerve compression injury.
Results: Fluorescence microscopy revealed that the transplanted cells survived,migrated, and differentiated in rats. At two weeks post-operation, a large number of transplanted cells had migrated to the injured lesions; at six weeks post-operation, transplanted cells were visible around the injured nerve and several cells were observed to express a Schwann cell marker. Sciatic function index and electrophysiological outcomes of the transplantation group were better than those of the control group. Cell transplantation promoted the recovery of motor nerve conduction velocity and compound muscle action potential amplitude, and reduced gastrocnemius muscle atrophy.
Conclusions:Our experimental findings indicate that neural progenitor cells,differentiated from adipose tissue-derived stem cells, are potential seed stem cells that can be transplanted into lesions to treat sciatic nerve injury. This provides a theoretical basis for their use in clinical applications.
1 Introduction
Sciatic nerve injury is often caused by acute or chronic compression or cutting, as a result of accidents, knife injury, labor, and local muscle injection. Complete sciatic nerve transection injury results in muscle paralysis of the posterior femoral muscle, legs, and feet, hinders knee flexion, and reduces the motor function of ankle joint and toes. There is also a loss of sensory and nutritional function of the sciatic innervated area. These dysfunctions greatly reduce patient quality of life, increase labor loss and medical expenses, and heavily burden society and the patient's family. The existing treatment for sciatic nerve injury includesmedication with glucocorticoids, surgery, and physical therapy; however, these treatments fail to regenerate the injured nerve and to achieve an ideal therapeutic outcome[1]. Therefore, nerve regeneration and functional restoration following sciatic nerve injury are of priorities in research and clinical treatment[2].
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells isolated from fat tissue, with multi- differentiation potential. ADSCs have self-proliferation ability and can differentiate into mesodermal cell types, such as osteoblasts, adipocytes, chondrocytes, and myofibroblasts[3]. ADSCs express the markers of mesenchymal stem cells, including CD29, CD44, CD54, CD90, CD105, and stro-1, but do not express the markers of hematopoietic stem cells, CD14 and CD45[4, 5]. Increasing evidence has demonstrated that ADSCs also have the ability to differentiate into other cell types, including nerve cells, myocardial cells, liver cells, pancreatic cells, skeletal muscle cells, and vascular cells[6, 7]. The use of ADSCs has several advantages, including a convenient source, easy isolation and culture, strong and rapid proliferation, slow aging, and low immunity, which suggest great promise for their use as seed cells for transplantation treatment of various systemic diseases[8-11].
Our preliminary study demonstrated that rat ADSCs efficiently differentiated into neural progenitor cells and highly expressed nestin, a marker of neural stem cells[12]. Subsequent research revealed that ADSCs induced by different methods differentiated into ectodermal neural spheres[13, 14]and neuron-like cells[15]in vitro. The induced neural spheres exhibit similar morphology as brain-derived neural stem cells, and express nestin, neuron-specific enolase, neuron-specific nuclear protein, and glial fibrillary acidic protein (GFAP), a glial cell marker[3, 16]. Undifferentiated ADSCs were observed to promote axonal and myelin regeneration, and reduce muscular atrophy in rat models of peripheral nerve injury by a mechanism that may be dependent on the secretion of neurotrophic factors, such as nerve growth factor and brain-derived neurotrophic factor[17]. In addition, after ADSCs were induced to differentiate into Schwann cell-like cells and then transplanted into peripheral nerve injury models, they promoted axonal growth and nerve repair[18, 19]. However, few studies have focused on the efficacy of ADSC-derived neural progenitor cell transplantation for the treatment of sciatic nerve injury. In this study, ADSCs were transfected with a green fluorescent protein (GFP)-containing lentivirus, and were induced to differentiate into neuron-like progenitor cells. Then, the differentiated cells were injected into rats with sciatic nerve crush injury to observe their efficacy in the treatment of sciatic nerve injury.
2 Methods
2.1 Experimental animals
Twelve female Sprague-Dawley rats, aged 3-4 weeks, of clean grade, weighing 100-120 g, were provided by the Hubei Provincial Center for Disease Control and Prevention (Wuhan, Hubei Province, China). Forty-two male Sprague-Dawley rats, aged 8-10 weeks, of clean grade, weighing 230-280 g, were provided by the Experimental Animal Center of Tongji Hospital (Wuhan, Hubei Province, China). All experimental protocols were in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals, formulated by the Ministry of Science and Technology of China.
2.2 Isolation and preparation of ADSCs
The female Sprague-Dawley rats were sacrificed by decapitation under anesthesia and disinfected with 75% alcohol for 10 min. The inguinal skin was shaved and cut, and the fat tissue was isolated and rinsed with phosphate buffered saline (PBS) in a petri dish. Then, the fat tissue was transferred to a PBS-free petri dish and cut into pieces using scissors. Samples were digested with type I collagenase in a centrifuge tube at a volume ratio of 1:1 for 40 min at 37°C. Throughout the digestion, tissues were shaken and mixed two or three times to aid digestion. After digestion, Dulbecco's modified Eagle's medium (DMEM)/F12 medium containing 10% fetal bovine serum (HyClone, Logan, UT, USA) was added, and the samples were triturated and centrifuged at 1,100 rpm for 10 min. The supernatant was discarded, and fresh culture medium was added to the fat tissue prior to an additional centrifugation at 1,100 rpm for 5 min. The supernatant was discarded once more, and the fat tissue sample was resuspended in DMEM/F12 medium containing 10%fetal bovine serum, filtrated with a 40-μm mesh, and incubated in a 25 cm2culture flask at 37°C in a 5% CO2incubator. When cells reached 80%-90% confluence after seven days, cells were digested with trypsin containing EDTA (Genom, Hangzhou, China) for 2 min. When cells became round, the digestion was terminated with DMEM/F12 medium containing 10% fetal bovine serum, after which the cells were subcultured.
2.3 Labeling of ADSCs
ADSCs at passage two and three were incubated in three wells of a 6-well plate at a density of 2 × 105cells/9.6 cm2. ADSCs were transfected after complete adhesion. Cells in two wells were incubated with 25 μL lentivirus carrying GFP (Cyagen, Biosciences Inc, CA, USA) at 37°C in a 5% CO2incubator for 8 h. After transfection, complete culture medium containing the lentivirus was blotted with a pipette and rinsed twice with PBS. The culture medium was replaced with DMEM/F12 medium containing 10% fetal bovine serum, and cells were incubated at 37°C in a 5% CO2incubator. At 48 h post-transfection, the transfection efficacy was observed and cells were subcultured at a ratio of 1:2.
2.4 Differentiation of ADSCs
The GFP-labeled ADSCs at passage five were induced to differentiate into neural progenitor cells as we previously described[12], and digested with EDTA- containing trypsin. The digestion was terminated with ADSC culture medium. Then, cells (106cells/mL) were centrifuged at 800 rpm for 10 min and resuspended in induction medium. Cells were further incubated in a culture flask at 37°C in 5% CO2. Epidermal growth factor (Pepro Tech, Inc., Rocky Hill, NJ, USA) and fibroblast growth factor-2 (.) were added to the culture medium every day. The quantity and size of neural spheres were observed under an inverted phase contrast microscope every day. At 3-4 days post-induction, neural progenitor-like cells were removed from the incubator and centrifuged at 800 rpm for 6 min. Harvested cells were digested with 0.5 mL of 0.25% trypsin containing 0.02% EDTA for 30-60 s; 1.5 mL culture medium was added to terminate the digestion. Cells were pipetted into a single cell suspension and the number of cells/2 mL was calculated. After centrifugation, the cell density was adjusted to 2 × 107/mL using culture medium.
2.5 Immunofluorescence analysis
At four days post-induction, neural progenitor cells were fixed in 4% paraformaldehyde (pH 7.4) at room temperature for 30 min, washed three times with PBS, and incubated at room temperature for 15 min. After an additional PBS wash, cells were blocked for 1 h, incubated with rabbit-anti-nestin monoclonal antibody (1:150; Sigma Aldrich, St. Louis, MO, USA) at 4°C overnight, and then incubated with goat-anti rabbit- Cy3 antibody (1:200; Earthox, CA ,USA) in the dark for 1 h. Between all steps, cells were washed three times with PBS. The cell nuclei were stained with DAPI (Wuhan Google Biotechnology Co., Ltd., Wuhan, Hubei Province, China) for 10 min, washed three times with PBS, and then observed under a fluorescent microscope.
2.6 Surgery
Forty-two Sprague-Dawley rats were randomly divided into two groups of 21 rats: a cell transplantation group and a control group. Sciatic nerve crush injury was performed according to the method of Santiago et al. with some modifications[17]. Sprague-Dawley rats were anesthetized with 10% chloral hydrate (0.5 mL/100 g) via intraperitoneal injection, and fixed in the prone position. A 20 mm oblique incision was made at the right posterior thigh under sterile conditions and bluntly separated along the biceps femoris and vastus lateralis, exposing and freeing the sciatic nerve. The sciatic nerve shaft was clamped for 30 s at 5 mm lateral to the inferior margin of the piriformis, producing a 2 mm crush injury area. All operations were performed by the same individual. The model was defined successful upon the transparent appearance of the clamped nerve stem under the microscope and the disappearance of the unfolded paw reflex after awakening.
In the transplantation group, a 5 μL suspension containing the neural progenitor cells differentiated from ADSCs (2 × 107/mL) was injected in the injured sciatic nerve of rats by using a micro-syringe and a surgical microscope. The injection lasted 5 min; then, the micro-syringe was withdrawn, the sciatic nerve was replaced to its original location, and the muscles and skin were sutured and disinfected. In the control group, rats were injected with an equal volume of suspension without cells.
2.7 Functional assessment
A self-made footprint walking box was used (length 50 cm, width 10 cm, height 10 cm) with white paper at the bottom. Rats were allowed a unidirectional walk in the box every week after operation, after their bilateral metapedes were dipped in red link. The numbers of clear podograms were recorded and the following indexes were measured: EPL, experimental print length (from the third toe to the heel); NPL, normal print length; ETS, experimental toe spread (from the first toe to the fifth toe); NTS, normal toe spread; EIT, experimental intermediary toe spread (from the second toe to the fourth toe); and NIT, normal intermediary toe spread. Two or three clear footprints selected from each rat were used to calculate the mean value of each index. The sciatic nerve function index (SFI) of rats in each group was calculated according to the Bain formula[18, 19]: SFI = [-38.3 × (EPL - NPL)/NPL] + [109.5 × (ETS - NTS)/NTS] + [13.3 × (EITS - NITS)/NITS] - 8.8. SFI has a range of 0 to -100, where 0 indicates normal function and -100 represents complete nerve transection. The SFI of rats in the two groups was calculated and recorded each week after surgery, for a total of six weeks.
2.8 Electrophysiological study
Three rats in each group were randomly selected at 1-6 weeks after surgery. Rats were anesthetized with 10% chloral hydrate by intraperitoneal injection and fixed in the prone position. A recording electrode with a 0.3 mm diameter monopolar needle was placed in the plantar muscle belly, while a reference electrode was placed on the Achilles tendon. The anode and cathode of the distal stimulus electrode were placed at the superior and inferior edges of the popliteal fossa, at an interval of 0.5 cm. The anode and cathode of the proximal stimulus electrode were placed at the gluteus maximus, at an interval of 0.5 cm. A Type Nicolet- Viking-Select evoked potential instrument (Nicolet EDX, CA, USA) was used. Pulses were delivered at 20-25°C at a scanning speed of 5 ms/Div, pulse width of 0.1 ms, and frequency of 1 Hz. Electrophysiological determinations were also performed by the same physician. The motor conduction velocity (MCV) and compound muscle action potential (CMAP) of bilateral sciatic nerves were recorded. The ratio of MCV and CMAP of the lesion side compared to that of the normal side was calculated as the recovery rate of the sciatic nerve for a total of six weeks.
2.9 Wet weight ratio of gastrocnemius muscle
At six weeks post-transplantation, rats were anesthetized and fixed in a prone position. The bilateral gastrocnemius muscle was isolated in rats of both groups. The wet weight of the gastrocnemius muscle was measured on a precise electronic balance. The wet weight ratio of the gastrocnemius muscle = (wet weight of the right gastrocnemius muscle)/(wet weight of the left gastrocnemius muscle) × 100%.
2.10 Survival and differentiation of transplanted cells by histological detection
At day 1, and weeks 1-6 post-transplantation, three rats in each group were selected and anesthetized. The right sciatic nerve was isolated, fixed in isopentane for 10 min and stored in a -80°C freezer. Sciatic nerve specimens were sliced into 40 longitudinal frozen sections at 6 μm thickness. In this experiment, GFAP was used as a marker of differentiated Schwann cells. Slices were fixed in 4% paraformaldehyde for 15 min, rinsed three times with PBS for 5 min, and incubated with 30 μL permeabilization solution at 37°C for 15 min. The slices were then washed with PBS and incubated with 30 μL blocking solution at 37°C for 1 h and 30 μL rabbit anti-rat GFAP antibody (1:1,000; Abcam, Cambridge, UK) at 4°C overnight. After another PBS wash, slices were incubated with goat anti-rabbit Cy3 antibody (1:200; Earth, USA) at room temperature in the dark for 1 h. Samples were stained with 30 μL DAPI for 15 min.
2.11 Statistical analysis
Data are expressed as the mean ± SD. SPSS 17.0 software was used for t-tests. The resulting correlation was analyzed using linear correlation analysis. A P < 0.05 value was considered statistically significant.
3 Results and discussion
3.1 Culture and GFP-expressing lentiviral transfection of ADSCs
After seven days of culture, ADSCs began to adhere and exhibit fibroblast-like morphology (Figure 1a).After ADSCs were transfected with the GFP-expressing lentivirus for 48 h, fluorescent expression was observed. Microscopy revealed that transfection efficacy was good and the GFP expression rate was 85% (Figure 1b).
3.2 Induction and identification of ADSCs
After ASDCs had been cultured in the induction medium for one day, neurosphere-like neural progenitor cells were observed. Over the next five days, the number and size of neurospheres increased, and the fluorescent intensity did not observably decrease (Figure 2a). Neural progenitor cells were detected by immunofluorescence analysis, which revealed that cells expressed the neuronal marker, nestin (Figure 2b).
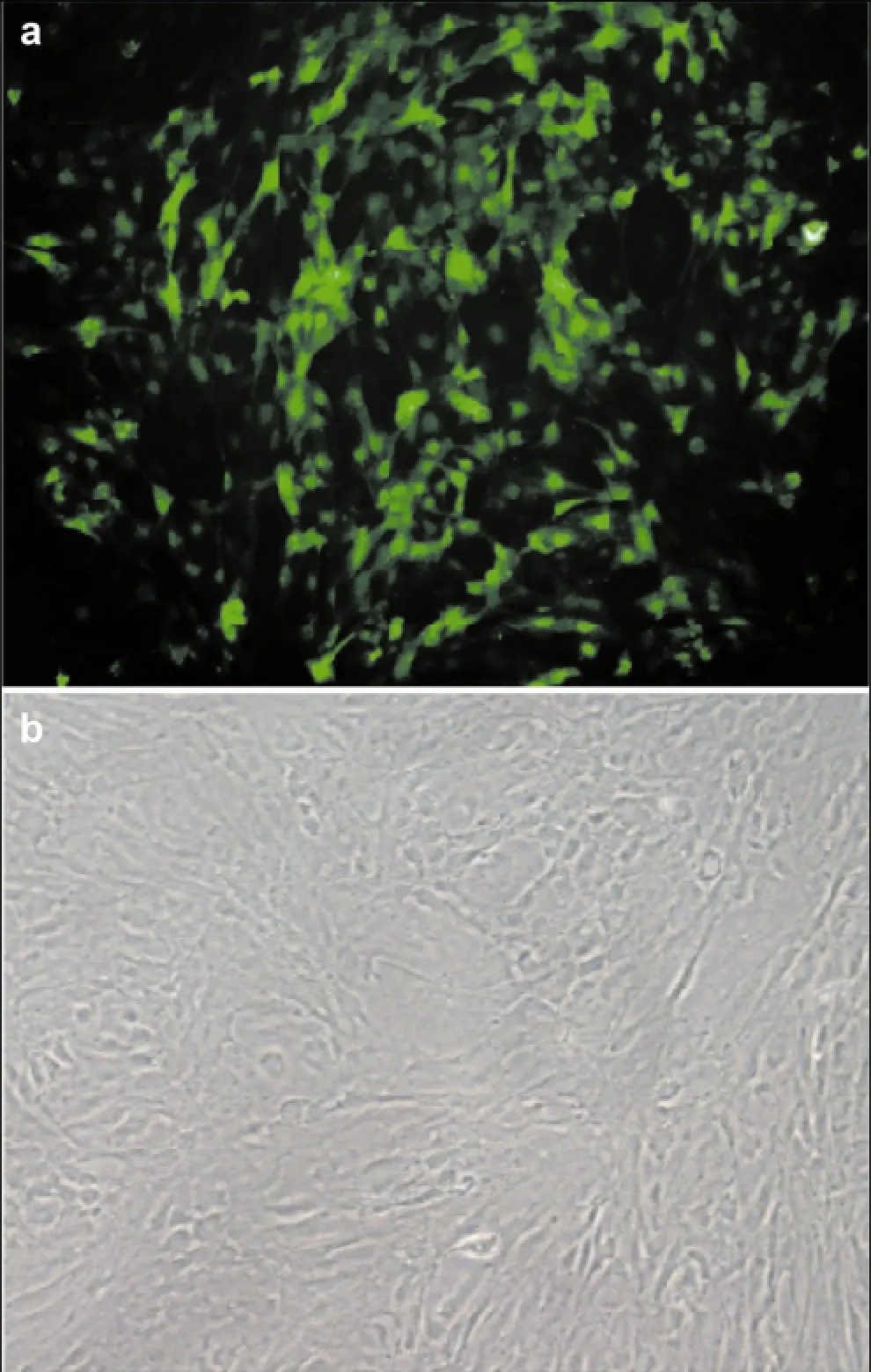
Figure 1 GFP expression in ADSCs infected with a GFP- expressing viral vector. (a) After seven days of culture, adipose- derived stem cells began to adhere and exhibit fibroblast-like morphology (200× magnification). (b) Adipose-derived stem cells were transfected with the GFP-containing lentivirus for 48 h (200× magnification).
3.3 Transplantation of neural progenitor cells promotes nerve regeneration after injury
The regeneration of sciatic nerve function after surgery was assessed using the SFI. The typical gait of rats in the two groups was observed at postoperative week 1; no significant differences were found in the gait between the transplantation group and the control group. At six weeks postoperative, the gait of the transplantation group had recovered compared to that of the control group. The gait curve was plotted at each time point, revealing no significant differences in the SFI between the two groups at postoperative weeks 1-3. At 3 weeks postoperative, the recovery of rat gait in the control group remained the same, while that of the experimental group rapidly recovered. At postoperative weeks 4, 5, and 6, there was a significant difference in SFI between the two groups (P < 0.05) (Figure 3).
3.4 Electrophysiological detection
Electrophysiological analysis revealed no recovery of CMAP amplitude in the two groups at one week postoperative (Figure 4a). At six weeks, the CMAP amplitude in the ipsilateral side of the transplantation group was greater than that of the control group (Figure 4b). The MCV and CMAP curves were plotted. At 1-4 weeks postoperative, there were no differences observed in the MCV recovery rate. At 5-6 weeks, the MCV ratio in transplantation group (0.8402 ± 0.0495) was significantly higher than that of the control group (0.5598 ± 0.1435) (P < 0.01; Figure 4c). At 6 weeks, the CMAP recovery ratio in the transplantation group (0.8453 ± 0.0805) was significantly higher than in the control group (0.4783 ± 0.1856) (P < 0.01; Figure 4d).
3.5 Wet weight ratio of gastrocnemius muscle
At six weeks postoperative, the bilateral gastrocnemius muscle in the two groups was isolated and weighed. The wet weight ratio was calculated as follows: wet weight ratio of gastrocnemius muscle = (wet weight of the right gastrocnemius muscle)/(wet weight of the left gastrocnemius muscle) × 100%. At six weeks postoperative, the gastrocnemius muscle in the ipsilateral side was intact in the transplantation group and was atrophied in the control group. The wet weight ratio of the gastrocnemius muscle in the transplantation group was greater than that in the control group (Figure 5).

Figure 2 Immunocytochemistry analysis of the nestin and GFP expression in cells differentiated from neurospheres. (a) Neural progenitor cells expressing GFP (green, 200× magnification). (b) Neural progenitor cells expressing nestin (red, 200× magnification). (c) Cellnuclei stained with DAPI (blue, 200× magnification). (d) Merged image of a, b and c.

Figure 3 Comparison of gait footprints in the transplantation and control groups. (a) Ipsilateral side in the transplantation group at postoperative week 1. (b) Ipsilateral side in the control group at postoperative week 1. The toe-spreading reflex narrowed and the footprint length was longer. There were no significant differences between the two groups. (c) Ipsilateral side in the transplantation group at postoperative week 6. (d) Ipsilateral side in the control group at postoperative week 6. The toe-spreading reflex was wider and footprint length was shorter in the transplantation group, indicating a better recovery than the control group. (e) Normal side. (f) Comparison of sciatic nerve functional index (SFI) in the transplantation group and control group. * indicates a significant difference at postoperative weeks 4, 5, and 6 (P < 0.05).
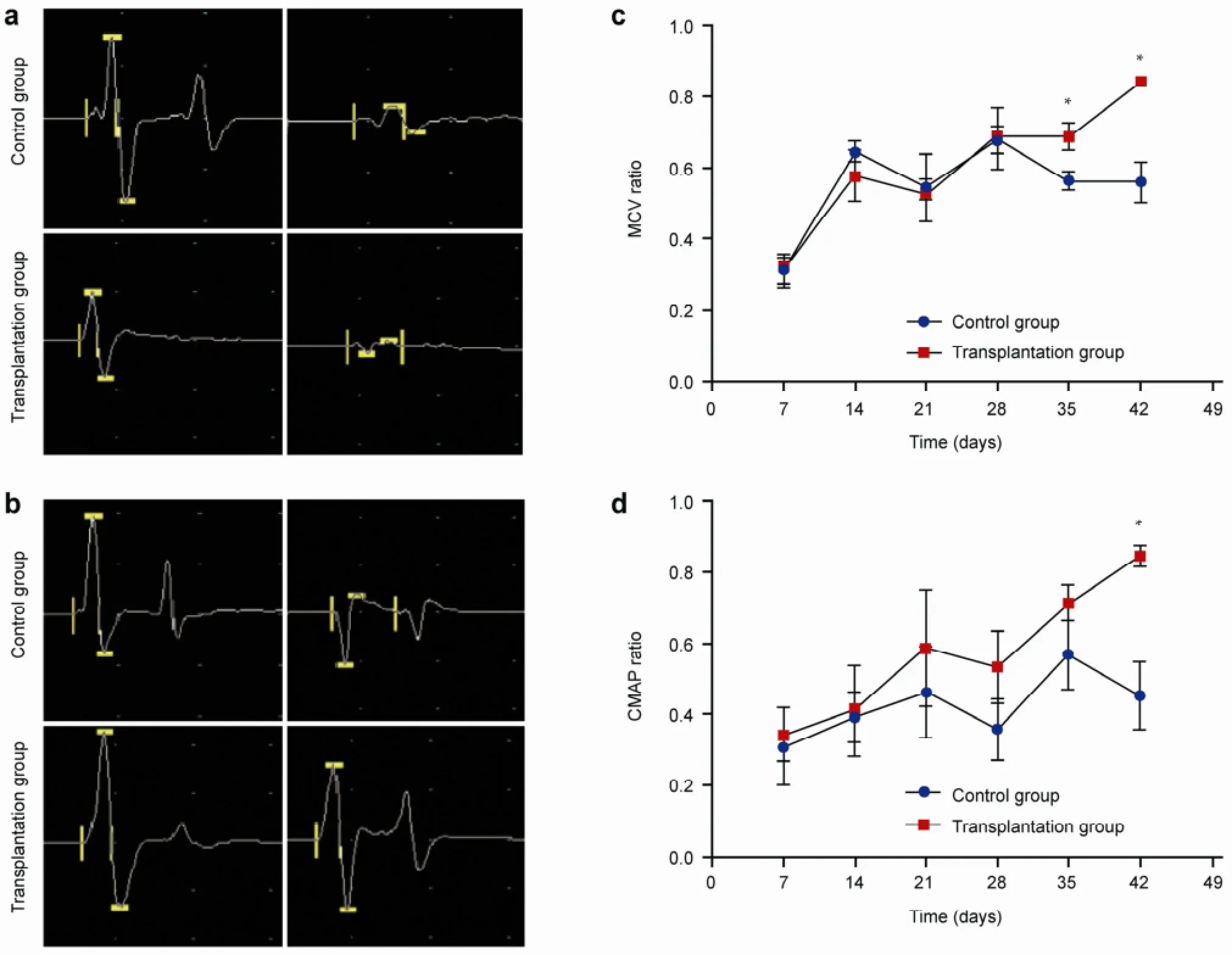
Figure 4 Motor conduction velocity (MCV) and compound muscle action potential (CMAP) at different time points. (a) CMAP in the two groups seven days postoperative. The left side is normal, and the right side is the sciatic nerve crush injury side. The recovery of CMAP amplitude was not apparent after seven days, and no significant difference was found between two groups. (b) CMAP in the two groups at 42 days postoperative. The left side is normal, and the right side is the sciatic nerve crush injury side. The CMAP amplitude in the transplantation group was higher than that of the control group, indicating a better recovery. (c) Comparison of MCV recovery rate in the two groups at 1-6 weeks postoperative. *indicates a significant difference at 5-6 weeks (P< 0.05). (d) Comparison of CMAP recovery rate in the two groups at 1-6 weeks after surgery. *indicates a significant difference at 6 weeks (P < 0.05).
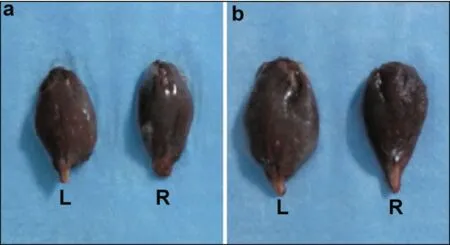
Figure 5 Comparison of gastrocnemius muscle in the two groups at six weeks postoperative. (a) Ipsilateral side (right) and contralateral side (left) in the transplantation group. (b) Ipsilateral side (right) and contralateral side (left) in the control group.
3.6 Survival, migration, and differentiation of neural progenitor cells differentiated from ADSCs
After GFP-expressing neural progenitor cells differentiated from ADSCs were transplanted into rats, green fluorescence was observed under a fluorescent microscope in vitro. At 1 day and every week postoperatively, sciatic nerve specimens were harvested, and neural progenitor cells differentiated from ADSCs and expressing GFP were observed. Transplanted cells were located under the neurilemma and elicited strong fluorescence one day after surgery in vitro. Transplanted cells began to migrate towards the injury site after one week, and a large number of transplantedcells migrated to the injury site after two weeks. The migration of transplanted cells was still visible at six weeks, and the number of cells and fluorescent intensity decreased (Figure 6). At two weeks postoperative, some transplanted cells expressed GFAP, a marker of Schwann cells. At six weeks, the number of cells expressing GFAP increased (Figure 7).

Figure 6 Survival and migration of transplanted cells in the sciatic nerve. GFP expression is labeled by green fluorescence. Arrows represent the neurilemma. At day one postoperative, the transplanted cells were located under the neurilemma. At seven days, cells began to migrate toward the nerve fibers. At 14 days, many cells had migrated, and at 42 days, the number of transplanted cells decreased and the fluorescent intensity was weaker than that at previous time points.
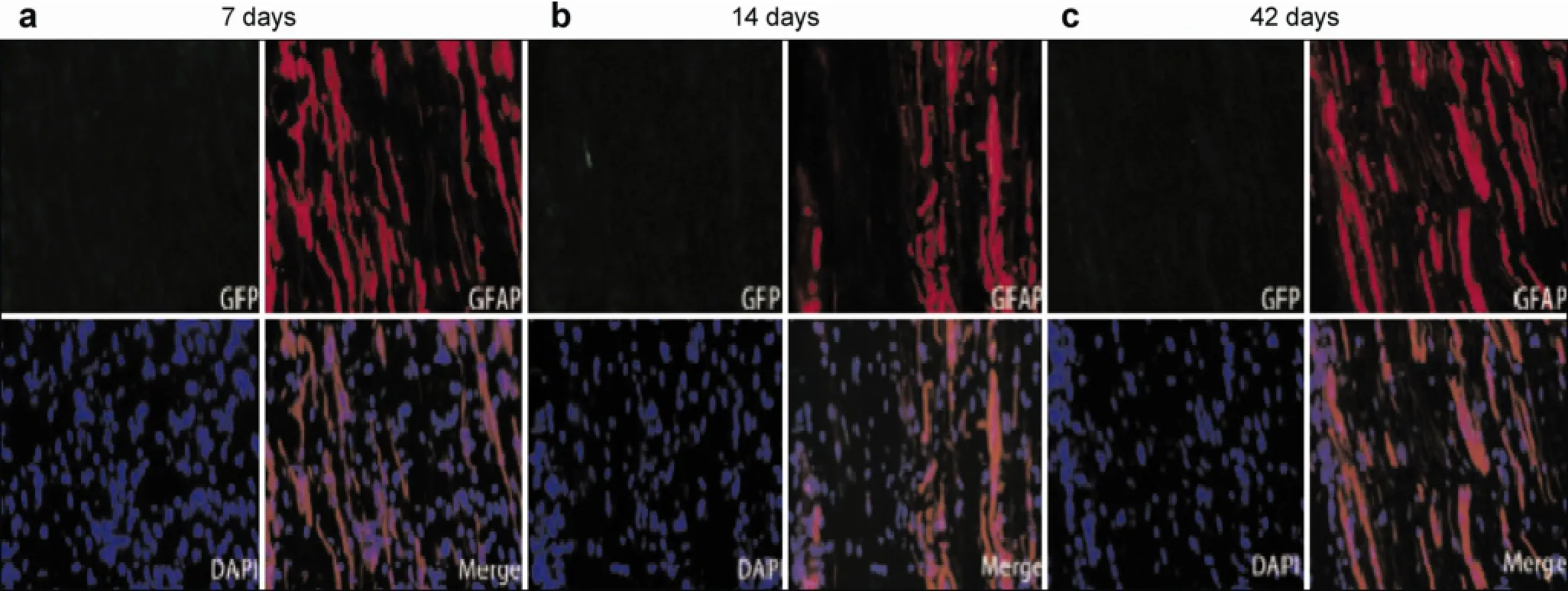
Figure 7 GFP (green) and GFAP (red) double staining of transplanted cells. GFP positive cells are labeled by green fluorescence and GFAP positive cells are labeled by red fluorescence. At 7 days after surgery, nearly no GFAP positive cells were observed. At 14 days after surgery, some transplanted cells co-expressed GFAP and GFP, which increased at 42 days.
Many studies have investigated the use of nervestem cell transplantation for treating sciatic nerve injury, including the transplantation of artificial nerves constructed using tissue engineering methods in vitro, intramuscular or intravenous injection of stem cells, and focal injection of stem cells after nerve injury[20]. The application of embryonic stem cells is controversial due to ethical considerations, and because the isolation of neural stem cells from the brain, spinal cord, and bone marrow induces great trauma. Furthermore, non- autologous transplantation may result in immune rejection; therefore, the transplantation of adult stem cells has attracted increasing attention. The most frequent adult stem cells used in the treatment of nervous system diseases include ectoderm-derived hair follicle stem cells, dental pulp stem cells, neural stem cells, retinal stem cells, bone marrow-derived mesenchymal stem cells, peripheral blood mononuclear cells, skin-derived precursor cells, umbilical cord mesenchymal stem cells, adipose-derived mesenchymal stem cells, placenta-derived mesenchymal stem cells, and Muse cells[21]. Increasing numbers of animal experiments and clinical studies have demonstrated that adult stem cell transplantation is effective in the treatment of various nervous system diseases, such as peripheral nerve injury, Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, multiple system atrophy, cerebral infarction, cerebral hemorrhage, and spinal cord injury[18], opening up new possibilities for treating these conditions. Therefore, it is imperative to identify an accessible adult stem cell suitable for autologous transplantation.
ADSCs, first discovered in 2001 by Zuk et al., are derived from human fat cells, have multi-differentiation potential, and can be amplified in vitro[22]. ADSCs can differentiate into mesodermal cells in vitro, as well as ectoderm cells (e.g., neural cells) and endoderm cells (e.g., liver cells). Compared to other cell sources, ADSCs have the following advantages: they are widely abundant in vivo, are suitable for large-scale culture, are easy to harvest by liposuction surgery with less damage to the body, can undergo autologous transplantation with no immune rejection, and require no ethical considerations[8-11]. Therefore, ADSCs are considered one of the most promising adult stem cells for clinical use.
The use of ADSCs in the treatment of nervous system diseases has been reported using animal experiments and in clinical research. In a recent randomized triple-blind placebo-controlled clinical trial performed on healthy participants, ADSC-rich autologous adipose tissue was subcutaneously transplanted into subjects' arms. The transplanted cells survived for 121 days and the size was significantly higher than that of the control group (autologous adipose tissue without ADSCs), and no severe adverse events were observed[23, 24]. Furthermore, bone marrow mesenchymal stem cells, which exhibit similar biological characteristics to ADSCs, were effective in the treatment of ischemic cardiomyopathy[9]and drug-resistant tuberculosis[10]. Ra et al.[25]demonstrated that intravenous transplantation of autologous ADSCs improved the neurological function of eight patients with spinal cord injury, with no tumorigenicity observed. Yang et al.[26]demonstrated that the intravenous transplantation of human ADSCs improved neurological function in rats with cerebral hemorrhage, which was consistent with a preliminary study by our group[27]. This evidence suggests that the transplantation of ADSCs is reliable, safe, and stable for the treatment of nervous system diseases.
Our group has previously investigated the use of ADSCs for transplantation. We demonstrated that ADSCs were induced to differentiate into neuron-like cells and glial-like cells in vitro and in an in vivo injury microenvironment. For example, rat ADSCs were induced to differentiate into neural precursor cells in vitro and accordingly into terminal nerve cells expressing markers of neurons and glial cells. This differentiation trend was also observed in ADSCs after in vitro culture and transfection with GFP-containing lentivirus[12]. A preliminary study by our group revealed that after ADSCs were injected into the lateral ventricle of rats with cerebral hemorrhage 48 h after modeling, the transplanted cells migrated to areas around the hematoma and differentiated to nerve terminal cells, accompanied by the rapid recovery of neurological function[27]. In 2013, Tomita et al.[19]induced human ADSCs to differentiate into astrocyte- like cells in vitro; the differentiated cells secreted the neurotrophic factors: brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and glial cell- derived neurotrophic factor (GDNF), and promoted the production of more and longer processes, compared to non-differentiated cells. In rats with a tibial nerve crush injury, the differentiated ADSCs improved nervemyelin growth rate after injury. Kingham et al.[18]also confirmed that astrocyte-like cells differentiated from human ADSCs in vitro could increase the secretion of the neurotrophic factors BDNF, GDNF, VEGF-A and angiopoietin-1 protein, and promote the axonal regeneration of dorsal root ganglion neurons. Two weeks after differentiated cells were transplanted into the injured sciatic nerve in rats, the regenerated axons of sciatic nerves were longer than those of the control group. Therefore, we hypothesized that ADSC- differentiated neural precursor cells can be used as seed stem cells for the transplantation treatment of sciatic nerve injury.
Under normal physiological conditions, motor neurons bind with the motor endplate and skeletal muscle fibers to innervate a variety of skeletal muscles. When sciatic nerve injury occurs, the proximal axons retrogradely degenerate, neurons are damaged and die, skeletal muscle fibers degenerate due to the loss of neurotrophic support, and enzyme activity and distribution are changed. The distal axons undergo Wallerian degeneration, decreasing acetylcholinesterase activity in the motor endplate and altering the ultrastructure significantly. When peripheral nerve injury occurs, nerve fibers begin to regenerate 2-3 weeks after injury, Nissl bodies in the injured nerve fibers gradually return to a normal morphology, cell nuclei return to the center of cells, and the proximal axons connected to the cell body extend several shoots. These shoots transverse the injury area and grow alongside Schwann cells, ultimately reaching the original tissues and organs, while other shoots are degraded or lost. The regeneration of the peripheral nerve is affected by many micro-environmental factors. First, the proliferation of Schwann cells is important. If Schwann cells are not accompanied by axonal growth, the axonal growth is not meaningful. The growth of Schwann cells is regulated by the increasing expression of fibronectin. At the same time, Schwann cells are an important source of neurotrophic factors, including NGF, BDNF, CNF, and FGF. These factors play crucial roles in maintaining the growth and development of central and peripheral neurons, protecting tissues from injury, and promoting axonal regeneration. When sciatic nerves begin to regenerate, motor endplate acetylcholinesterase activity and ultrastructure are restored to normal[23]. Therefore, from the viewpoint of pathophysiological mechanisms, maximizing neural regeneration and repair is the key to improving functional recovery after sciatic nerve injury.
In this study, rat ADSCs were isolated and cultured in vitro, then transfected with GFP-containing lentivirus and induced to differentiate into neuron-like progenitor cells, which highly expressed nestin. This indicates that neural progenitor cells can express a phenotype marker of neural stem cells. After GFP- labeled ADSC-derived neural progenitor cells were transplanted into the sciatic nerve injury model, cells expressing GFP were detected 1-6 weeks after surgery and began to migrate towards the injured area one week after surgery. This indicates that transplanted cells can survive in rats and migrate to the injured nerve fiber area. The SFI value was maintained in the control group at three weeks postoperative, while the SFI recovery rate in the transplantation group was significantly higher at 4-6 weeks postoperative.
As rats have limited sciatic nerve self-repair ability, cell transplantation can promote the repair of sciatic nerve. At 5-6 weeks postoperature, the MCV recovery rate of rat sciatic nerves in the transplantation group was significantly higher than that of the control group, indicating that cell transplantation can promote myelin sheath regeneration in sciatic nerves. At six weeks, the CMAP recovery rate and wet weight ratio of the gastrocnemius muscle in the transplantation group were significantly higher than those in the control group, indicating that cell transplantation may promote axonal regeneration of sciatic nerves. The present study confirmed that the recovery of sciatic nerves in the transplantation group was greater than that in the control group, as assessed by SFI, electrophysiological tests, and muscle atrophy.
During the peripheral nerve regeneration process, the proliferation of Schwann cells is important and is regulated by fibronectin expression. Furthermore, Schwann cells are an important source of neurotrophic factors, which play a crucial role in the growth and development of central and peripheral neurons, maintaining normal conditions, as well as protection and axonal regeneration after injury. The outer membrane of Schwann cells and basement membrane are called neurilemma, which can be observed by light microscopy. In this study, we transplanted cells by neurilemma injection.
At present, the underlying mechanism of how transplanted ADSCs promote the regeneration of peripheral nerves remains unclear. Studies have demonstrated that the secretion of BDNF, NT-3, GDNF, and other neurotrophic factors increased after ADSCs transplantation, suggesting that transplanted ADSCs directly secrete neurotrophic factors, which promote neurite growth and have a neuroprotective effect[5, 28, 29]. In addition, the transplanted cells expressed Schwann cells markers, suggesting the transplanted cells had differentiated into Schwann cells[23]. Cell transplantation also increases the production of angiogenic factors at the injury site[30]. We observed that several transplanted cells expressed GFAP, a marker of Schwann cells, by immunofluorescence double staining (GFP and GFAP). This suggests that neural progenitor cells transplanted into the injury site may differentiate into Schwann cells to promote recovery. However, these preliminary findings regarding the mechanism of ADSC-derived neural progenitor cell transplantation for the treatment of sciatic nerve injury require further study. Recovery after nerve injury is a complex process, and may involve other factors, such as nutritional factors, the micro-environment, and angiogenesis.
Conflict of interests
All contributing authors have no conflict of interests.
[1] Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat 1999, 194(Pt 1): 1-14.
[2] Berrocal YA, Almeida VW, Gupta R, Levi AD. Transplantation of Schwann cells in a collagen tube for the repair of large, segmental peripheral nerve defects in rats. J Neurosurg 2013, 119: 720-732.
[3] Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002, 13: 4279-4295.
[4] Faroni A, Terenghi G, Magnaghi V. Expression of functional gamma-aminobutyric acid type A receptors in Schwann-like adult stem cells. J Mol Neurosci 2012, 47: 619-630.
[5] Kalbermatten DF, Schaakxs D, Kingham PJ, Wiberg M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res 2011, 344: 251-260.
[6] Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol 2003, 58: 137-160.
[7] Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007, 100: 1249- 1260.
[8] Tobita M, Mizuno H. Adipose-derived stem cells and periodontal tissue engineering. Int J Oral Max Impl 2013, 28: e487-e493.
[9] Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, Wang Y, Tong L, Tong X. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med 2011, 28: 565-572.
[10] Kim EH, Heo CY. Current applications of adipose-derived stem cells and their future perspectives. World J Stem Cells 2014, 6: 65-68.
[11] Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 2007, 327: 449-462.
[12] Zhang Y, Liu N, Tang Y, Yang E, Dong S, Huang M, Pan C, Zhang Y, Zhang P, Chen H, et al. Efficient generation of neural stem cell-like cells from rat adipose derived stem cells after lentiviral transduction with green fluorescent protein. Mol Neurobiol 2014, 50: 647-654.
[13] Nagase T, Matsumoto D, Nagase M, Yoshimura K, Shigeura T, Inoue M, Hasegawa M, Yamagishi M, Machida M. Neurospheres from human adipose tissue transplanted into cultured mouse embryos can contribute to craniofacial morphogenesis: A preliminary report. J Craniofac Surg 2007, 18: 49-53.
[14] Xu Y, Liu Z, Liu L, Zhao C, Xiong F, Zhou C, Li Y, Shan Y, Peng F, Zhang C. Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neurosci 2008, 9: 21.
[15] Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol 2004, 187: 319-328.
[16] Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 2002, 294: 371- 379.
[17] Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant 2009, 18: 145-158.
[18] Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cellsdifferentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 2007, 207: 267-274.
[19] Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience 2013, 236: 55-65.
[20] Nachum MD, Melamed E, Offen D. Stem cells treatment for sciatic nerve injury. Expert Opin Biol Ther 2011, 12: 1591-1597.
[21] Kanno H. Regenerative therapy for neuronal diseases with transplantation of somatic stem cells. World J Stem Cells 2013, 5: 163-171.
[22] Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 2001, 7: 211-228.
[23] Mohammadi R, Azizi S, Amini K. Effects of undifferentiated cultured omental adipose-derived stem cells on peripheral nerve regeneration. J Surg Res 2013, 180: e91-e97.
[24] Kolle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382: 1113-1120.
[25] Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011, 20: 1297-1308.
[26] Yang KL, Lee JT, Pang CY, Lee TY, Chen SP, Liew HK, Chen SY, Chen TY, Lin PY. Human adipose-derived stem cells for the treatment of intracerebral hemorrhage in rats via femoral intravenous injection. Cell Mol Biol Lett 2012, 17: 376-392.
[27] Chen J, Tang YX, Liu YM, Chen J, Hu XQ, Liu N, Wang SX, Zhang Y, Zeng WG, Ni HJ, et al. Transplantation of adipose- derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther 2012, 18: 847-854.
[28] Chung JY, Kim W, Im W, Yoo DY, Choi JH, Hwang IK, Won MH, Chang IB, Cho BM, Hwang HS, Moon SM. Neuroprotective effects of adipose-derived stem cells against ischemic neuronal damage in the rabbit spinal cord. J Neurol Sci 2012, 317(1-2): 40-46.
[29] Lattanzi W, Geloso MC, Saulnier N, Giannetti S, Puglisi MA, Corvino V, Gasbarrini A, Michetti F. Neurotrophic features of human adipose tissue-derived stromal cells: In vitro and in vivo studies. J Biomed Biotechnol 2011, 2011:468705.
[30] Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev 2013, 23(7): 741-754.
Dong SS, Liu N, Hu Y, Zhang P, Pan C, Zhang YP, Tang YX, Tang ZP. Transplantation of neural progenitor cells differentiated from adipose tissue-derived stem cells for treatment of sciatic nerve injury. Transl. Neurosci. Clin. 2016, 2(2): 108-119.
✉ Corresponding author: Yang Hu, E-mail: huyang081991@126.com; Zhouping Tang, E-mail: ddjtzp@163.com
Supported by the National Natural Science Foundation of China (Nos. 81171089 and 81471201) and the Scientific and Technological Projects of Wuhan City of China (No. 2013060602010240).
 Translational Neuroscience and Clinics2016年2期
Translational Neuroscience and Clinics2016年2期
- Translational Neuroscience and Clinics的其它文章
- A newly developed open-end intracranial hematoma drainage tube
- New frontiers in biomaterials research for tissue repair and regeneration
- A study of the effects of 3,5-diiodo-L-thyronine in the tail suspension and forced swim models of depression
- Behavioral features of mice fed with a cholesterol-enriched diet: Deficient novelty exploration and unaltered aggressive behavior
- Preliminary analysis of cellular sociology of co-cultured glioma initiating cells and macrophages in vitro
- Antiparkinsonian treatment for depression in Parkinson's disease: Are selective serotonin reuptake inhibitors recommended?
