焙烤对澳洲坚果果仁挥发性成分特征的影响
静 玮,刘义军,彭芍丹,林丽静,李积华(中国热带农业科学院农产品加工研究所,广东 湛江 524001)
焙烤对澳洲坚果果仁挥发性成分特征的影响
静 玮,刘义军,彭芍丹,林丽静,李积华
(中国热带农业科学院农产品加工研究所,广东 湛江 524001)
采用HS-SPME-GC-MS自动分析方法,研究了利用烤箱焙烤澳洲坚果果仁后形成的香气成分,并检测不同焙烤工艺条件下焙烤香气成分的变化。两种不同形式的澳洲坚果果仁原料(整果仁和碎果仁)分别按照不同温度、时间条件焙烤:130℃ 40 min,140℃ 40 min,150℃ 30 min 以及 170℃ 20 min。结果表明:澳洲坚果焙烤果仁中的33种香气成分和原料果仁中的4种香气成分被定性鉴定。总体上,随着焙烤温度从130℃升至170℃,焙烤果仁中的香气成分的数量和含量显著增加,特别是吡嗪类和呋喃类两类物质。2,5-二甲基吡嗪是所有焙烤澳洲坚果果仁香气成分中含量最高的物质。在相同的焙烤工艺条件下,焙烤碎果仁中的香气成分含量高于焙烤整果仁。2-乙基-6-甲基吡嗪可以作为一个很好的指示物,能够反映所有焙烤碎果仁和焙烤整果仁之间的焙烤工艺差异显著性。
香气;焙烤;澳洲坚果果仁
Macadamia tree(Macadamia integrifolia)belonged to the botanical family Proteaceae. Macadamia nut was rich in monounsaturated fatty acids,with oleic acid,which was claimed to be a potent inhibitor of fatty acid and cholesterol synthesis[1],contributing to more than 70% of the total fatty acids. Australia,the United States and Brazil were the top three producers of macadamia nut kernels worldwide. Similarly to hazelnuts,macadamia nut kernels could be processed into several products: smooth or granulate nuts paste,confectionary ingredients,or direct consumption of the whole kernels[2].
Roasting is a dry heating technology,inducing several chemical reactions and producing a series of compounds. The main purpose of roasting is to improve the flavor,the color,and the crispy and crunchy texture of the product[3-4]. Volatile compounds produced during roasting are mainly due to reactions of precursors in macadamia nut including lipids,sugars and amino acids. Volatile compounds generated from Maillard reactions (including Strecker degradation)were good indicator of the effectiveness of roasting,as they played an important role in determining overall flavor[5].
The solvent-free HS-SPME allowed the extraction of more odor-active regions,required very little sample handling and shorter time for sampling. HS-SPME presented better values of withinday repeatability and between-days repeatability (intermediate precision)of the chromatographic areas,than the traditional direct solvent extraction followed by high-vacuum transfer[6]. Solid phase microextraction(SPME)fibers,coated with several different combinations of stationary phase and film thickness,were selected depending on the molecular weight of the compounds of interest.
This study aimed to identify the volatile components in roasted macadamia nut kernels. An applicable testing method was determined to effectively detect the volatile components by automated HS-SPME-GC-MS. In particular,the focus was on finding the indicator volatile components in roasted kernels,whose concentration changes could indicate the differences under different roasting conditions. These indicator volatile components could further optimize the roasting processing and improve the sensory quality by monitoring changes in volatile composition fingerprint.
1 Materials and methods
1.1Reagents
Pure reference compounds used for linear retention index(RI)determination was n-alkanes saturated alkanes standard(n-C7 to n-C30,Supelco,USA)at 1000 μg/mL in hexane. Pure α & β thujone standard mixture(Sigma-Aldrich,USA)were diluted to 1μg/L in n-hexane(Merck,Germany)as Internal Standards for quantification.
1.2Macadamia nut samples and roasting
Dehulled raw macadamia nut kernels(var. Own Choice)were obtained from Moveheart,Inc. (Dehong prefecture,Yunnan province,China)and vacuum packaged with plastic cans. All raw kernels were divided into two parts prior to roasting:whole-kernels and cut-kernels. Part of the raw kernels were ground in a food blender(Philips,Suzhou,PRC)for 5 s and used for cut-kernels. The kernels were placed uniformly in aluminium trays and roasted by circulating hot air oven. Two parts of kernels were roasted following different protocols to achieve light(at 130℃ for 40 min),medium (at 140℃ for 40 min),dark(at 150℃ for 30 min)and very dark(at 170℃ for 30 min). Three replicate samples were processed separately for each roasting condition. Samples of whole-kernels were ground immediately before analysis. 4.0 g ground kernel samples were sealed in a 20 mL headspace vial added with 1 mL Internal Standards(α & β thujone).
1.3 Automated HS-SPME device setup
Volatile aroma compounds were sampled by automated HS-SPME device using a CombiPAL sampler(CTC Analytics AG,Switzerland). A df 50/30 μm,2 cm long SPME fiber coated with divinylbenzene/carboxen/ polydimethylsiloxane (Supelco,USA)was chosen for aroma compounds analysis,and conditioned before sampling as recommended by the manufacturer. The SPME fiber was installed and conditioned in the automated HSSPME device. The SPME fiber was pre-incubated for 5 min at 70℃. After thermal equilibrium,the SPME fiber was inserted to the PTFE/Silicone liner to a depth of 30 mm and exposed above the headspace of the sample. Following headspace extraction for 40 min at 70℃,the SPME fiber was injected into the GC and desorbed for 5 min at 250℃.
1.4Automated HS-SPME-GC-MS setup
The CombiPAL sampler was integrated with an SHIMADZU QP-2010 Plus gas chromatography coupled with a mass spectrometer(SHIMADZU,Japan). Volatile components desorbed from the fiber were separated by a VF-WAX column(30 m ×0.25 mm id,0.25μm film thickness,Agilent Technologies)and under the following conditions:splitless mode and injector temperature 250℃. The carrier gas was helium at a constant flow rate of 1.0 mL/min. The temperature program was as follows:40℃ for 1 min,3℃/min to 170℃,20℃/min to a final temperature of 240℃,with a final holding time of 3 min. Mass spectra were collected by the detector of an electron impact ionization source at 70 eV,scanning from m / z 30 to 400 for 2 s. The ion source and transfer line temperatures were set at 200℃ and 250℃,respectively.
1.5Identification of volatile components
Total ion chromatograms(TIC)were performed by data processing software GCMSSolutions Shimadzu(LabSolutions,Version2.0,Japan). Peaks identification was calculated with their retention times from samples and C7-C40 n-alkanes,and comparison of their mass spectrograms with those in the spectrum libraries of NIST08 and Wiley9(Scientific Instrument Services,USA)with > 80% similarity index to match compounds.
1.6Relative quantification of volatile components
Concentrations of volatile components were calculated as follows:the peak area of identified volatile component was divided by those of Internal Standards; the area ratio obtained above was subsequently converted to relative concentration of the identified volatile component in a 4.0 g sample based on the concentration of appropriate Internal Standards. Concentrations were recorded on a dry weight basis.
1.7Statistical analysis
IBM SPSS statistics 19 software was used for data processing. Results were analyzed by one-way ANOVA,followed by the Duncan’s multiplerange test.
2 Results and analysis
2.1Pyrazines
A typical HS-SPME-GC-MS chromatograms of roasted macadamia nut kernels was showed in Fig. 1. 33 aroma compounds and 2 Internal Standards were marked by their retention time. 12 pyrazines were identified in roasted macadamia nut kernels(Table 1). Pyrazines have been previously reported in roasted almonds,which contributed to nutty and roasty odours[7]. Other researchers also found that pyrazines were important compounds formed during thermal processing(Maillard reaction between amino acids and reducing sugars,Strecker and pyrolysis of amino acids). The data listed in Table 2 showed a marked increase in pyrazines content over increasing roasting time.
Relatively,the pyrazine contents in kernels roasted for 40 min at 130℃ were lower than those in other treatments. High temperature induced thedevelopment of large amount of pyrazines(from 388.37 to 3 249.40 μg/g). 2,5-dimethylpyrazine,contributing to chocolate and roasty aromas,accounted for the highest concentration in pyrazines. The contents of 2-methylpyrazine and trimethylpyrazine were also high,and were contributors of nutty and earthy aromas,respectively. 3-ethyl-2,5-dimethylpyrazine,2,5-and 2,6-dimethylpyrazine were also detected in fresh roasted almonds[8]. 2,6-dimethylpyrazine,ethylpyrazine and 3-ethyl-2,5-dimethylpyrazine,were responsible for nutty,earthy and herbaceous aromas,regarded as characteristic for roasted coffee aroma[9-10].

Table 1 Identification and characteristics of the volatile components in roasted macadamia nut kernels at 150 ℃ for 30 min
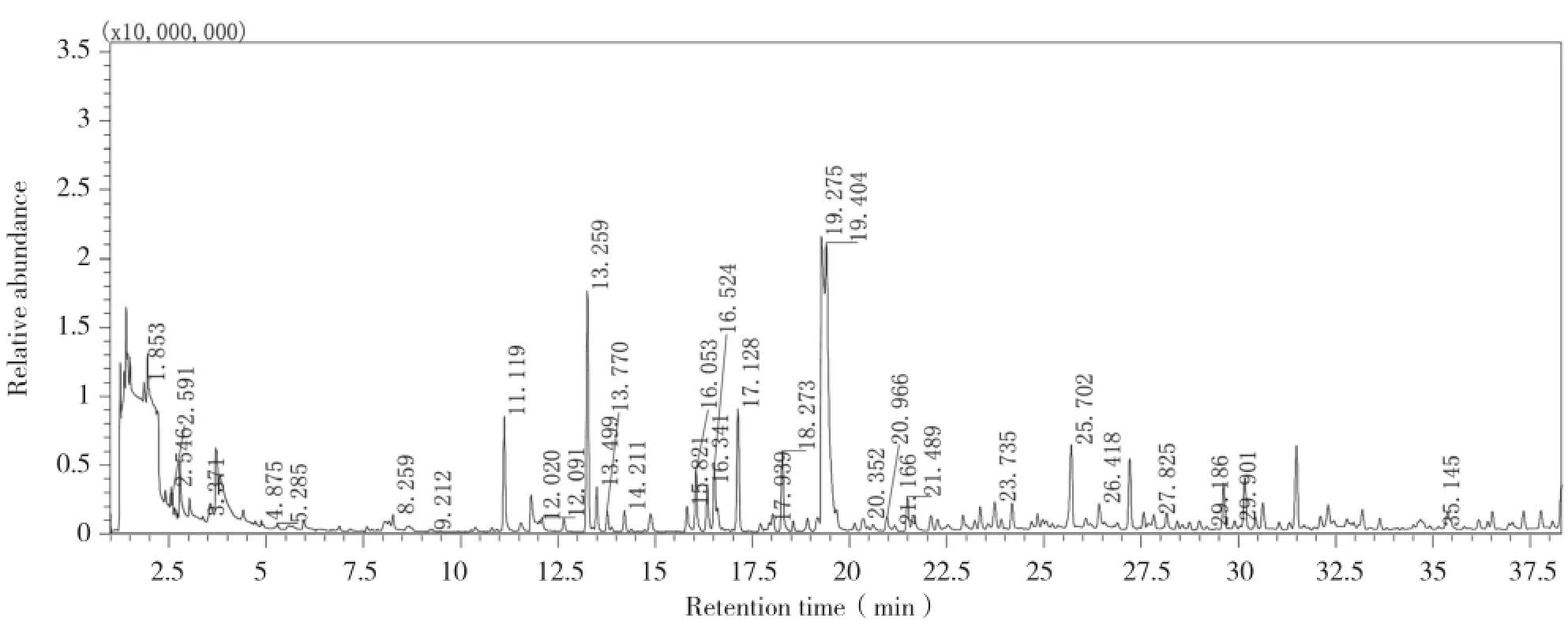
Fig. 1 Typical HS-SPME-GC-MS chromatograms of roasted macadamia nut kernels at 150℃ for 30 min
In our study,the roasted aroma compounds between whole macadamia nut kernels and ground nuts after different roasting temperatures were compared(Table 2). The concentration of pyrazines in roasted whole nuts ranged from 92.52 to 1 114.13μg/g,and being lower than that in roasted ground nuts(from 193.22 to 3 249.40 μg/g under the same roasted condition. The results might be due to the larger area of heating surface in ground kernels. The greater amount of reactants would create a greater amount of volatiles,such as pyrazines. By contrast,for the whole nut,its surface was sufficiently heating rather than internal heating. In general,pyrazines could be considered as an indicator of volatile compounds from roasted macadamia nut kernels.
2.2Aldehydes
Nine aldehydes were identified in roasted macadamia nut kernels(Table 1). In general,long chain aldehydes have typically pleasant fruity and flowery aromas[11]. The formation of aldehydes was complex,such as Strecker degradation or oxidative degradation of amino acids,pyrolysis of proteins or amino acids,and auto-oxidation of lipids. 2-methylpropanal,2-methyl- and 3-methyl butanal were remarkable in roasted hazelnuts,being responsible for fruity,nutty and malty aroma. In addition to those aldehydes,benzeneacetaldehyde might be produced from the Strecker degradation of amino acids during Maillard reaction[12]. Benzaldehyde was described to strongly contributed to the aroma for a pleasant almond aroma in roasted almonds[13]and was produced from phenylalanine under heat[14]. Hexanal,heptanal,octanal and nonanal were lower concentration of lipid aldehydes,which contributed to the aroma of green,grassy and slightly fruity. Those aldehydes were also detected in the roasted aroma of pumpkin seeds[15].
From Table 2,the concentration of aldehydes in roasted whole or ground macadamia nut kernels increased with roasting temperature increasingfrom 130℃ to 150℃,but slightly decreased at 170℃. Among the 9 aldehydes,the concentration of 2-methylpropanal was higher than others,and it seemed to be an important marker for aldehydes in roasted macadamia nut kernels,which significantly affected the total aldehydes content in roasted nuts. 2-methylpropanal was not detected in roasted macadamia nut kernels at 130℃. In the roasted whole kernels from 140℃ to 170℃,the content of 2-methylpropanal increased at first and then decreased,from 397.67μg/g,632.58 μg/g to 360.19 μg/g,respectively. In the roasted ground nuts,2-methylpropanal was detected to be higher at 140℃ than that at 150℃,but which was not detected at 170℃. Compared with the roasted whole kernels,the ground kernels could be heated much more enough,which induced fully reaction of the Strecker degradation. High roasting temperature might induce the volatilization of some temperature-sensitive aroma compounds,such as 2-methylpropanal being partly or completely lost during roasting at 170℃. Hexanal,heptanal,octanal and nonanal were the major by-products of unsaturated fatty acid oxidation,which contributed to the cardboard,painty and oxidized flavors at high concentration associated with flavor fade when lipid oxidation intensified. Macadamia nut kernels contain approximately 72% lipids in edible nuts(USDA,2009; Table 85.1). Typically,two monounsaturated fatty acids(MUFA),oleic and palmitoleic acid account for over 77% of the fat(USDA,2009;Table 85.2). High content of MUFA is susceptible to the rancidity from the reaction of unsaturated fatty acids with oxygen followed by the degradation of fatty acid peroxides with production of off-flavor compounds[16]. We observed that the concentration of hexanal and heptanal in roasted ground kernels increased steadily with roasting temperature increasing,and be remarkably high at 170℃. The increasing of hexanal and nonanal concentration during storage could indicate the shelf life of roasted almonds better than peroxide value[8]. The saturated lipid aldehydes as secondary products of lipidperoxidation could provide information on aroma and technological profile[17]. The concentration of lipid aldehydes was higher in roasted ground kernels than in the whole ones at the same roasted temperature,which might be due to the deep development of lipid oxidation.

Table 2 Comparison of concentration (μg/g) in the volatile components between raw and roasted whole / ground kernels
2.3Ketones
There were only 3 ketones identified in roasted macadamia nut kernels(Table 1). They delivered a special aroma profile such as buttery and caramel from 2,3-butanedione and 2,3-pentandione,fatty and meat from 3-hydroxy-2-butanone,respectively. Thermal pyrolysis of carbohydrate and sucrose,and lipid oxidation are two main pathways for the formation of ketones in thermally processed foods. Yaylayan and Keyhani indicated that 2,3-butanedione was formed through cleavage of the D-glucose moiety,while 2,3-pentandione was formed both from glucose and the reaction of glucose and alanine[18]. 3-hydroxy-2-butanone was formed directly from the reduction of 2,3-butanedione contributing to the possibility of disproportionation with R-hydroxycarbonyl compounds[19]. The roasted temperature high up to 150-170℃ could induce the production of ketones,especially the increasing of 2,3-butanedione. The threshold of 2,3-butanedione is 6.5 μg/kg in water,and it could be perceived more intensely in roasted defective coffee seeds[20-21].
2.4Furans
Six furans were identified in roasted macadamia nut kernels(Table 1). Furans were considered as a major kind of volatile compounds in roasted coffee,due to the number of compounds and aroma contribution to the heat processing[21]. Furans were often found in heat-treated foods saved in jars and cans[22]. High temperature produced large amount of furans in roasted macadamia nut kernels,such as dihydro-3(2)H-furanoneand furfural that being the top two high content of furans. In general,furans are produced by thermal degradation of carbohydrates,such as fructose and glucose,except for dihydro-3(2)H-furanone,which derived from lipid oxidation[23]. Acetylfuran and 5-methyl furfural,being responsible for sweet and cameral flavor,also increased over temperature. Vázquez et al.[24]found that acetyl furan and 5-methyl furfural increased in toasted almonds with heating time during the manufacture of turrón (a typical Spanish confectionery product). The contents of Furfural,5-methyl furfural,dihydro-3 (2)H-furanone and 5-methyl-2-furanmethanol significantly increased in roasted ground kernels at 170℃,which made furan content even higher than aldehyde content and just behind pyrazine content. In our study,furans might be considered as an important volatile marker for roasted ground nuts at 170℃. Meanwhile,the concentration of furans in roasted ground kernels was higher than that in roasted whole ones at the same temperature(Table 2). It could be inferred that sufficient roasting for the whole kernels occurred externally rather than internally,which being less uniform than for the ground nuts. The greater amount of reactants would create a greater total amount of volatiles[23]. Furans were produced by Maillard and Strecker degradation reactions,and thermal degradation of sugars such as fructose and glucose[24]. Even that some furans have been reported to contribute to burnt,sweet,bitter,cooked meat and coconut flavor,they were not indicated as a kind of significant aroma due to the high-odor threshold values in toasted almonds[24-26]. These indicated that the differences among thermal treatments could be better distinguished through the change of furans content quantitatively detected by SPME-GC-MS rather than the sensory evaluation of furan compounds.
2.5Pyrroles
Some pyrroles are known to be responsible for a peculiar sweet and slightly flared smell[20]. There were only two pyrroles,1H-pyrroles and 2-acetyl-1H-pyrroles,identified in roasted macadamia nut kernels(Table 1). They were responsible for nutty and roasted bread flavor,respectively. It would be difficult to understand the form of pyrroles,including Maillard reaction,caramelization,pyrolysis and degradation of trigonelline[27]. In our study,the content of 1H-pyrroles in roasted ground kernels was higher than that in roasted whole ones,but showed no linear regularity with temperature. The content of 2-acetyl-1H-pyrroles increased with temperature increasing,and was significantly higher in roasted ground kernels than in roasted whole ones at the same temperature above 140℃(Table 2).
3 Conclusion
In this study,33 volatile components were identified in roasted macadamia nut kernels,including pyrazines,aldehydes,furans,ketones,pyrroles and acids. These aroma components contributed to the aroma characteristics of nutty,bakey and buttery. The changes of volatile components from different temperature/time treatments were quantitatively compared. In general,the number and concentration of aroma components in roasted macadamia nut kernels significantly increased with roasting temperature rising from 130℃ to 170℃,especially for pyrazines and furans. In addition,pyrazines and furans could be potential indicators to better qualification of overall roasting quality. Especially,2-ehtyl-6-methylpyrazine could be a better indicator to show the significant differences between roasted ground and whole kernels under different roasting conditions. Volatile components were higher in roasted ground kernels than those in roasted whole kernels under the same roasting condition,due to larger surface of kernels during roasting. Future studies should focus on finding the relationship between volatile fingerprint and other sensory characteristics,such as the browning intensityof external color,to better monitor the on-line processing of macadamia nut kernels products.
[1] Francesco N,Luisa S,Serafina S,et al. Oleic acid is a potent inhibitor of fatty acid and cholesterol synthesis in C6 glioma cells[J]. Journal of Lipid Research,2007,48(9):1966-1975.
[2] Nicolotti L,Cordero C,Bicchi C,et al. Volatile profiling of high quality hazelnuts(Corylus avellana L.):Chemical indices of roasting[J]. Food Chemistry,2013,138(2-3):1723-1733.
[3] Saklar S,Katnas S,Ungan S. Determination of optimum hazelnut roasting conditions[J]. International Journal of Food Science & Technology,2001,36(3):271-281.
[4] Burdack-Freitag A,Schieberle P. Changes in the key odorants of Italian hazelnuts(Corylus avellana L. var. Tonda Romana)induced by roasting [J]. Journal of Agricultural and Food Chemistry,2010,58(10):6351-6359.
[5] Srichamnong W,Wootton M,Srzednicki G. Effect of nut-in-shell storage conditions on volatile profile in macadamia nuts[J]. 10th International Working Conference on Stored Product Protection,2010:270-274.
[6] Aceña L,Vera L,Guasch J,et al. Comparative study of two extraction techniques to obtain representative aroma extracts for being analysed by gas chromatography-olfactometry:Application to roasted pistachio aroma[J]. Journal of Chromatography A,2010,1217(49):7781-7787.
[7] Vázquez-Araújo L,VerdúA,Navarro P,et al. Changes in volatile compounds and sensory quality during toasting Spanish almonds [J]. International Journal of Food Science & Technology,2009,44(11):2225-2233.
[8] Yang J H,Pan Z L,Takeoka G,et al. Shelflife of infrared dry-roasted almond[J]. Food Chemistry,2013,138(1):671-678.
[9] Akiyama M,Murakami K,Ikeda M,et al. Characterization of flavor compounds released during grinding of roasted robusta coffee beans [J]. Food Science & Technology International Tokyo,2005,11(3):298-307.
[10] Baltes W,Bochmann G. Model reactions on roast aroma formationⅠ:Reaction of serine and threonine with sucrose under the conditions of coffee roasting and identification of new coffee aroma compounds[J]. Journal of Agricultural and Food Chemistry,1987,35(3):340-346.
[11] Toci A T,Farah A. Volatile fingerprint of Brazilian defective coffee seeds:Corroboration of potential marker compounds and identification of new low quality indicators[J]. Food Chemistry,2014,153(24):298-314.
[12] Bi H X,Ma C W. Synthesis of 3-methylbutanal by Strecker reaction at unelevated temperature and in acidic systems[J]. Chinese Chemical Letters,2006,17(8):1041-1044.
[13] Vazquez-Araujo L,Verdu A,Navarro P,et al. Changes in volatile compounds and sensory quality during toasting of Spanish almonds [J]. International Journal of Food Science & Technology,2009,44(11):2225-2233.
[14] Chu F L,Yaylayan V A. Model Studies on the oxygen-induced formation of benzaldehyde from phenylacetaldehyde using pyrolysis GC-MS and FTIR[J]. Journal of Agricultural and Food Chemistry,2008,56(22):10697-10704.
[15] Siegmund B,Murkovic M. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil(Part 2:volatile compounds)[J]. Food Chemistry,2004,84(3):367-374.
[16] García-Pascual P,Mateos M,Carbonell V,et al. Influence of storage conditions on the quality of shelled and roasted almonds[J]. Biosystems Engineering,2002,84(2):201-209.
[17] Cordero C,Liberto E,Bicchi C,et al. Profiling food volatiles by comprehensive two-dimensionalgas chromatography coupled with mass spectrometry:advanced fingerprinting approaches for comparative analysis of the volatile fraction of roasted hazelnuts(Corylus avellana L.)from different origins[J]. Journal of Chromatography A,2010,1217(37):5848-5858.
[18] Yaylayan V,Keyhani A. Origin of 2,3-pentanedione and 2,3-butanedione in d-glucose/l-alanine maillard model systems[J]. Journal of Agricultural & Food Chemistry,1999,47(8):3280-3284.
[19] Wnorowski A,Yaylayan V. Influence of pyrolytic and aqueous-phase reactions on the mechanism of formation of Maillard products[J]. Journal of Agricultural & Food Chemistry,2000,48(8):3549-3554.
[20] Flament,I. Coffee flavor chemistry[M]. London:John Wiley & Sons,2001.
[21] Toci A T,Farah A. Volatile fingerprint of Brazilian defective coffee seeds:corroboration of potential marker compounds and identification of new low quality indicators[J]. Food Chemistry,2014,153(24):298-314.
[22] Crews C,Castle L. A review of the occurrence,formation and analysis of furan in heat-processed foods[J]. Trends in Food Science & Technology,2007,18(7):365-372.
[23] Agila A,Barringer S. Effect of roasting conditions on color and volatile profile including HMF level in sweet almonds(Prunus dulcis)[J]. Journal of Food Science,2012,77(4):461-468.
[24] Hasnip S,Crew C,Castle L. Some factors affecting the formation of furan in heated foods [J]. Food Additives & Contaminants,2006,23 (3):219-227.
[25] Maga,J A,Katz I. Furans in food[J]. CRC Critical Reviews in Food Science & Nutrition,1979,11(4):355-400.
[26] Alasalvar C,Shahidi F,Cadwallader K R. Comparison of natural and roasted Turkish tombul hazelnut(Corylus avellana L.)volatiles and flavor by DHA/GC/MS and descriptive sensory analysis[J]. Journal of Agricultural and Food Chemistry,2003,51(17):5067-5072.
[27] Baltes W,Bochmann G. Model reactions on roast aroma formation III:Mass spectrometry identification of pyrroles from the reaction of serine and threonine with sucrose under the conditions of coffee roasting[J]. Zeitschrift für Lebensmittel-Untersuchung und Forschung,1987,184(6):478-484.
(责任编辑 储霞玲)
date:2016-03-28
JING Wei(1982-), female, master,assistant-researcher,E-mail:163email26@163.com
LI Ji-hua(1979-),male,doctor,researcher,E-mail:foodpaper@126.com
Projects funded:Natural Science Foundation of Hainan Province(20153164)

