A study of the effects of 3,5-diiodo-L-thyronine in the tail suspension and forced swim models of depression
Nataliia Markova, Anton Chernopiatko, Aslan Kubatiev, Sergey Bachurin, Harry M. W. Steinbusch, Tatyana Strekalova(✉)
1Laboratory of Biomolecular screening, Institute of Physiologically Active Compounds, Russian Academy of Sciences, Severnii Proezd 1, Chernogolovka, Moscow Region 142432, Russia
2Laboratory of Cognitive dysfunctions, Federal State Budgetary Scientific Institution “Institute of General Pathology and Pathophysiology”, Baltiyskaia Str. 8, Moscow 125315, Russia
3Department of Neuroscience, School for Mental Health and Neuroscience, Maastricht University, Universiteitssingel 40, Maastricht NL 6229 ER, the Netherlands
4Section of Neuropharmacology Research, Timantti AB, Sundbyberg 104, Stockholm 17407, Sweden
A study of the effects of 3,5-diiodo-L-thyronine in the tail suspension and forced swim models of depression
Nataliia Markova1,2,3, Anton Chernopiatko4, Aslan Kubatiev2, Sergey Bachurin1, Harry M. W. Steinbusch3, Tatyana Strekalova3(✉)
1Laboratory of Biomolecular screening, Institute of Physiologically Active Compounds, Russian Academy of Sciences, Severnii Proezd 1, Chernogolovka, Moscow Region 142432, Russia
2Laboratory of Cognitive dysfunctions, Federal State Budgetary Scientific Institution “Institute of General Pathology and Pathophysiology”, Baltiyskaia Str. 8, Moscow 125315, Russia
3Department of Neuroscience, School for Mental Health and Neuroscience, Maastricht University, Universiteitssingel 40, Maastricht NL 6229 ER, the Netherlands
4Section of Neuropharmacology Research, Timantti AB, Sundbyberg 104, Stockholm 17407, Sweden
ARTICLE INFO
Received: 20 April 2016
Revised: 9 May 2016
Accepted: 18 May 2016
© The authors 2016. This article
is published with open access
at www.TNCjournal.com
forced swim test;
tail suspension test;
depression;
animal models;
thyroid hormones
Objectives: Recent findings have further highlighted the role of the thyroid system in the pathophysiology of depression and revealed new physiologically relevant elements of the thyroid system. Our previous study showed an antidepressant-like effect of 3,5-diiodo-L-thyronine (T2), which was previously considered to be a physiologically inactive molecule, in mice. Here, we aimed to investigate the antidepressant-like effects of T2 further.
Methods: We studied the effects of bolus injections of T2 to C57Bl6J mice at doses of 0.25 or 0.75 mg/kg with the tail suspension and forced swim models. The effects of the higher dose were investigated in CD1 mice in the forced swim test. Potential behavioral effects of these treatments were also studied using the novel cage and dark-light box tests.
Results: A reduction of depressive-like behavior was found in mice treated with 0.75 mg/kg of T2 in the tail suspension test, but not in the forced swim test. Locomotion and anxiety variables were unaltered following treatment with T2. There were no significant changes after bolus administration of 0.25 mg/kg T2 in either test for depressive-like behavior. Thus, bolus injection of T2 at the dose 0.75 mg/kg can induce antidepressant-like effects without affecting other behaviors.
Conclusions: A discrepant result in the forced swim test may be due to its different sensitivity to T2 compared with the tail suspension paradigm. Furthermore, the development of procedural modifications of this model can be useful in itsapplication in pre-clinical studies.
1 Introduction
Thyroid hormones are important in brain function and dysfunction, including affective disorders such as depression. It is well established that thyroid hormones are essential for crucial processes in the brain and areimplicated in neuronal processing, proliferation and integration, central nervous system (CNS) myelination, and the synthesis of key enzymes required for neurotransmitter synthesis. Therefore, they play important roles in the development and adaptive responses of the nervous system[1, 2]. It is commonly accepted that thyroid hormones are important regulators of mechanisms that are relevant to the pathogenesis of affective conditions. This view is particularly supported by numerous studies demonstrating the efficacy of adjuvant therapy with 3,3,5-triiodo-L-thyronine (T3) and/or 3,5,3',5'-tetraiodo-L-thyronine (T4) thyroid hormones in depressed patients[3-6].
However, the cellular response to both T3 and T4 is known to be delayed[7, 8]. In contrast to T3 and T4, 3,5-diiodo-L-thyronine (T2), which has been recently identified as a functionally inactive metabolite of T3 in the periphery in in vivo and in vitro models, exerts rapid effects on mammalian cells and, as such, might possess unique antidepressant-like properties to be exploited in a clinical setting[7]. It has been shown that T3, whose antidepressant-like properties are well documented and known to be more pronounced than those of T4, evokes lasting effects on the metabolic rate and mitochondrial respiration, with a delayed onset of 48 h. In contrast, the action of T2 begins within the first few hours, does not last for longer than 48 h, and is not sensitive to actinomycin D[7, 9, 10]. Unlike T3, T2 increases mitochondrial respiration by a nuclear- independent mechanism, which is also independent of thyroid hormone receptor beta and AMP-activated protein kinase. Other physiological activities of T2 were shown to be distinct from the effects of T3, such as effects on mitochondrial pathways, where T2 was demonstrated to stimulate reactions involved in substrate oxidation[9, 11]and increase the downstream mechanisms that are involved in mitochondrial biogenesis[12, 13]. Furthermore, T2 was found to evoke selective thyromimetic activity in vitro and in vivo that is divergent from that of T3[14]. The growing evidence suggests that the effects of T2 do not merely mimic those of T3, but, instead, involve discrete mechanisms that seem likely to have rapid dynamics.
Antidepressants with a fast onset of therapeutic effects are currently in high demand in the clinical management of depressive disorders. Ketamine is a unique example of an effective antidepressant of this kind. Unfortunately, its practical use is largely limited due to serious side effects such as euphoria, confusion, cognitive impairment, and transient dissociative states[15]. In light of these challenges, studies of the potential applications of T2 as an antidepressant treatment with rapid onset therapeutic effect are of high interest, because of its high physiological activity that allows low doses to be used, and its endogenous origin that decreases the risk of adverse effects during treatment. However, to date, the antidepressant-like effects of T2 have been little investigated.
The latest evidence suggests that T2 may be important for antidepressant effects. For instance, chronic treatment of rats with the tricyclic antidepressant desipramine induces the expression of T2 in the amygdala that is paralleled by elevated levels of T2 expression in the mitochondria, while concentrations of T3 are increased in the nuclei[16]. Pinna and colleagues[16]have shown the presence of T2 metabolites in several brain areas, including the structures of the limbic system, such as the hippocampus and septum, that points to the presence of T2 itself in these brain areas. Recently, we have demonstrated that mice acutely treated with T2 display a shortened duration of immobility in the two-day tail suspension test, suggesting antidepressant-like effects of T2[17].
To further study the antidepressant-like effects of T2, we employed the forced swim test, another commonly used model of depressive-like behavior in mice[18-20]. Like the tail suspension model, the forced swim test is based on the induction of helplessness and despair behavior in rodents, which are placed in an inescapable stressful situation for a few minutes[18, 21]. In the context of these tests, the posture of floating/ immobility of the tested animals was originally described by Porsoltto as a state of “behavioral despair” that was largely based on the assumption that the animals had “given up hope of escaping”. It is currently regarded that behavioral responses of animals in these two paradigms are manifestations of a preserved evolutionary coping strategy, where the floating/immobility behavior in animals parallels a human state of “entrapment” and psychomotor impairment during clinical depression, and is due to motivational rather than physical factors[22]. This view is supported by the fact that animals can adopt the floating/immobility posture rather quickly anddrugs that may suppress activity, e.g., antidepressants, counteract the immobility response[19].
While both tail suspension and forced swim paradigms are regarded to involve similar neurobiological factors and show a substantial overlap in the pharmacological sensitivity to various drugs[23, 24], the available literature suggests differences between these models in the induction and measuring of depressive-like features in tested rodents. These disparities were found while comparing the effects of gender differences[25], SSRI dosages[26], and genetic manipulations[27], among other factors[19, 20, 28, 29].
In the present study, we investigated the antidepressant-like effects of T2 administered at doses of either 0.25 or 0.75 mg/kg to C57Bl6J mice in the forced swim and tail suspension tests. Additionally, we used T2 at 0.75 mg/kg in CD1 mice in the forced swim test. Finally, potential effects of T2 at both doses on general behavior were assessed in the dark-light box anxiety paradigm and a novel cage vertical activity task.
2 Experimental
2.1 Animals
All studies were performed using 3-month-old male C57BL/6J and CD1 mice from RAS, Moscow, a provider licensed by Charles River (http://www.spf-animals.ru/ about/providers/animals). Mice were single housed under a reversed 12-hour light-dark cycle (lights on: 20:00) starting from the day of the animals' transportation to the laboratory, with food and water provided ad libitum under controlled laboratory conditions (22 ± 1°C, 55% humidity). All experiments were carried out in accordance with the European Communities Council Directive for the care and use of laboratory animals 2010/63/EU.
2.2 Experimental design
We investigated the effects of a bolus oral gavage of T2 to C57BL6J mice at doses of 0.25 mg/kg and 0.75 mg/kg 30 min before the first testing session, as described previously[17], in the two-day tail suspension paradigm (Figure 1a), and in the two-day forced swim model (Figure 1b). Additionally, CD1 mice were treated with T2 at a dose of 0.75 mg/kg via the above-described method and tested in the forced swim model. Control mice received a vehicle 30 min before the first session of tail suspension or forced swim tests. The selection of doses of T2 and timing of the dosing were based on our previous results, which showed higher efficacy of treatment, which was carried out 30 min prior to the experiment rather than treatment performed 2 h after the first testing session[17]. Additionally, we addressed the question of whether the administration of T2 in the investigated doses influences the anxiety and locomotion of mice in the dark-light box and novel cage tasks. These experiments were aimed to rule out potential confounds with the evaluation of behavioral despair in the models of depression after treatment with T2. The dark-light box and novel cage tests were performed with a 5 min interval, 30 min after T2 administration (Figure 1c). Except for the novel cage assay, all behavioral data were normalized to mean values of the control group and expressed as a percentage. For each experiment, the number of mice in each group is indicated in the figure legends.

Figure 1 Schematic timeline of tail suspension (a) and forced swim (b) experiments, and (c) tests for anxiety and locomotion.
2.3 Tail suspension test
The protocol used in this study has been described previously[30]. In the tail suspension test, mice werehung by their tails, attached to a rod 50cm above the floor with adhesive tape for 6 min. The latency of the first episode of immobility, and the durations spent immobile in 2-min intervals and for the entire 6-min period were scored manually using criteria that were previously validated by automated scoring with Noldus Etho Vision XT 8.5 (Noldus Information Technology, Wageningen, the Netherlands) and Clever Sys (Clever Sys, Reston, VA, USA), as described elsewhere[24, 30]. Immobility was defined as the absence of any movement of the animals' head or body. The latency of immobility was determined as the time between the onset of the test and the first episode of immobility. This procedure was carried out twice with a 24 h interval between tests. The trials were recorded by a video camera positioned directly in front of the mice while the experimenter observed the session from a distance in a dark area of the experimental room.
2.4 Forced swim test
Mice were subjected to two swimming sessions spaced 24 h apart, as previously described[30]. All sessions were 6 min long and consisted of placing a mouse in a transparent cylinder (Ø 17 cm) filled with water (temperature of water, 23°C; depth of water, 13 cm; height of cylinder, 20 cm). The latency of the first episode of floating, and time spent floating in 2-min intervals and for the entire 6-min period was scored manually using criteria, which were previously validated by automated scoring with Noldus Etho Vision XT 8.5 (Noldus Information Technology) and Clever Sys(Clever Sys) as described elsewhere[24, 30]. The floating behavior was defined as the absence of any movements of the animals' head and body. The latency of floating was determined as the time between the onset of the test and the first episode of immobility. The trials were recorded by a video camera positioned directly in front of the mice while the experimenter observed the session from a distance in a dark area of the experimental room.
2.5 Dark-light box
The dark-light box (Techno Sm Art, Rome, Italy) consisted of two plexiglass compartments, one black (dark compartment) (15 cm × 20 cm × 25 cm) and one white (light compartment) (30 cm × 20 cm × 25 cm), connected by a tunnel. Anxiety-like behavior was assessed using previously validated measures[31]. Mice were placed into the dark compartment, from where they could visit the lit box (light intensity, 25 Lux). The latency of the first exit to the light compartment, the total time spent in the lit compartment, and the number of visits to the lit compartment were scored using visual observation over 5 min.
2.6 Novel cage
The novel cage test was performed to assess vertical activity[30, 31]. Mice were introduced into a standard plastic cage the same size as their home cage filled with small amounts of fresh sawdust. The number of exploratory rearing behaviors was counted per each minute under a red light during a 5 min period. The testing was carried out in a dark quiet room in the morning hours.
2.7 Statistics
Data were analyzed using Graph Pad Prism version 5.00 for Windows (San Diego, CA). The Mann-Whitney test was applied for two-group data sets, as data did not have a normal distribution. The confidence level was set at 95% (P < 0.05) and data were normalized to control values. Data are shown as mean ± SEM.
3 Results
3.1 Effects of 0.25 mg/kg T2 on immobility and floating behaviors
In the tail suspension test, the latency and total duration of immobility in C57Bl/6 mice dosed with 0.25 mg/kg T2, in comparison to a vehicle-treated group, did not differ on Day 1 (P = 0.4175 and P = 0.6425, respectively, Mann-Whitney test; Figure 2a) or Day 2 (P = 0.7711 and P = 0.3969, respectively, Mann-Whitney test; Figure 2b). As compared with control mice, the group of animals subjected to the administration of 0.25 mg/kg T2 did not show any significant difference in the duration of immobility on Day 1 (1-2 min: P = 0.3533, 3-4 min: P = 0.4515, and 5-6 min: P = 0.4515, Mann-Whitney test; Figure 2a) or Day 2 (1-2 min: P = 0.3969, 3-4 min: P = 0.2968, and 5-6 min: P = 0.8619, Mann-Whitney test; Figure 2b).
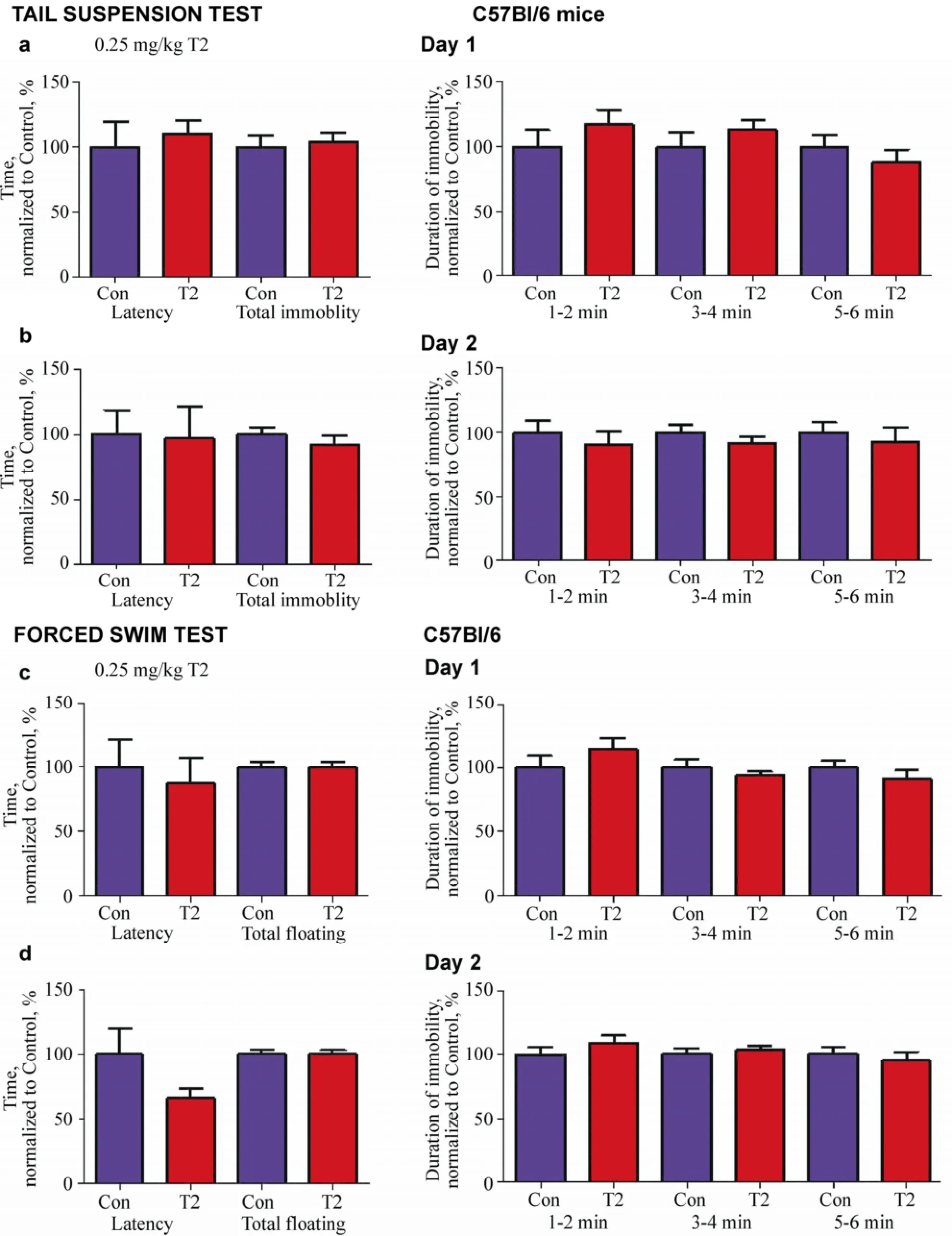
Figure 2 Effects of 0.25 mg/kg T2 in the tail suspension and forced swim tests. On Day 1 (a) and Day 2 (b) of the tail suspension test, mice treated with 0.25 mg/kg T2 (n = 8) showed no difference in the latency or duration of immobility compared with vehicle-treated control mice (n = 7). On Day 1 (c) and Day 2 (d) of the forced swim test, mice treated with 0.25 mg/kg T2 (n = 20) showed no difference in the latency or duration of floating as compared with vehicle-treated control mice (n = 20). Mann-Whitney test.
In the forced swim test, in comparison with vehicle- treated mice, dosing with 0.25 mg/kg T2 did not lead to a difference in the latency or total duration of floating of C57Bl/6 mice on Day 1 (P = 0.8698, and P = 0.9767, respectively, Mann-Whitney test; Figure 2c) or Day 2 (P = 0.2136 and P = 0.9031, respectively, Mann- Whitney test; Figure 2d). In comparison with vehicle- treated mice, there was a tendency for less time spent floating during the second 2-minute testing interval on Day 1 (2-3 min: P = 0.0771, Mann-Whitney test; Figure 2c). No significant differences between the groups were found in the duration of floating during other periods of observation on Day 1 (1-2 min: P = 0.3132, and 5-6 min: P = 0.3574, Mann-Whitney test; Figure 2c) or Day 2 (1-2 min: P = 0.3643, 3-4 min: P = 0.6846, and 5-6 min: P = 0.8497, Mann-Whitney test; Figure 2d).
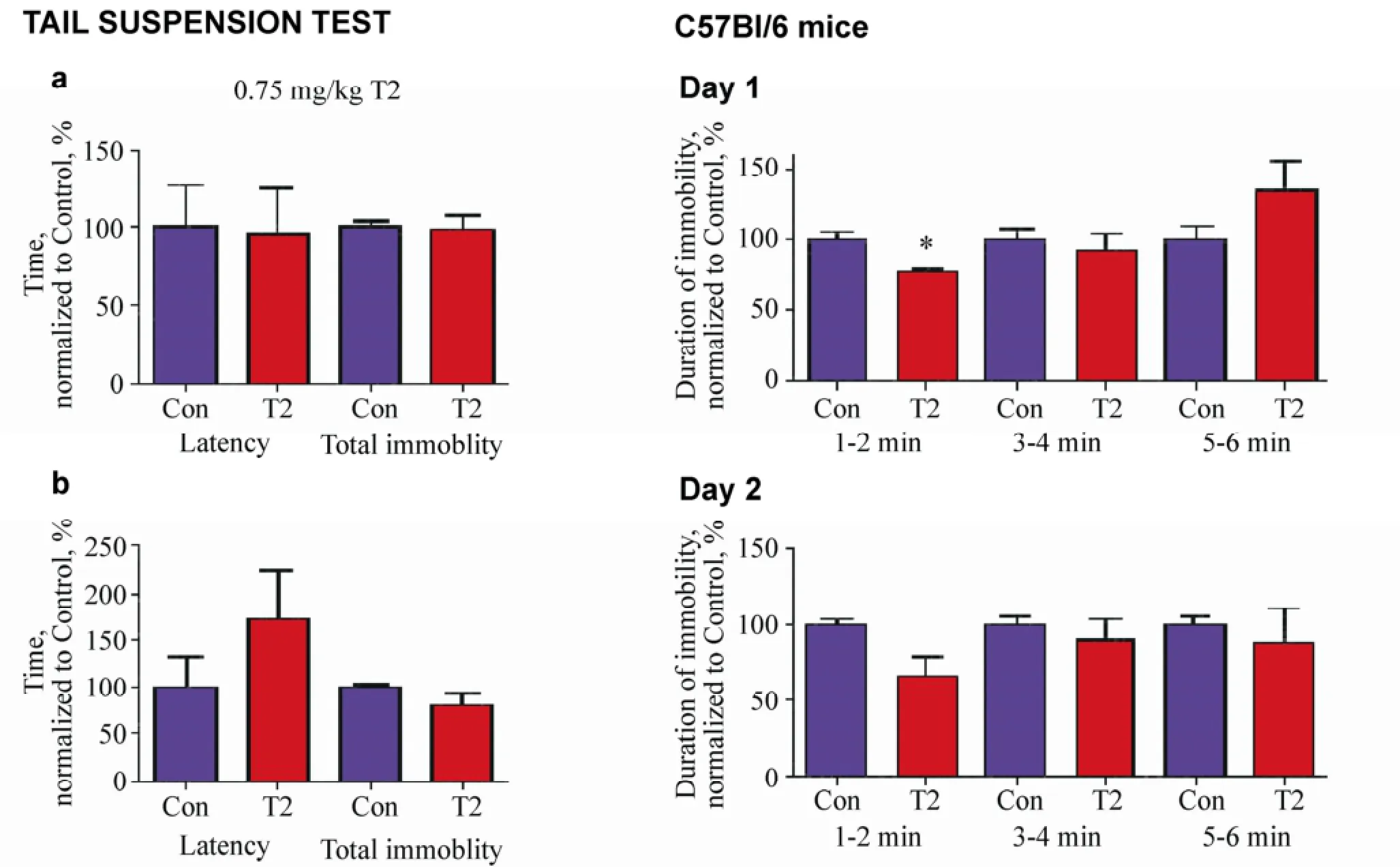
Figure 3(a, b) Effects of 0.75 mg/kg T2 in the tail suspension and forced swim tests. (a) In comparison with vehicle-treated control animals (n = 5), mice treated with 0.75 mg/kg T2 (n = 8) showed no difference in the latency or duration of immobility on Day 1 of the tail suspension test. (b) The duration of immobility was significantly reduced during the first two-minute testing interval of the test on Day 2.
3.2 Effects of 0.75 mg/kg T2 on immobility and floating behaviors
In the tail suspension test, bolus administration of 0.75 mg/kg T2 did not affect the latency or total duration of immobility, in comparison with injection of a vehicle, on Day 1 (P = 0.9166 and P = 0.6905, respectively, Mann-Whitney test; Figure 3a) or Day 2 (P = 0.2812 and P= 0.2222, respectively, Mann-Whitney test; Figure 3b). A significantly reduced duration of immobility was found in T2-treated compared with vehicle-treated animals during the first two-minute testing interval of test on Day 1 (1-2 min: P = 0.009, Mann-Whitney test; Figure 3a) and Day 2 (1-2 min: P = 0.0317, Mann-Whitney test; Figure 3b). Immobility in T2-treated mice did not significantly differ during the remaining periods of scoring, in comparison with a control group, on Day 1 (3-4 min: P = 0.4005, and5-6 min: P = 0.2492, Mann-Whitney test; Figure 3a) or Day 2 (3-4 min: P = 1.000 and 5-6 min: P = 0.6905, Mann-Whitney test; Figure 3b).
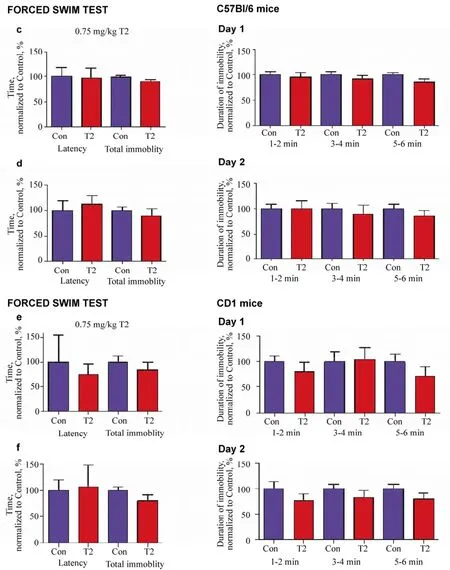
Figure 3(c-f) Effects of 0.75 mg/kg T2 in the tail suspension and forced swim tests.In the forced swim test, mice treated with 0.75 mg/kg T2 (n = 8) showed no difference in the latency or duration of floating in comparison with control mice (n = 8) on Day 1 (c) or Day 2 (d). In the forced swim test, CD1 mice treated with T2 (n = 5) showed no difference in the latency or duration of floating compared with control mice (n = 5) on Day 1 (e) and Day 2 (f). * -P <0.05 versus control; Mann-Whitney test.
In the forced swim test carried out on C57Bl/6 mice, in comparison with a vehicle-treated group, animals treated with 0.75 mg/kg T2 showed unchanged latency of floating on Day 1 (P = 0.9360 Mann-Whitney test, Figure 3c) and Day 2 (P = 0.5738, Mann-Whitney test; Figure 3d). As compared with controls, pharmacologically treated mice exhibited a tendency to decreased total duration of floating on Day 1 (P = 0.0777, Mann-Whitney test; Figure 3c), but not on Day 2 (P = 0.9372, Mann-Whitney test; Figure 3d). The administration of 0.75 mg/kg T2 did not affect the duration of floating of mice during any testing intervals, as compared with the injection of vehicle, on Day 1 (1-2 min: P = 0.8089, 3-4 min: P = 0.3939, and 5-6 min: P = 0.1087, Mann-Whitney test; Figure 3c). Similarly, on Day 2, no significant differences were found in the duration of floating between T2- and vehicle-treated groups (1-2 min: P = 0.8182, 3-4 min: P = 1.000, and 5-6 min: P = 0.6304, Mann-Whitney test; Figure 3d).
In the forced swim test performed on CD1 mice, as compared with the vehicle-treated group, mice treated with 0.75 mg/kg T2 showed a similar latency and total duration of floating on Day 1 (P = 0.8092 and P = 0.4848, respectively, Mann-Whitney test; Figure 3e) and Day 2 (P = 0.6858 and P = 0.2403, respectively, Mann-Whitney test; Figure 3f). In comparison with controls, no significant difference in the time spent floating was found in T2-treated mice during any of the predefined 2 min time intervals on Day 1 (1-2 min: P= 0.3776, 3-4 min: P = 0.7483, and 5-6 min: P = 0.2290, Mann-Whitney test; Figure 3e). CD1 mice treated with T2, as compared with vehicle-treated animals, had a tendency to decreased time floating during the first two-minute testing interval on Day 2 (1-2 min: P = 0.0651, Mann-Whitney test; Figure 3f), but not during either of the other two testing intervals (3-4 min: P = 0.2971 and 5-6 min: P = 0.2403, Mann- Whitney test; Figure 3f).
3.3 T2-treated mice display unaltered anxiety and locomotor behaviors
Treating C57Bl/6 mice with 0.75 mg/kg T2 did not alter anxiety-like behavior in mice, as shown by the unaffected latencies of the first exit to the lit compartment in dosed animals, in comparison with a vehicle-treated group (P = 0.4059, Mann-Whitney test; Figure 4a). No significant differences were found between the groups in time spent therein (P = 0.5830, Mann-Whitney test) or in the number of exits (P = 0.7112, Mann-Whitney test; Figures 4b and 4c). In the novel cage, C57Bl/6 mice that received 0.25 mg/kg T2 and CD1 mice treated with 0.75 mg/kg T2 did not differ from vehicle-treated mice in the number of rearing behaviors at any time point of scoring (0.25 mg/kg: 1 min: P = 0.9095, 2 min: P = 0.6201, 3 min: P = 0.3423, 4 min: P = 0.5188, 5 min: P = 0.9696; 0.75 mg/kg: 1 min: P = 0.9271, 2 min: P = 0.5219, 3 min: P = 0.4286, 4 min: P = 0.3120, 5 min: P = 0.0948, Mann- Whitney test; Figures 4d and 4e).
4 Discussion
In line with previous results, treatment with 0.75 mg/kg T2 decreased depressive-like behavior as measured by the duration of immobility in the tail suspension test during the first minutes of testing[17]. However, no changes in the floating behavior in the forced swim test were observed in mice that received treatment that improved depressive-like behavior in the tail suspension test. A lack of significant behavioral effects of T2 in the forced swim test was evidenced in both C57Bl/6 and CD1 mouse strains. The administration of T2 did not change anxiety-like or vertical locomotor activity suggesting that the general behavioral activity of mice was not altered by T2 and reported changes in immobility are unlikely to be due to its general effects on locomotion. The administration of 0.25 mg/kg T2 did not induce any effects in either paradigm.
Discrepant effects of compounds with antidepressant activity in the tail suspension and forced swim test are not uncommon in the literature. For example, administration of the tricyclic antidepressant imipramine was shown to decrease immobility in the tail suspension test at 4 mg/kg, while in the forced swim test, a dose of 30 mg/kg was effective[32]. Studies with the SSRI antidepressant, reboxetine, revealed a decrease in floating behavior in the forced swim test, but found no change in the tail suspension test[26]. Several other studies have reported discrepant effects of antidepressants in the two depression paradigms[23].
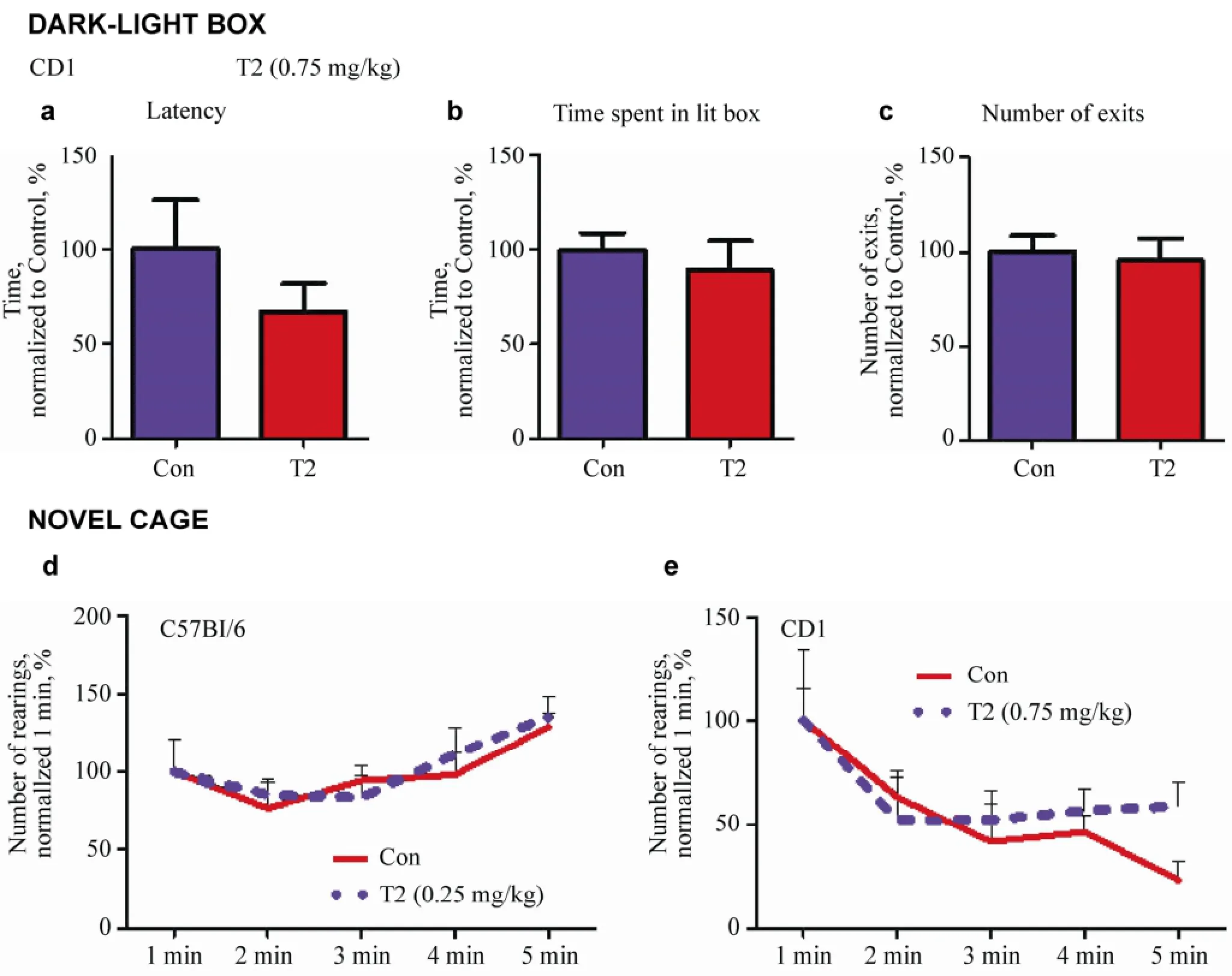
Figure 4 Effects of T2 on anxiety and locomotor behavior. In the dark-light box, C57Bl/6 mice treated with 0.75 mg/kg T2 (n = 5) did not differ from vehicle-treated mice (n = 6) in the latency of the first exit (a), time spent in (b), or number of exits to (c) the light compartment. In the novel cage (d), C57Bl/6 mice that received 0.25 mg/kg T2 (n = 5) and CD1 mice treated with 0.75 mg/kg T2 (n = 5) did not differ from vehicle-treated control C57Bl/6 mice (n = 10) or vehicle-treated CD1 mice (n = 6), respectively, in the number of rearing behaviors. Mann-Whitney test.
A difference in the behavioral changes after T2 administration in the present study may be due to hypothermia in the forced swim test, which can interfere with metabolic effects of T2. Given the rapid effects of T2 on mitochondrial activation, it can be hypothesized that this treatment counteracts the hypothermic effect of swimming procedure that can decrease floating in small rodents[7, 33]. Other drugs, including imipramine, are known to produce the opposing effect to T2, decreasing body temperature and potentiating swimming in rodents and thus, thermogenesis, helping the animals' physiological adaptation to cold. Hypothermia is considered one of the major factors affecting rodent behavior when subjected to forced swimming during the Porsolt test, where pharmacological agents can interfere with this factor and confound the evaluation of depressive-like behavior in rodents[32, 33]. For instance, independent studies have shown that the effects of amitriptyline and desipramine on floating behavior can be detectable or more pronounced when the water in the tank is warm rather than cold[33].
Moreover, floating and immobility behaviors observed in small rodents during repeated tail suspension and forced swim tests can also implicate elements of contextual learning. As such, drugs orother challenges that interfere with this form of memory may affect behavioral consequences in these models[34]. Among the two paradigms, the impact of contextual learning is regarded to be somewhat higher in the forced swim test, because of the generally more stressful effects of the procedure. However, given the fact that thyroid hormones are generally required for normal neuronal plasticity and learning, and their exogenous supply can improve cognition, it is unlikely that this factor can explain the difference between the tail suspension and forced swim experiments with T2.
In a broader context, floating and immobility behaviors induced in rodents in the two depression paradigms employed here can play distinct roles in the behavioral repertoire of animals and be affected differentially by various challenges. For example, parallels have been made between these behaviors and the freezing behavior following exposure to aversive stimuli, such as a predator or a shock, or the environmental context of where a stressful experience had previously taken place[35]. As such, floating and immobility behaviors displayed by animals may depend on the immediate adverse effects of external stressors. Taking into account the rather weak antidepressant-like effect of T2 reported in this and previous studies with the tail suspension test, one can suggest that a lack of antidepressant-like changes in the forced swim test was due to the higher stress impact of the latter paradigm. A tendency to a significant effect of T2 at the higher dose of 0.75 mg/kg suggests a possibility that the antidepressant-like action of T2 could likely occur under modified conditions of testing in the forced swim model.
The present study further suggests that floating and immobility behaviors are complex and their accurate analysis requires a detailed assessment. For instance, our results suggest that scoring depressive- like behaviors in these tests in short time intervals can increase the accuracy of the analysis. This study supports previously reported observations that the analysis of the first 2-min interval of scoring of a depressive-like behavior can be more sensitive in detecting group differences in both tests, rather than the frequently used analysis of the entire 6-min observation period. Finally, development of methodological variants of these tests can be of use in their pre-clinical applications. In particular, given the presen and other facts of limited sensitivity of the forced swim test with the analysis of the antidepressan effects in small rodents, one can suggest that a modification of existing protocols in this paradigm could be potentially beneficial for neurobiologica and translational studies on depression using thi model[19, 23, 36].
5 Conclusions
Given the rapid cellular response evoked by T2 in comparison with the effects of other thyroid hormones, the present data suggest clinical benefits from the potential application of T2 as an adjuvant therapy of depression, particularly in cases where early onset of therapeutic effect is of relevance.
Conflict of interests
All contributing authors have no conflict of interests.
[1] Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev 1993, 14(1): 94-106.
[2] Bernal J, Nunez J. Thyroid hormones and brain development. Eur J Endocrinol 1995, 133(4): 390-398.
[3] Baumgartner A. Thyroxine and the treatment of affective disorders: An overview of the results of basic and clinical research. Int J Neuropsychopharmacol 2000, 3(2): 149-165.
[4] Moreau X, Jeanningros R, Mazzola-Pomietto P. Chronic effects of triiodothyronine in combination with imipramine on 5-HT transporter, 5-HT1A and 5-HT2A receptors in adult rat brain. Neuropsychopharmacology 2001, 24(6): 652-662.
[5] Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol Psychiatry 2002, 7(2): 140-156.
[6] Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord 2006, 91(2-3): 211-215.
[7] Goglia F. Biological effects of 3, 5-diiodothyronine (T2). Biochemistry (Mosc) 2005, 70(2): 164-172.
[8] Schroeder AC, Privalsky ML. Thyroid hormones, T3 and T4, in the brain. Front Endocrinol (Lausanne) 2014, 5 (40). DOI: 10. 3389/fendo. 2014. 00040.
[9] Lombardi A, Lanni A, Moreno M, Brand MD, Goglia F. Effect of 3, 5-di-iodo-L-thyronine on the mitochondrial energy-transduction apparatus. Biochem J 1998, 330(1): 521-526.
[10] Moreno M, Lombardi A, Beneduce L, Silvestri E, Pinna G, Goglia F, Lanni A. Are the effects of T3 on resting metabolic rate in euthyroid rats entirely caused by T3 itself? Endocrinology 2002, 143(2): 504-510.
[11] Lanni A, Moreno M, Cioffi M, Goglia F. Effect of 3, 3'- di-iodothyronine and 3, 5-di-iodothyronine on rat liver mitochondria. J Endocrinol 1993, 136(1): 59-64.
[12] De Lange P, Cioffi F, Senese R, Moreno M, Lombardi A, Silvestri E, De Matteis R, Lionetti L, Mollica MP, Goglia F, et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3, 5-diiodo-L-thyronine in rats. Diabetes 2011, 60(11): 2730-2739.
[13] Del Viscovo A, Secondo A, Esposito A, Goglia F, Moreno M, Canzoniero LMT. Intracellular and plasma membrane- initiated pathways involved in the [Ca2+]i elevations induced by iodothyronines (T3 and T2) in pituitary GH3 cells. Am J Physiol Endocrinol Metab 2012, 302(11): 1419-1430.
[14] Ball SG, Sokolov J, Chin WW. 3, 5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J Mol Endocrinol 1997, 19(2): 137-147.
[15] Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment- resistant major depression. Arch Gen Psychiatry 2006, 63(8): 856-864.
[16] Pinna G, Broedel O, Eravci M, Stoltenburg-Didinger G, Plueckhan H, Fuxius S, Meinhold H, Baumgartner A. Thyroid hormones in the rat amygdala as common targets for antidepressant drugs, mood stabilizers, and sleep deprivation. Biol Psychiatry 2003, 54(10): 1049-1059.
[17] Markova N, Chernopiatko A, Schroeter CA, Malin D, Kubatiev A, Bachurin S, Costa-Nunes J, Steinbusch HM, Strekalova T. Hippocampal gene expression of deiodinases 2 and 3 and effects of 3, 5-diiodo-L-thyronine T2 in mouse depression paradigms. Biomed Res Int 2013, DOI: 10. 1155/ 2013/565218.
[18] Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977, 229(2): 327-336.
[19] Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 1997, 8(6-7): 523-532.
[20] Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci 2002, 23(5): 238-245.
[21] Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85(3): 367-370.
[22] Weingartner H, Silberman E. Models of cognitive impairment: Cognitive changes in depression. Psychopharmacol Bull 1982, 18(2): 27-42.
[23] Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav Rev 2005, 29(4-5): 571-625.
[24] Costa-Nunes JP, Cline BH, Araújo-Correia M, Valença A, Markova N, Dolgov O, Kubatiev A, Yeritsyan N, Steinbusch HWM, Strekalova T. Animal models of depression and drug delivery with food as an effective dosing method: Evidences from studies with Celecoxib and Dicholine succinate. BioMed Res Int 2015, 2015: Article ID 596126.
[25] Jones MD, Lucki I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology 2005, 30(6): 1039-1047.
[26] Ampuero E, Luarte A, Santibañez M, Varas-Godoy M, Toledo J, Diaz-Veliz G, Cavada G, Rubio FJ, Wyneken U. Two chronic stress models based on movement restriction in rats respond selectively to antidepressant drugs: Aldolase C as a potential biomarker. Int J Neuropsychopharmacol 2015, 18(10): pyv038.
[27] Yoshikawa T, Watanabe A, Ishitsuka Y, Nakaya A, Nakatani N. Identification of multiple genetic loci linked to the propensity for “behavioral despair” in mice. Genome Res 2002, 12(3): 357-366.
[28] Guardiola-Lemaître B, Lenègre A, Porsolt RD. Combined effects of diazepam and melatonin in two tests for anxiolytic activity in the mouse. Pharmacol Biochem Behav 1992, 41(2): 405-408.
[29] Liu XQ, Gershenfeld HK. Genetic differences in the tail- suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry 2001, 49(7): 575-581.
[30] Malatynska E, Steinbusch HWM, Redkozubova O, Bolkunov A, Kubatiev A, Yeritsyan NB, Vignisse J, Bachurin S, Strekalova T. Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: Implications for modeling elderly depression. Exp Gerontol 2012, 47(8): 552-564.
[31] Strekalova T, Evans M, Costa-Nunes J, Bachurin S, Yeritsyan N, Couch Y, Steinbusch HMW, Eleonore Köhler S, Lesch KP, Anthony DC. Tlr4 upregulation in the brain accompanies depression- and anxiety-like behaviors inducedby a high-cholesterol diet. Brain Behav Immun 2015, 48: 42-47.
[32] Porsolt RD, Deniel M, Jalfre M. Forced swimming in rats: Hypothermia, immobility and the effects of imipramine. Eur J Pharmacol 1979, 57(4): 431-436.
[33] Bogdanova OV, Kanekar S, D'Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav 2013, 118: 227-239.
[34] Naudon L, Jay TM. Opposite behaviours in the forced swimming test are linked to differences in spatial working memory performances in the rat. Neuroscience 2005, 130(2): 285-293.
[35] Blanchard RJ, Griebel G, Henrie JA, Blanchard DC. Differentiation of anxiolytic and panicolytic drugs by effects on rat and mouse defense test batteries. Neurosci Biobehav Rev 1997, 21(6): 783-789.
[36] Perrault GH, Morel E, Zivkovic B, Sanger DJ. Activity of litoxetine and other serotonin uptake inhibitors in the tail suspension test in mice. Pharmacol Biochem Behav 1992, 42(1): 45-47.
Markova N, Chernopiatko A, Kubatiev A, Bachurin S, Steinbusch HMW, Strekalova T. A study of the effects of 3,5-diiodo-L-thyronine in the tail suspension and forced swim models of depression. Transl. Neurosci. Clin. 2016, 2(2): 96-107.
✉ Corresponding author: Tatyana Strekalova, Email: t.strekalova@maastrichtuniversity.nl
Supported by RFBR, research project No. 16-34-01165.
 Translational Neuroscience and Clinics2016年2期
Translational Neuroscience and Clinics2016年2期
- Translational Neuroscience and Clinics的其它文章
- A newly developed open-end intracranial hematoma drainage tube
- New frontiers in biomaterials research for tissue repair and regeneration
- Transplantation of neural progenitor cells differentiated from adipose tissue-derived stem cells for treatment of sciatic nerve injury
- Behavioral features of mice fed with a cholesterol-enriched diet: Deficient novelty exploration and unaltered aggressive behavior
- Preliminary analysis of cellular sociology of co-cultured glioma initiating cells and macrophages in vitro
- Antiparkinsonian treatment for depression in Parkinson's disease: Are selective serotonin reuptake inhibitors recommended?
