不同磺酸掺杂聚苯胺的制备及在超级电容器中的应用
林有铖 钟新仙 黄寒星 王红强 冯崎鹏 李庆余(广西师范大学化学与药学学院,药用资源化学与药物分子工程教育部重点实验室,广西低碳能源材料重点实验室,广西桂林541004)
不同磺酸掺杂聚苯胺的制备及在超级电容器中的应用
林有铖钟新仙*黄寒星王红强冯崎鹏李庆余*
(广西师范大学化学与药学学院,药用资源化学与药物分子工程教育部重点实验室,广西低碳能源材料重点实验室,广西桂林541004)
以MnO2为氧化剂,采用乳液聚合法,用三种不同的磺酸型表面活性剂制备掺杂聚苯胺(PANI)。通过扫描电子显微镜(SEM)、傅里叶变换红外(FTIR)光谱以及X射线衍射(XRD)等手段对其结构及形貌进行表征;用所得的掺杂聚苯胺制作电极,组装成对称扣式超级电容器,用循环伏安法(CV)、电化学阻抗(EIS)和恒电流充放电技术进行电化学性能研究。结果表明,磺酸表面活性剂的引入有利于PANI纳米纤维的形成和分散,掺杂Nafion的PANI纤维直径在30-40 nm之间,纤维交织成多孔的疏松结构;当放电电流为0.1 A∙g-1时,以PANI-Nafion、PANI-SDS(十二烷基磺酸钠)、PANI-SDBS(十二烷基苯磺酸钠)为电极材料的超级电容器比容量分别为385.3、359.7、401.6 F∙g-1,均高于未掺杂PANI的比容量(235.8 F∙g-1);其中,PANINafion的循环稳定性最好,1000次循环后其比容量保持率高达70.7%。
聚苯胺;有机磺酸;乳液聚合;纳米纤维;超级电容器
doi:10.3866/PKU.WHXB201511104
1 Introduction
Supercapacitors are a new type of energy storage devices with good electrochemical performance between traditional capacitors and secondary batteries.Supercapacitors have aroused highly attention because of their high power density,light weight,high rates of charge-dischage,long cycle life,good voltage memory, etc1-3.At present,supercapacitor electrode materials can be mainly divided into three categories:conducting polymers,carbon materials,numerous transition metal oxides1,4,5.Conducting polymers (e.g.,polyaniline(PANI),polythiophene(PTh),polypyrrole(PPy), polyfuran(PF)and their derivatives,etc.)have been extensively studied in recent years because they not only have high specific capacity,wide voltage windows,and highenergydensity,butalso arecheaperthanpreciousmetaloxide6-12.As an interesting member of conducting polymers,PANI has been considered to be a promising electrode material of supercapacitors due to its environmental stability,easy synthesis,high capacitance,and relatively low cost13-15.
However,poor electrical conductivity and repeatedly insertion/ deinsertion process of the dopant ions accompanying swelling/ shrinkage of volume restrict the application of PANI,which destroys the polymer chains and further decays the specific capacitance as well as weakens the charge-discharge cycle life13,16. Furthermore,the swelling/shrinkage degree of PANI nanostructure is influenced by dopant ion.The macromolecules functionalized protonic acids such as camphorsulfonic acid(CSA)17,dodecylbenzenesulfonic acid(DBSA)18,p-toluenesulfonic acid(p-TSA)19, etc.have been used for doping into PANI through the introduction of larger hydrophobic group and counter-ions20.These organic sulfonic acids generally work as both surfactant and protonic agent during the process of emulsion polymerization.To the best of our knowledge,emulsion polymerization is an effective synthesis for PANI21-24,because it not only affects polymerization of monomer, chain growth,size and distribution of polymer,but also is a highly effective method,simple and low cost.
Therefore organic sulfonic acids arouse our interest once again. Nafion,a kind of perfluorosulfonic acid,has high chemical and mechanical stability and good proton conductivity25,and was commonly used to fabricate proton conducting membranes26. Nafion was used for the electrodeposition of PPy and was doped into PPy27.Our research group applied Nafion as a dopant to improve the electrochemical properties of PANI28.As far as we know,there are few reports about the comparison between Nafion and common surfactants(e.g.,sodium dodecyl benzene sulfonate (SDBS)and sodium dodecyl sulfate(SDS))29-32used for preparing PANI so far.
In this study,we adopted emulsion polymerization to prepare PANI at room temperature with Nafion as dopant and emulsifier and MnO2as oxidant.For comparison,SDBS and SDS were applied to synthesize PANI composites under the same condition. The structure and morphology of the resulting materials were examined by Fourier transform infrared(FTIR)spectrometry, scanningelectronmicroscope(SEM),andX-raydiffraction(XRD). These products were used as active electrode material for fastenertype supercapacitor cells and their electrochemical performances were evaluated by electrochemical test technique with the electrolyte of 1.0 mol∙L-1H2SO4aqueous solution.
2 Experimental
2.1Materials
Perfluorinatedsulfonicacidionexchangeresin(Nafion)(5%(w, massfraction),Sigma-Aldrich).Sodiumdodecylbenzenesulfonate andsodiumdodecylsulfate(CP,XilongChemicalCo.,China).HCl, H2SO4,aniline(AN),MnO2,acetone,acetylene,polytetrafluoroethylene(AR,Xilong Chemical Co.,China).Water was doubledistilled.
2.2Synthesis
Firstly,4.0 mL of fresh aniline was purified by vacuum distillation before the experiment dissolved in 100 mLof 1.0 mol∙L-1HCl with magnetic stirring at room temperature for 20 min to obtain a uniform solution.Then a small quantity of Nafion,SDS, and SDBS surfactant were added into the aqueous solution(the molecular structures of Nafion,SDS,and SDBS are shown in Fig.1).After that,MnO2was added slowly into the above mixture (the molar ratios of AN/MnO2is 1/1).A distinct color change of the mixture from transparent white to dark green was observed during the polymerization reaction.Then,the mixture was stirred for 10 h reaction.Finally,the product was filtered,washed with acetone and deionized water until Mn2+ion was completely removed from solution,dried under vacuum at 80°C for 24 h,and then these materials were marked as PANI-Nafion,PANI-SDS, and PANI-SDBS in the paper,respectively.PANI powder was obtained without adding the surfactant.
2.3Characterization
The morphology of the samples was analyzed by Fourier transform infrared spectroscopy(PE Spectrum One,Perkin Elmer, the wavenumber range 400-4000 cm-1with KBr as compressed slices)andField-emission-scanningelectronmicroscope(FE-SEM, FEI Quanta 200 FEG).The crystal structures of the samples were analyzed by X-ray diffraction(Rigaku D/max 2500 ν/pc,Cu Kα1,λ=0.15406 nm,the operation voltage and current maintained at 40 kV and 30 mA,respectively).The surface area of the samples was studied using BET measurements(ASAP2020,Micromeritics).
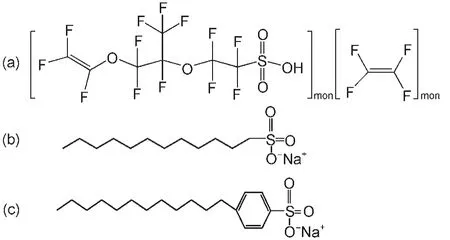
Fig.1 Molecular structures of Nafion(a),SDS(b),and SDBS(c)
2.4Electrochemical measurements
The as-prepared PANI and doped PANI,polytetrafluoroethylene, and acetylene black(mass ratio,80:10:10)were mixed in absolute alcohol.The slurry was turned into a mud-like by magnetic stirring for 3 h,and then was rolled into a film with 0.2 mm thick. The film was cut into disk electrodes with diameter of 10 mm and dried under vacuum at 80°C for 4 h.Then fastener-type symmetric supercapacitor cells were assembled with the electrolyte of 1.0 mol∙L-1H2SO4aqueous solution and two equal quality of disk electrodes divided by a piece of porous separator paper(shown in Fig.2).The electrochemical properties of cells were measured by cyclic voltammograms(CVs)(Zahner IM6,Germany),constant current charge-discharge test(Neware,Shenzhen,China),and electrochemical impedance spectroscopy(EIS)at ambient temperature.

Fig.2 Assembly diagrams of button supercapacitor(a)and fastener-type supercapacitor cell(b)
3 Results and discussion
3.1Microstructure characterizations
SEM images of different doped PANI and PANI materials are shown in Fig.3.Uniform interwoven nanofibers morphology with averagediametersofabout30-40nmandporousnetworkstructure isobtainedforPANI-Nafion(Fig.3(a)).Meanwhile,thefibrousand uniform morphology displays three-dimensional network with distributive porosity.These features are conducive to full contact withtheelectrolyteandtheelectrodematerials,andconsequently canimproveelectrochemicalperformanceofthematerials.PANISDS(Fig.3(b))also shows that nanofiber structures with average diameterof30-80nmareseverecross-linked.Similarmorphology canbeobservedforPANI-SDBS(Fig.3(c)),but the fibers appear uneven thickness(diameters of 20-110 nm)and more holes with sizes in wide range.Nevertheless,granular and fibrous PANI (Fig.3(d))agglomerates clearly with diameters between 40 and 100 nm.
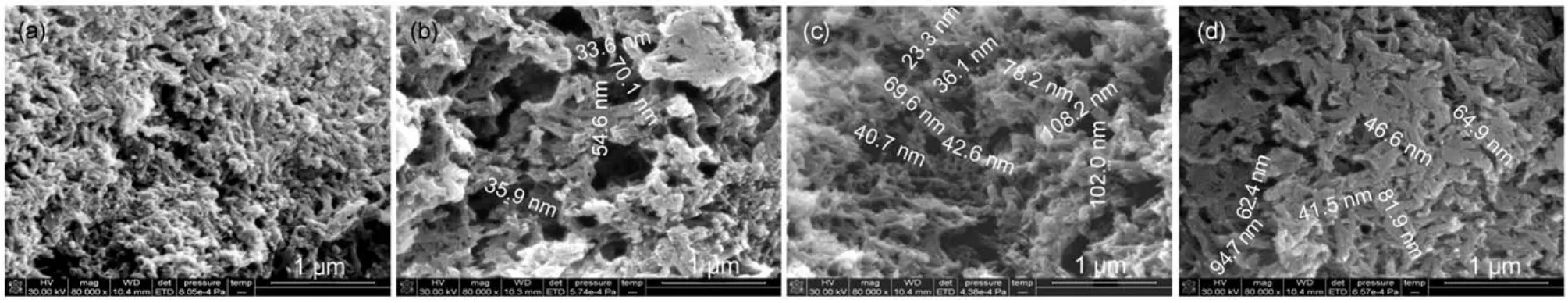
Fig.3 SEM images of PANI-Nafion(a),PANI-SDS(b),PANI-SDBS(c),and PANI(d)
In order to further understand the changes of doped PANI morphology,we analyzed surface area of the samples by the BET measurements.The BET results of materials are shown in Table 1 and Fig.4.The results show that the doped PANI materials have a higher appreciably surface area than PANI.This finding may be attributed to the adding of small amount organic sulfonate,the anionic surfactants interact with cationic of imine groups via electrostatic force,which is favorable for reducing the interaction between PANI chain and forming longer PANI fibers with good dispersion33.These results suggest that the introduction of surfactant has an effect on the morphology of PANI to enable the formation of a fiber structure and increases dispersity of PANI. The bigger surface area and porous network structure of PANINafion may be the reason of the better electrochemical properties.
3.2Molecular characterizations
The FTIR spectra of PANI-Nafion,PANI-SDS,PANI-SDBS, and PANI are shown in Fig.5.The spectra of doped PANI exhibit the main IR bands similar to those of PANI.For PANI,the characteristic bands around 1578 and 1502 cm-1correspond to the C=C and C―N stretching vibrations of quinoid and benzenoid rings(N=Q=N),respectively.The other peaks at 1301,833,and 1147 cm-1belong to C―N stretching vibration of PANI,the external plane of C―H bending vibration in 1,4 disubstituted benzenes,and C=N stretching vibration of PANI,respectively. The FTIR spectrum of PANI studied in this work is consistent with the literature data34,35.Apparently a distinguishable band is present at 1000 cm-1in the doped PANI spectra which is attributed to the stretching vibrations of the sulfonic acid groups(―SO3H),indicating that surfactants successfully doped on the PANI during the synthesis.These results agree with the previous study36. Meanwhile,compared with PANI the main peaks of doped PANI occur a slight red-shift,it is suggested that doping reaction occurs on the nitrogen atom of quinine.Since―SO3H group is a strongelectron withdrawing group and reduces the electron density of the polymer chains through the delocalization of charge.As a result, the electron of transition energy is reduced and the group shifts to low vibration frequency37.

Table1 BET test results of materials
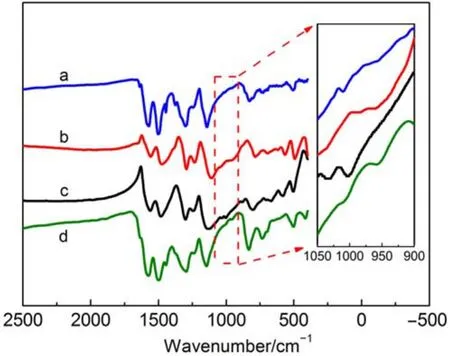
Fig.5 FTIR spectra of PANI-Nafion(a),PANI-SDS(b), PANI-SDBS(c),and PANI(d)
Fig.6 shows XRD patters of PANI-Nafion,PANI-SDS,PANISDBS and PANI.The XRD pattern of doped PANI is similar to that of PANI.Two intense diffraction peaks at 2θ=21.3°,25.1° attribute to the groups paralleling to polymer chains and the groups perpendicular to polymer chains,respectively.The diffraction peak at 2θ=14.4°conforms to the scattering paralleling to polymer chains and represents partial amorphous characteristics of PANI.
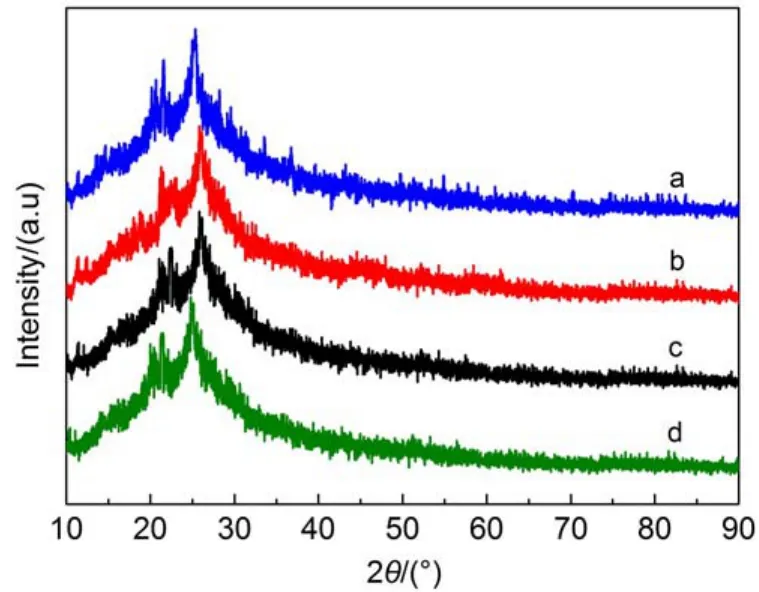
Fig.6 XRD patterns of PANI-Nafion(a),PANI-SDS(b), PANI-SDBS(c),and PANI(d)
3.3Electrochemical performance
The cyclic voltammograms of doped PANI and PANI shown in Fig.7werecarriedoutin1.0mol∙L-1H2SO4aqueoussolutionwith potentialwindowfrom-0.2to0.8Vatascanrateof20mV∙s-1. Fig.7 illustrates that a pair of anodic and cathodic current peaks attributed to PANI leucoemeraldine/emeraldine pair are clearly observed38.TheresponsecurrentofdopedPANIishigherthanPANI, aswellasthesymmetryofCVcurveofdopedPANIisbetterthan PANI.Meanwhile,the specific capacity of doped PANI is higher thanthatofPANIaccordingtotheareasoffourcurves,whichisin theorder:PANI-SDBS˃PANI-Nafion˃PANI-SDS˃PANI.This fact may be due to the larger surface area and porous network structureofdopedPANIallowingabetterelectrolyteaccessaswell asprovidinglowerinternalresistance.
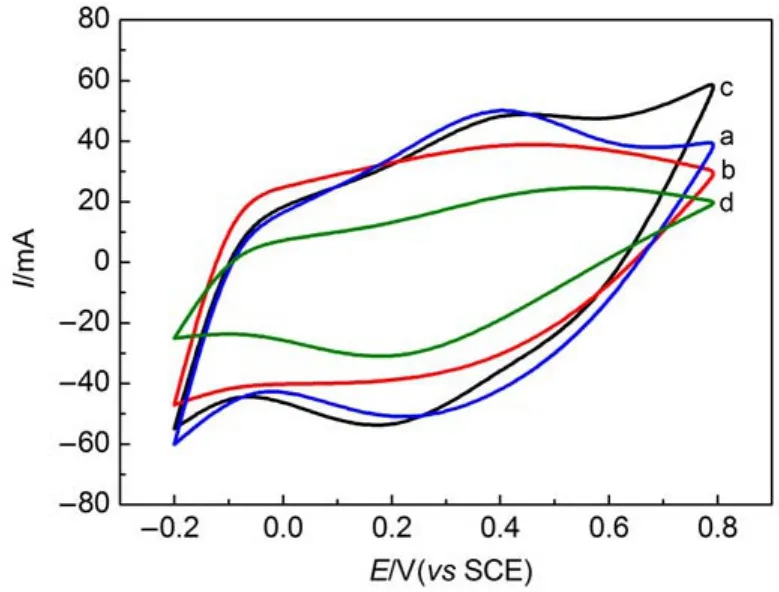
Fig.7 Cyclic voltammograms of PANI-Nafion(a),PANI-SDS(b), PANI-SDBS(c),and PANI(d)electrodes
Fig.8showsthegalvanostaticcharge-dischargecurvesofelectrodesin1.0mol∙L-1H2SO4solutionat0.1A∙g-1withthepotential window ranging from 0.0 to 0.7V.From Fig.8,charge-discharge curvesexpressroughlytrianglerepresentativeofthegoodcapacitive propertiesandreversibleredoxofPANI.Butallthecurvesarenot idealstraightlineoutofthewell-knownohmic-dropphenomenon causedbyinternalresistance39.
The specific capacity value of single electrode can be calculated according to the following Eqs.(1)and(2):

where CTis the total capacitance of button supercapacitor,I is the charge-discharge current,Δt is the discharge time,ΔV is the po-tential window set in the experiment,CSis the specific capacitance of single electrode(F∙g-1),m1and m2are the masses of electrodes, respectively.
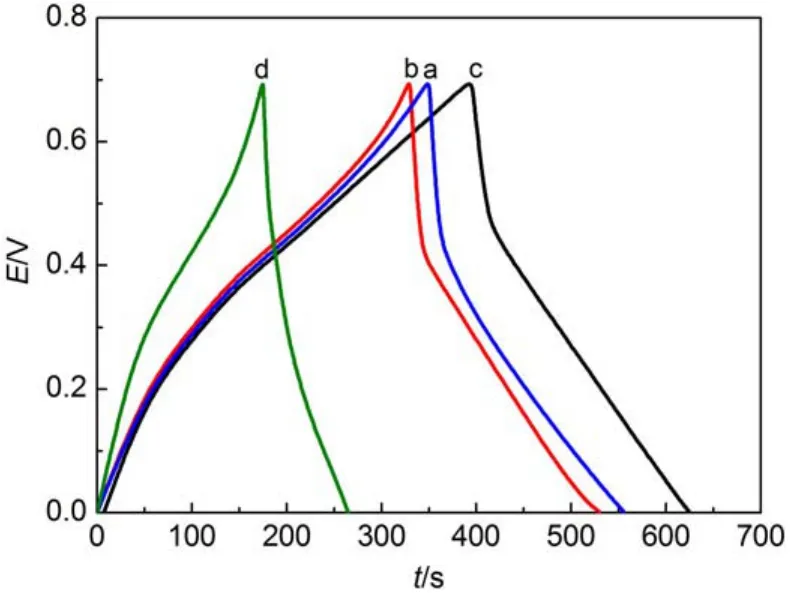
Fig.8 First cycle charge-discharge curves of PANI-Nafion(a), PANI-SDS(b),PANI-SDBS(c),and PANI(d)electrodes
The obtained specific capacity values are 385.3,359.7,401.6, and 235.8 F∙g-1for PANI-Nafion,PANI-SDS,PANI-SDBS,and PANI,respectively.The results are consistent with CV test results and show that polyaniline nanomaterials doped with organic sulfonic acid have a higher capacitance than PANI.
Electrochemical impedance spectroscopy is a powerful tool to understand the charge transfer of materials.Herein the performance of electrodes was tested in the frequency range of 10 mHz to 100 kHz with anAC perturbation voltage of 5 mV.Fig.9 shows the Nyquist plots of doped PANI and PANI.It is evident that the plots exhibit two parts:a low frequency region on the upper right portion and a high frequency region on the lower left portion of the plot.The resistance at the semi-circular portion at the high frequency range is commonly known as charge transfer resistance (Rct)between electrode and electrolyte.Impedance solution resistance(Rs)of doped PANI is equivalent to that of PANI electrode,both reaching to 0.7 Ω.Rctvalues of PANI-Nafion,PANISDS,PANI-SDBS,and PANI electrodes in the intermediate zone are 1.87,2.08,1.69,and 2.70 Ω,respectively,which show that PANI charge transfer resistance is decreased obviously after doping organic sulfonic acid.Because the sulfonic acid doped polyaniline forms porous network structure,which increases the contact area between electrodes and electrolytes,accelerate the proton diffusion rate,thereby possibly improving the electrochemical properties of the material.At the low frequency,the slope of diffusion impedance curve reflects the capacitance characteristics of an electrode.The slope of PANI-SDBS(curve c)is obviously greater than that of others,which shows that PANISDBS has better capacitance characteristics1.

Fig.9 Electrochemical impedance spectroscopy of PANI-Nafion(a),PANI-SDS(b),PANI-SDBS(c),and PANI(d)electrodes
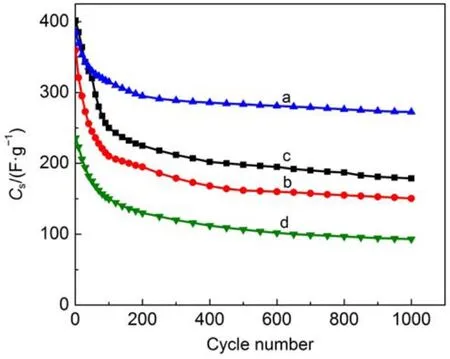
Fig.10 Relationship between cycle number and specific capacitance of PANI-Nafion(a),PANI-SDS(b), PANI-SDBS(c),and PANI(d)electrodes
The cyclic stability is a crucial parameter for supercapacitors electrode materials.The electrochemical stability of materials was tested by charge-discharge cycling tests between 0.0 and 0.7 V at 0.1 A∙g-1for 1000 cycles.The relationship between specific capacitance of the different electrode materials and cycle numbers is depicted in Fig.10.The specific capacitances of the four electrode materials decrease gradually with increasing cycle numbers, which could be attributed to the swelling and shrinkage of conducting PANI during the long-term charge-discharge processes40. Interestingly,doped PANI delivers higher specific capacitance than PANI,among those materials PANI-Nafion basically shows the highest capacitance especially from 200 to 1000 cycles.After 1000 cycles,the specific capacities of PANI-Nafion,PANI-SDS, PANI-SDBS,and PANI are 272.4,150.4,178.7,and 93.1 F∙g-1with capacitance retention calculated to 70.7%,41.8%,44.5%,and 39.5%,respectively.The possible reason of the result is the intervention of the macromolecular organic sulfonic acid anion, which increases the PANI molecular chain pitch so that the polymer chains PANI uniformly dispersed,thereby facilitating anion diffusion inside the electrode material,which is effective to inhibit the swelling phenomenon during dedoping process and improve the cycle performance of doped PANI.These results show that the stability of PANI-Nafion is the highest in the three doped PANI materials,from which we can deduce that Nafion provides mechanical reinforcement thus promoting to the formation the radical cations formed during the redox reaction41.
4 Conclusions
PANI-Nafion,PANI-SDS,and PANI-SDBS composite nanomaterials were prepared at room temperature by emulsion po-lymerization using manganese dioxide(MnO2)as oxidant and Nafion,SDS and SDBS as surfactant and dopant,respectively.The results suggest that the introduction of surfactant has an effect on the morphology of PANI and is in favour of forming a fiber structure,and increases dispersity of PANI.PANI-Nafion network with distributed porosity and average diameter of 30-40 nm was obtained.The electrochemical test results show that the capacitance and conductivity of PANI have been significantly improved after the doping of organic sulfonic acid.Among these,PANINafion delivers a higher specific capacity as well as an excellent cyclic stability far superior to other doped PANI and undoped PANI.The specific capacity of PANI-Nafion is 385.3 F∙g-1at 0.1 A∙g-1,its capacitance retention is 70.7%after 1000 cycles.All the electrochemical performance demonstrates that PANI-Nafion will be a promising electrode material for supercapacitors.
References
(1)Wang,L.L.;Xing,R.G.;Zhang,B.W.;Hou,Y.Acta Phys.-Chim.Sin.2014,30(9),1659.[汪丽丽,邢瑞光,张邦文,侯渊.物理化学学报,2014,30(9),1659.]doi:10.3866/PKU. WHXB201406162
(2)Fan,T.J.;Tong,S.Z.;Zeng,W.J.;Niu,Q.L.;Liu,Y.D.;Kao, C.H.;Li,J.Y.;Huang,W.;Min,Y.;Arthur,J.E.Synth.Met. 2015,199,79.doi:10.1016/j.synthmet.2014.11.017
(3)Simon,P.;Gogotsi,Y.Nat.Mater.2008,7,845.doi:10.1038/ nmat2297
(4)Zhang,Y.;Feng,H.;Wu,X.B.;Wang,L.Z.;Zhang,A.Q.;Xia, T.C.;Dong,H.C.;Li,X.F.;Zhang,L.S.Int.J.Hydrog. Energy 2009,34,4889.doi:10.1016/j.ijhydene.2009.04.005
(5)Brownson,D.A.C.;Kampouris,D.K.;Banks,C.E.J.Power Sources 2011,196,4873.doi:10.1016/j.jpowsour.2011.02.022
(6)Tapan,K.D.;Smita,P.Polym.Plast.Technol.Eng.2012,51, 1487.doi:10.1080/03602559.2012.710697
(7)Wang,J.D.;Peng,T.J.;Xian,H.Y.;Sun,H.J.Acta Phys.-Chim.Sin.2015,31(1),90.[汪建德,彭同江,鲜海洋,孙红娟.物理化学学报,2015,31(1),90.]doi:10.3866/PKU. WHXB201411202
(8)Yang,S.;Xu,G.Y.;Han,J.P.;Bing,H.;Dou,H.;Zhang,X.G. Acta Phys.-Chim.Sin.2015,31(4),685.[杨硕,徐桂银,韩金鹏,邴欢,窦辉,张校刚.物理化学学报,2015,31(4), 685.]doi:10.3866/PKU.WHXB201502022
(9)Gnanakan,S.R.P.;Murugananthem,N.;Subramania,A.Polym. Adv.Technol.2011,22(6),788.doi:10.1002/pat.1578
(10)Liao,Y.;Gao L.;Zhang,X.H.;Chen,J.H.Mater.Res.Bull. 2012,47(7),1625.doi:10.1016/j.materresbull.2012.03.047
(11)Peng,H.;Ma,G.F.;Ying,W.M.;Wang,A.D.;Huang,H.H.; Lei,Z.Q.J.Power Sources 2012,211,40.doi:10.1016/j. jpowsour.2012.03.074
(12)Gao,L.;Liao,Y.;Zhang,X.H.;Chen,J.H.Chem.J.Chin. Univ.2013,34(2),2845.[高磊,廖奕,张小华,陈金华.高等学校化学学报,2013,34(2),2845.]doi:10.7503/ cjcu20130366
(13)Cong,H.P.;Ren,X.C.;Wang,P.;Yu,S.R.Energy Environ. Sci.2013,6,1185.doi:10.1039/C2EE24203F
(14)Graeme,A.S.;Pon,K.;Adam,S.B.J.Power Sources 2011, 196,1.doi:10.1016/j.jpowsour.2010.06.084
(15)David,V.;Pavel,L.;Kimberly,A.S.;Fred,W.;Alan,J.H.Adv. Mater.2014,26(30),5095.doi:10.1002/adma.201400966
(16)Xin,G.X.;Wang,Y.H.;Liu,X.X.;Zhang,J.H.;Wang,Y.F.; Huang,J.J.;Zang,J.B.Electrochim.Acta 2015,167,254.doi: 10.1016/j.electacta.2015.03.181
(17)Mohd,K.;Milton,A.T.;Iuri,S.B.;Vinicius,C.Z.;Jose,J.S. A.;Andre,A.P.Indian J.Mater.Sci.2013,2013,7.doi.org/ 10.1155/2013/718304
(18)Salma,B.;Salma,G.;Khurshid,A.;Shah,A.A.Synth.Met. 2012,162,2259.doi:10.1016/j.synthmet.2012.11.003
(19)Girija,T.C.;Sangaranarayanan,M.V.Synth.Met.2006,156, 244.doi:10.1016/j.synthmet.2005.12.006
(20)Jan,P.;Jaroslav,S.Polym.Degrad.Stab.2004,86,187.doi: 10.1016/j.polymdegradstab.2004.04.012
(21)Gaikwad,P.D.;Shirale,D.J.;Gade,V.K.;Savale,P.A.; Kharat,H.J.;Kakde,K.P.;Hussaini,S.S.;Dhumane,N.R.; Shirsat,M.D.Bull.Mater.Sci.2006,29(2),169.doi:10.1007/ BF02704611
(22)Chen,T.;Dong,C.F.;Li,X.G.;Gao,J.Polym.Degrad.Stab. 2009,94(10),1788.doi:10.1016/j. polymdegradstab.2009.06.011
(23)Chen,W.;Rakhi,R.B.;Alshareef,H.N.J.Mater.Chem.A 2013,1,3315.doi:10.1039/C3TA00499F
(24)Xie,H.G.;Ma,Y.M.;Guo,J.S.Polymer 1999,40(1),261. doi:10.1016/S0032-3861(98)00224-9
(25)Smitha,B.;Sridhar,S.;Animesh,K.J.Membr.Sci.2005,259, 10.doi:10.1016/j.memsci.2005.01.035
(26)Birgit,S.;Soowhan,K.;Vijayakumar,M.;Yang,Z.G.;Liu,J. J.Membr.Sci.2011,372,11.doi:10.1016/j.memsci.2011.01.025 (27)Kim,B.C.;Ko,J.M.;Wallace,G.G.J.Power Sources 2008, 177,665.doi:10.1016/j.jpowsour.2007.11.078
(28)Huang,Y.G.;Zhong,X.X.;Huang,H.X.;Li,Q.Y.;Wang,Z. H.;Feng,Q.P.;Wang,H.Q.Int.J.Hydrog.Energy 2014,39 (28),16132.doi:10.1016/j.ijhydene.2014.06.013
(29)Sudipta,C.;Fabien,S.;Christine,C.;Suzy,V.;Alexandre,B. Carbohydr.Polym.2012,90(2),967.doi:10.1016/j. carbpol.2012.06.028
(30)Yang,M.;Li,J.X.;Li,H.H.;Su,L.W.;Wei,J.P.;Zhou,Z. Phys.Chem.Chem.Phys.2012,14,11048.doi:10.1039/ C2CP41604B
(31)Qiu,Y.;Lu,L.;Wang,S.S.;Zhang,X.H.;He,S.T.;He,T. J.Power Sources 2014,253,300.doi:10.1016/j. jpowsour.2013.12.061
(32)Gu,Y.S.;Tsai,J.Y.Synth.Met.2012,161(23-24),2743.doi: 10.1016/j.synthmet.2011.10.013
(33)Zhou,D.H.;Li,Y.H.;Wang,J.Y.;Xu,P.;Han,X.J.Mater. Lett.2011,65(23-24),3601.doi:10.1016/j.matlet.2011.08.021
(34)Zhang,L.Y.;He,S.J.;Chen,S.L.;Guo,Q.H.;Hou,H.Q.Acta Phys.-Chim.Sin.2010,26(12),3181.[张雷勇,何水剑,陈水亮,郭乔辉,侯豪情.物理化学学报,2010,26(12),3181.]doi: 10.3866/PKU.WHXB20101135
(35)Trchová,M.;Sédeňková,I.;Tobolková,E.;Stejskal,J.Polym. Degrad.Stab.2004,86(1),179.doi:10.1016/j. polymdegradstab.2004.04.011
(36)Lu,X.H.;Ng,H.Y.;Xu,J.Y.;He,H.B.Synth.Met.2002,128, 167.doi:10.1016/S0379-6779(01)00668-3
(37)Liu,W.;Ashok,L.C.;Ramaswamy,N.;Jayant,K.;Sukant,T.; Ferdinando,F.B.;Lynne,S.J.Am.Chem.Soc.1999,121(49), 11345.doi:10.1021/ja9926156
(38)David,E.S.;Su-Moon,P.J.Electrochem.Soc.1988,135(9), 2254.doi:10.1149/1.2096248
(39)Mi,H.Y.;Zhang,X.G.;Yang,S.D.;Ye,X.G.;Luo,J.M. Mater.Chem.Phys.2008,112(1),127.doi:10.1016/j. matchemphys.2008.05.022
(40)Yan,J.;Wei,T.;Fan,A.J.;Qian,W.Z.;Zhang,M.L.;Shen,X. D.;Wei,F.J.Power Sources 2010,195,3041.doi:10.1016/j. jpowsour.2009.11.028
(41)Kima,B.C.;Too,C.O.;Kwon,J.S.;Ko,J.M.;Wallace,G.G. Synth.Met.2011,161,1130.doi:10.1016/j. synthmet.2011.01.015
Preparation and Application of Polyaniline Doped with Different Sulfonic Acids for Supercapacitor
LIN You-ChengZHONG Xin-Xian*HUANG Han-XingWANG Hong-Qiang FENG Qi-PengLI Qing-Yu*
(Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources,Ministry of Education, Guangxi Key Laboratory of Low Carbon Energy Materials,School of Chemistry and Pharmaceutial Sciences, Guangxi Normal University,Guilin 541004,Guangxi Zhuang Autonomous Region,P.R.China)
Polyaniline(PANI)nanomaterials doped with three different sulfonic acid surfactants(perfluorinated sulfonic acid ion exchange resin(Nafion),sodium dodecyl sulfate(SDS),and sodium dodecyl benzene sulfonate (SDBS))were prepared using an emulsion polymerization method with manganese dioxide(MnO2)as the oxidant.The structure and morphology of the products were studied by scanning electron microscopy(SEM), Fourier transform infrared(FTIR)spectroscopy,and X-ray diffraction(XRD).Symmetric redox supercapacitor was assembled with doped PANI as the active electrode material.The electrochemical performances of the materials were evaluated by cyclic voltammetry(CV),electrochemical impedance spectroscopy(EIS),and galvanostatic charge-discharge tests.These results suggest that the introduction of surfactant is beneficial for the formation of a fiber structure and increases the dispersion of PANI.APANI-Nafion network with distributed porosity and average diameters of 30-40 nm is obtained.The specific capacitances of PANI-Nafion,PANI-SDS, and PANI-SDBS electrodes at 0.1A∙g-1are 385.3,359.7,and 401.6 F∙g-1,respectively.Among these electrodes PANI-Nafion delivers the best cycle performance,maintaining 70.7%of its initial capacitance after 1000 cycles.
July 20,2015;Revised:November 6,2015;Published on Web:November 10,2015.
Polyaniline;Organicsulfonic acid;Emulsion polymerization;Nanofiber;Supercapacitor
O646
*Corresponding authors.ZHONG Xin-Xian,Email:zhongxx2004@163.com;Tel:+86-773-5849641.LI Qing-Yu,Email:liqingyu62@126.com.
The project was supported by the China Postdoctoral Science Foundation(2014M562499XB)and National Natural Science Foundation of China (U1401246,51474110).
中国博士后科学基金(2014M562499XB)及国家自然科学基金(U1401246,51474110)资助项目
©Editorial office ofActa Physico-Chimica Sinica

