Effects of Predation by Invasive Western Mosquitofi sh (Gambusia affi nis) on Survival of Eggs, Embryos and Tadpoles of Pelophylax nigromaculatus and Duttaphrynus melanostictus in South China
Xiaoli FAN, Zhihua LIN, Xiang LI, Li WEI and Guohua DING
College of Ecology, Lishui University, Lishui 323000, China
Effects of Predation by Invasive Western Mosquitofi sh (Gambusia affi nis) on Survival of Eggs, Embryos and Tadpoles of Pelophylax nigromaculatus and Duttaphrynus melanostictus in South China
Xiaoli FAN, Zhihua LIN*, Xiang LI, Li WEI and Guohua DING
College of Ecology, Lishui University, Lishui 323000, China
Alien species are one of the most serious threats to the decline and extinction of native amphibian populations. In this study, we examined the predation of invasive Western Mosquitofi sh Gambusia affi nis on the eggs, embryos, and tadpoles of Duttaphrynus melanostictus and Pelophylax nigromaculatus in south China. Our results suggested that the survival of eggs and embryos remaining in the egg capsules of P. nigromaculatus and D. melanostictus was signifi cantly higher than those removed from the egg capsule at 12-h intervals within 72 h in the presence of G. affi nis. The survival of P. nigromaculatus eggs and embryos without egg capsules was signifi cantly lower than those of D. melanostictus without egg capsules. The survival of P. nigromaculatus eggs and embryos with egg capsules was signifi cantly higher than those of D. melanostictus with egg capsules from 24 h to 72 h except for 12 h. The survival of D. melanostictus tadpoles was significantly higher than that of P. nigromaculatus tadpoles in the presence of G. affinis. The survival of Gosner stage 26 tadpoles of P. nigromaculatus was signifi cantly higher than that of Gosner stage 30 tadpoles from 12 h to 60 h, but there were no signifi cant differences at 72 h. In contrast, the survival of Gosner stage 26 tadpoles of D. melanostictus was signifi cantly lower than that of Gosner stage 30 tadpoles within 72 h, recording every 12h. The increasing temperature caused a significant increase in predation by G. affinis on P. nigromaculatus eggs and embryos. The outer jelly capsule surrounding anurans eggs might serve as a mechanical defense against predation by G. affi nis due to its large diameter, relatively stationary state and unpalatability. The differences in the vulnerability of P. nigromaculatus and D. melanostictus embryos and tadpoles to G. affi nis probably due to differences in the unpalatability,black skin and activity. Based on the magnitude of predation by G. affi nis on the eggs, embryos and tadpoles of these two species and the combined impact of temperature, we might speculate that invasive G. affi nis and global warming would have more detrimental impacts on population viability of P. nigromaculatus than D. melanostictus in China.
amphibian anura, tadpoles, eggs, embryos, predation risk
1. Introduction
Alien species are a potential threat to the survival of amphibian larvae globally. In particular, the introduction of non-native fish often results in the rapid decline and extinction of native amphibian populations (Collins and Storfer, 2003; Hussain and Pandit, 2012). Such nonnative predators are more of a threat to native speciesbecause native species are often incapable of recognizing non-native predators (Gomez-Mestre and Díaz-Paniagua,2011; Polo-Cavia and Gomez-Mestre, 2014; Salo et al., 2007; Smith et al., 2007). Western Mosquitofish,Gambusia affi nis, one of the most widespread introduced fish, is native to the Atlantic coast of North America(Lowe et al., 2000; Pyke, 2005). Their widespread introduction can be attributed to their purported effectiveness at consuming larval mosquitoes (Pyke,2008). G. affi nis was introduced to China for this reason in 1927 and has now become widespread throughout the southern water bodies of the Yangtze River (Li andJie, 2002). Previous studies have shown that G. affinis readily preys on amphibian eggs and larvae, both within(Grubb, 1972; Baber and Babbitt, 2003; Gunzburger and Travis, 2005; Zeiber et al., 2008; Kerby et al., 2012) and outside of its native range (Komak and Crossland, 2000;David and Craig, 2006; Gregoire and Gunzburger, 2008;Segev et al., 2009; Shulse and Semlitsch, 2014). The introduction of G. affinis caused a serious threat to the diversity and population dynamics of amphibians, leading the International Union for Conservation of Nature to list it among the 100 worst invasive species (Lowe et al.,2000). To date, there have been no intensive studies on the degree of damage caused to Chinese anurans by the invasive G. affi nis.
There is an abundance of vegetation and water bodies at the Lishui University campus (latitude 28°27′ N,longitude 119°53′ E) situated in Zhejiang, South China. The advantageous environment provides living and breeding habitats for a large number of amphibians. Among them, Duttaphrynus (formerly Bufo) melanostictus and Pelophylax nigromaculatus breed in the same permanent ponds (Figure 1A) from March to May every year, so the two species both belong to spring-breeders. However, there are some differences in their clutch structure. The amplectant pairs of D. melanostictus spawn long egg strings entwining the vegetation, including Nymphaea alba, Hydrilla verticillata, and Alternanthera philoxeroides (Figure 1B). In contrast, the amplectant pairs of P. nigromaculatus spawn globular egg masses, and this species lays a single egg mass on vegetative cover (Figure 1C) or a large number of egg masses in the open water(Figure 1D). However, their fertilized eggs are enveloped by transparent jelly capsules. The invasive G. affi nis also inhabits in the breeding ponds, so the eggs, embryos, and tadpoles of these two anurans may face predation risk.
In this study, we examined the survival of the eggs,embryos, and tadpoles of D. melanostictus and P. nigromaculatus exposed to the visual cues of the invasive predator G. affi nis. Our aims were to evaluate (1) whether the jelly capsule of eggs plays a role in protecting from the predators, (2) whether there are differences in G. affinis predation on different development stages of tadpoles, and (3) the effect of temperature on predation.
2. Materials and Methods

Figure 1 Breeding water body and egg clutch of Duttaphrynus melanostictus and Pelophylax nigromaculatus, (A) a permanent pond, (B)amplexing and spawning of D. melanostictus, (C) single egg mass of P. nigromaculatus in the vegetative cover, (D) multiple egg masses of P. nigromaculatus in open water.
2.1 Collection of experimental animals Three freshly laid P. nigromaculatus egg masses and three D. melanostictus egg strings were collected from a permanent pond at Lishui University on March 22, 2014. All of the eggs were carried back to our herpetological laboratory. From each family, about 1200 eggs were directly used in two experiments, the remaining eggs of each specieswere separately incubated in two 700 mm × 500 mm × 400 mm (length × width × height) plastic containers fi lled with aged tap water to a 200 mm depth until tadpoles reached Gosner stages 26-30 (Gosner, 1960). The development stages of tadpoles were determined using an anatomical microscope (Nikon XTS30). The tadpoles were fed commercial fi sh food ad libitum every 2 days. The same pond retains water and contains G. affi nis yearround. We collected approximately 350 similar in size female G. affi nis with black embryo spots on both ventral sides using nets (mesh size: 2 mm) (Deaton and Cureton,2011; Pyke, 2005). They were transferred to an outdoor pool (3.0 m × 2.0 m × 1.5 m) to be raised. G. affi nis were starved for 24 h before the experiment.
Eggs with or without jelly capsules of P. nigromaculatus and D. melanostictus were randomly selected and transferred into Petri dishes (60 mm diameter, 10 mm depth) labeled with size standards, and photographed from above using a digital camera (Sony DSC-T100), then their egg diameters and jelly capsules diameters were analyzed using ImageJ 1.44p software (to the nearest 0.01 mm) (Fan et al., 2014).
2.2 Experimental design
Predation of G. affinis on eggs and embryos of P. nigromaculatus and D. melanostictus Between March 22 and 25, 2014, ten eggs with a jelly coat or ten eggs without a jelly coat (jelly coats were removed by slowly sucking out fertilized eggs with a capillary tube) of P. nigromaculatus and D. melanostictus were settled into separate plastic bowls. Plastic bowls with a 150 mm diameter were filled with water depth of approximately 80 mm and 30 replicates each treatment. After these were done, a fish was randomly assigned to each bowl with a 150 mm diameter. We estimated survivorship by counting the number of eggs or embryos remaining in the bowls of all treatments at 12 h intervals for consecutive 72 h. The environmental conditions in the laboratory were 12 L : 12 D photoperiod with an air temperature of 25 ± 1°C (mean ± SE).
Predation of G. affinis on different stage tadpoles of P. nigromaculatus and D. melanostictus Between 2-9 April, 2014, we performed a completely randomized 2 × 2 factorial design with 4 replicates of each treatment. The experiment had two tadpole levels (P. nigromaculatus and D. melanostictus) crossed with two levels of tadpole initial Gosner stage: 26 or 30. Tadpoles were firstly randomly assigned to each treatment, and then a similar sized fi sh was transferred into every bowl. We recorded the survival of tadpoles in each treatment by the same method above.
Predation of G. affinis on eggs and embryos of P. nigromaculatus at different temperatures According to data consecutively recorded hourly by HOBO temperature data loggers (USB, U12-006) in outdoor laboratory round-year, the average minimum and maximum air temperature from March to April in 2013 were 15.2 and 25.3°C, respectively, which were used as the basis of our experiment temperature designs. On 22 March, 2014,we fi rstly placed ten eggs into fi fteen plastic bowls, later placed fi fteen fi sh into the same bowls. The environment chambers were set at 15°C, 20°C, and 25°C, and the photoperiod was 12L: 12D. The survival of tadpoles in each treatment was recorded using the same method as above.
All of the eggs, embryos, tadpoles and fish of each treatment were used only once. After the experiments,excess eggs and tadpoles were released back to the site of capture. All of the G. affi nis were frozen to death, and their body length (the distance from snout to cloaca)and snout width (the distance between left and right jaw angles) were measured by a digital caliper (to the nearest 0.01 mm).
2.3 Statistical analysis The mean survival number of anuran eggs, embryos and tadpoles every 12 hours was calculated for each treatment. All statistical analyses were performed using Statistica 5.0 software. All variables were tested for normality and homogeneity using the Kolmogorov-Smirnov test and F-max test,respectively. Due to the inhomogeneity of variance (all P < 0.001), we used the Kruskal-Wallis test to examine the differences in the survivorship of P. nigromaculatus and D. melanostictus eggs, embryos (with and without egg capsules) and tadpoles (Gosner stage 26 and 30)coexisting with free-roaming G. affi nis at 12 h intervals within 72 h in the fi rst and second experiments. Date for the survival of P. nigromaculatus eggs and embryos in the presence of G. affi nis at three temperature treatments(15°C, 20°C, and 25°C) were compared with One-way MANOVA and Tukey's post hoc test with different times as factor. All results were expressed as a mean ± SE, with α = 0.05 considered as statistically signifi cant.
3. Results
G. affi nis used in the experiments had a mean body length of 35.70 mm (SE = 0.49, range = 30.81 to 44.22 mm)and snout width of 2.86 mm (SE = 0.06, range = 3.03 to 4.82 mm). Egg diameter of P. nigromaculatus was an average of 1.53 ± 0.03 (1.17-1.67) mm, and jelly capsules diameter was an average of 5.47 ± 0.08 (4.67-5.84) mm.Egg diameters of D. melanostictus was an average of 1.50 ± 0.13 (1.16-1.70) mm.
3.1 Predation of G. affi nis on eggs and embryos of P. nigromaculatus and D. melanostictus Kruskal-Wallis test revealed that the survival of P. nigromaculatus and D. melanostictus eggs and embryos remaining in an egg capsule was significantly higher than those removed from the egg capsule at different times within 12-72 h as shown in Figure 2 (all P < 0.007). The survival of P. nigromaculatus eggs and embryos without egg capsules was significantly lower than that of D. melanostictus without egg capsules in the same time (all P < 0.007,Figure 2). The survival of P. nigromaculatus eggs and embryos with egg capsules was signifi cantly higher than those of D. melanostictus with egg capsules within later 24-72 h (all P < 0.021), but there were no significant differences for their survival at early 12 h (H1,N=60= 2.03,P = 0.158, Figure 2).
3.2 Predation of G. affinis on tadpoles of P. nigromaculatus and D. melanostictus Kruskal-Wallis test indicated that the survival of Gosner stage 26 tadpoles of P. nigromaculatus was significantly higher than that of Gosner 30 tadpoles at 12 h intervals within fi rst 12-60 h (all P < 0.010), but there was no signifi cant difference during later 60-72 h (H1,N=60= 1.24, P = 0.265, Figure 3). In contrast, the survival of Gosner stage 26 tadpoles was significantly lower than that of Gosner stage 30 tadpoles of D. melanostictus at 12 h intervals within 12-72 h (all P < 0.040, Figure 3). The survival of D. melanostictus tadpoles was signifi cantly higher than that of P. nigromaculatus tadpoles at all different times within 72 h (all P < 0.001, Figure 3).
3.3 Predation of G. affi nis on eggs and embryos of P. nigromaculatus at different temperatures One-way MANOVA indicated that temperature had a significant effect on the predation of G. affi nis on P. nigromaculatus eggs and embryos (Wilks' λ = 0.18, df = 20, 66, P <0.001, Figure 4). Tukey's post hoc test suggested that there were no significant differences in the survival among 15°C, 20°C, and 25°C treatments at the point of 12 h and 24 h as shown in Figure 4. The survival of P. nigromaculatus embryos at 25°C was significantly lower than that at both 15°C and 20°C, while there were no significant differences in the survival between 15°C and 20°C at the point of 36 h and 48 h (Figure 4). The survival of P. nigromaculatus embryos at 20°C and 25°C were signifi cantly lower than that at 15°C, however, there were no signifi cant differences in the survival between 20 °C and 25°C from 60 h to 120 h (Figure 4).
4. Discussion
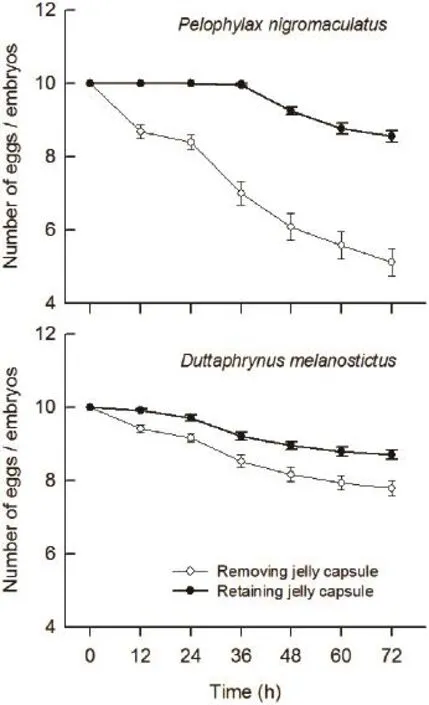
Figure 2 The survival of eggs and embryos of Pelophylax nigromaculatus and Duttaphrynus melanostictus coexisting with predator Gambusia affi nis at 12 h intervals within 3 days.
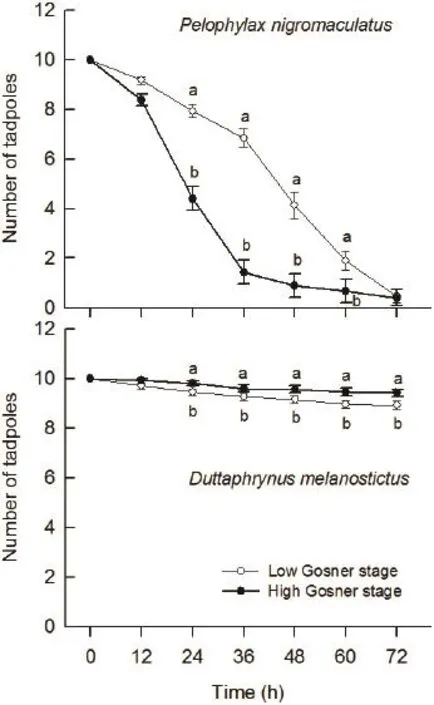
Figure 3 The survival of eggs and embryos of Pelophylax nigromaculatus and Duttaphrynus melanostictus coexisting with predator Gambusia affi nis at 12 h intervals within 3 days.
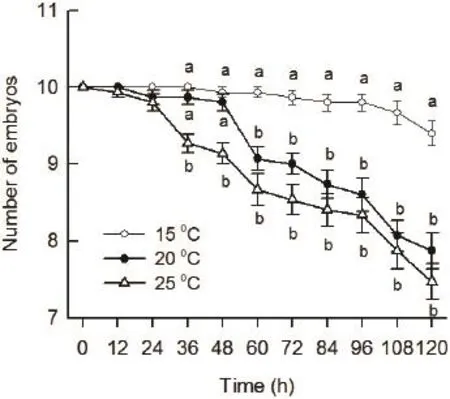
Figure 4 The survival of eggs and embryos of Pelophylax nigromaculatus coexisting with predator Gambusia affi nis at 15°C,20°C, and 25°C at 12 h intervals within 5 days.
Many anuran species enclose their fertilized eggs within transparent capsules which can attenuate environmental thermal variation (Méndez-Narváez et al., 2015), prevent damage from ultraviolet rays (Ovaska et al., 2011), protect the eggs from the harmful effects of water pollutants(Marquis et al., 2006), and serve as a mechanical defense against predation (Grubb, 1972; Zeiber et al., 2008). In our experiments, G. affi nis consumed rarely the eggs with jelly coats of P. nigromaculatus and D. melanostictus,and the survival of their embryos was higher than those without jelly coats, so this perhaps confi rm the outer jelly capsule of anurans eggs may play an important role in defensing against predators to a certain degree. Zeiber et al. (2008) found G. affi nis couldn't consume the eggs of Rana sylvatica, Bufo americanus, Ambystoma tigrinum tigrinum, and Pseudacris triseriata triseriata, and only observed a large female G. affinis temporarily stuck to the outer egg jelly coating, but was unable to successfully consume the egg. Grubb (1972) have demonstrated that predation was higher for smaller (< 3 mm), unattached anuran eggs and those with soft, loose capsules which facilitated egg extraction and consumption. The eggs of P. nigromaculatus with jelly capsule had a diameter larger than 3 mm, and they were often laid in clusters,so the firm jelly capsule most likely deterred western mosquitofish predation. The average snout width of G. affinis in our study was much shorter than the eggs diameter of P. nigromaculatus with egg capsules, so we believe that the failure of predation on the eggs in part,might be due to the morphological defects of G. affi nis,as a few studies described it a gape-limited predator(McCoy et al., 2012; Touchon and Wojdak, 2014; Zhao et al., 2014). However, there may be another reason is that the fertilized egg within the jelly coat remain stationary,which might lead to be failure to visually attract to potential predators. When the protective jelly layers of P. nigromaculatus and D. melanostictus eggs slowly began to dissolve together, and the embryos began to come out of egg capsules since about 36h. At this moment, the predator G. affi nis had access to prey on the embryos. Thus, we conclude that invasive predator G. affi nis poses little threat to the eggs of P. nigromaculatus and D. melanostictus, but only for a short period of time.
In our study, G. affinis preyed on a larger number of eggs and embryos without jelly capsules and tadpoles of P. nigromaculatus than those of D. melanostictus,for instance, G. affinis consumed nearly 95% P. nigromaculatus tadpoles but only 10% D. melanostictus ones within 72 h. Thus G. affinis's predation on anuran eggs and larvae might be species depentent. Several species belonging to the family Bufonidae were known to produce noxious or toxic compounds that deter native predators (such as dragonflies, Laurila et al., 1997;Karraker, 2011) or invasive predators (G. affi nis, Komak and Crossland, 2000; Pomacea canaliculata, Karraker and Dudgeon, 2014; Procambarus clarkii, Nunes and Richter-Boix, 2013). These toxins might render the larvae unpalatable to these predators, so unpalatability is often used as a common defensive strategy. Several previous studies have shown that the predatory weatern mosquitofish preyed selectively on palatable tadpoles,such as Limnodynastes ornatus and Pseudacris triseriata triseriata, avoiding unpalatable tadpoles of Bufo marinus and Bufo americanus (Komak and Crossland, 2000;Zeiber et al., 2008). Moreover, a tadpole's coloration is related to its anti-predator mechanism. Unpalatabletadpoles usually present black coloration, which is generally associated to aposematism (Heyer et al.,1975; Crossland and Alford, 1998; Hero et al., 2001). Additionally, unpalatable black tadpoles do not show strong reductions in foraging activity upon perceiving predation risk (D'Heursel and Haddad, 1999). The tadpoles of D. melanostictus studied in our experiments also have black skin and are fond of continuous swimming under real predation of G. affinis (personal observation). In contrast, tadpoles with brown coloration(just as P. nigromaculatus; Fei et al., 2009) usually stay motionless in the presence of a predator and moving from one point to another at high speeds if the predator attacks (Heyer et al., 1975; Azevedo-Ramos et al., 1992). Unpalatability mechanisms are the main defensive trait that makes the coexistence of tadpoles and fi sh possible(Hero et al., 2001) because fi sh are considered to be the main predators of tadpoles in permanent water bodies(Heyer et al., 1975). Therefore, G. affinis may well pose more threat to P. nigromaculatus larvae than to D. melanostictus larvae.
An interesting phenomenon happened in our laboratory studies is that predatory G. affi nis selectively consumed the different sizes of P. nigromaculatus and D. melanostictus tadpoles: more Gosner stage 26 tadpoles of P. nigromaculatus were consumed than Gosner stage 30 ones during the experiment, while it was just the opposite for D. melanostictus tadpoles in the same predation pressure. This is not consistent with the results of previous studies, weastern mosquitofi sh can consume tadpoles of all sizes up to metamorphosis by biting off their tails and visceral sections until the individual is immobilized and easier to consume (Baber and Babbitt, 2003). The reason should be further studied.
Overall, the higher the temperature was, the more the number of P. nigromaculatus embryos preyed upon by G. affinis was. It might be the results that the protective jelly capsules began to accelerate dissolution with the temperature increasing, which leading to the embryos in the high temperature treatments exposed to the predator early. As a result, it might be speculated that global warming (Araújo et al., 2006)would exacerbate the extent of the damage caused by G. affinis predation on the anuran larvae, which finally leading to the decline in anuran population.
In conclusion, the transparent jelly capsule surrounding eggs of P. nigromaculatus and D. melanostictus can serve as a mechanical defense against predator due to its large diameter, relatively stationary state and unpalatability. Based on the differences in vulnerability
of the eggs, embryos and tadpoles of P. nigromaculatus and D. melanostictus to predatory G. affi nis, we conclude invasive G. affi nis would pose more threat to the larvae of palatable P. nigromaculatus than to unpalatable D. melanostictus in China, and global warming would exacerbate the damage of predation by G. affinis on P. nigromaculatus larvae.
Acknowledgements We thank Prof. Xiang JI (Nanjing Normal University) for providing very useful comments on experimental design and earlier versions of the manuscript. This study was funded by grants from the National Natural Science Foundation of China (31270443,31500329), the Science Foundation of Zhejiang Provincial Committee of Education (Y201534237) and the Scientific Research Foundation of Ph.D., Lishui University (QD1423).
References
Araújo M. B., Thuiller W., Pearson R. G. 2006. Climate warming and the decline of amphibians and reptiles in Europe. J Biogeogr,33(10): 1712-1728
Azevedo-Ramos C., Van Sluys M., Hero J. M., Magnusson W. E. 1992. Infl uence of tadpole movement on predation by odonata naiads. J Herpet, 26: 335-338
Baber M. J., Babbitt K. J. 2003. The relative impacts of native and introduced predatory fish on a temporary wetland tadpole assemblage. Oecologia, 136(2): 289-295
Collins J. P., Storfer A. 2003. Global amphibian declines: sorting the hypotheses. Divers Distrib, 9(2): 89-98
Crossland M. R., Alford R. A. 1998. Evaluation of the toxicity of eggs, hatchlings and tadpoles of the introduced toad Bufo marinus (Anura: Bufonidae) to native Australian aquatic predators. Austral Ecol, 23: 129-137
David L. R., Craig A. S. 2006. Assessment of potential impacts of exotic species on populations of a threatened species, White Sands pupfi sh, Cyprinodon tularosa. Biol Invasions, 2006, 8(1):79-87
Deaton R., Cureton II J. C. 2011. Female masculinization and reproductive life history in the western mosquitofi sh (Gambusia affi nis). Environ Biol Fish, 92(4): 551-558
D'Heursel A., Haddad C. F. B. 1999. Unpalatability of Hyla semilineata tadpoles (Anura) to captive and free-ranging vertebrate predators. Ethol Ecol Evol, 11: 339-348
Fan X. L., Lin Z. H., Wei J. 2004. Effects of hydroperiod duration on developmental plasticity in tiger frog (Hoplobatrachus chinensis) tadpoles. Zool Res, 35(2): 124-131
Fei L., Hu S. Q., Ye C. Y., Huang Y. Z. 2009. Fauna Sinica:Amphibia, Vol. 3, Anura Ranidae. Beijing: Science Press, 320-1328 (In Chinese)
Gomez-Mestre I., Díaz-Paniagua C. 2011. Invasive predatory crayfish do not trigger inducible defences in tadpoles Ivan. P Roy Soc B, 278(1723): 3364-3370
Goodsell J. A., Kats L. B. 1999. Effect of introduced mosquitofi shon pacific treefrogs and the role of alternative prey. Conserv Biol, 13(3): 921-924
Gosner K. L. 1960. A simplifi ed table for staging anuran embryos and larvae with notes of identification. Herpetologica, 16(3):183-190
Gregoire D. R., Gunzburger M. S. 2008. Effects of predatory fi sh on survival and behavior of larval gopher frogs (Rana capito)and southern leopard frogs (Rana sphenocephala). J Herpet,42(1): 97-103
Grubb J. C. 1972. Differential predation by Gambusia affi nis on the eggs of seven species of anuran amphibians. Am Midl Nat,88(1): 102-108
Gunzburger M. S., Travis J. 2005. Critical literature review of the evidence for unpalatability of amphibian eggs and larvae. J Herpet, 39(4): 547-571
Hero J. M., Magnusson W. E., Rocha C. F. D., Catterall C. P. 2001. Antipredator defenses influence the distribution of amphibian prey species in the central Amazon rain forest. Biotropica, 33: 131-141
Heyer W. R., McDiarmid R. W., Weigmann D. L. 1975. Tadpoles,predation, and pond habitats in the tropics. Biotropica, 7: 100-111
Hussain Q. A., Pandit A. K. 2012. Global amphibian declines: A review. Internat J Biodivers Conserv, 4(10): 348-357
Karraker N. E, Dudgeon D. 2014. Invasive apple snails (Pomacea canaliculata) are predators of amphibians in South China. Biol Invas, 16(9): 1785-1789
Karraker N. E. 2011. Are toad tadpoles unpalatable: evidence from the behavior of a predatory dragonfl y in South China. Amphibia-Reptilia, 32(3): 413-418
Kerby J. L., Wehrmann A., Sih A. 2012. Impacts of the insecticide Diazinon on the behavior of predatory fi sh and amphibian prey. J Herpet, 46(2): 171-176
Komak S., Crossland M. R. 2000. An assessment of the introduced Mosquitofi sh (Gambusia affi nis holbrooki) as a predator of eggs,hatchlings and tadpoles of native and non-native anurans. Wildl Res, 27(2): 185-189
Laurila A., Kujasalo J., Ranta E. 1997. Different antipredator behaviour in two anuran tadpoles: Effects of predator diet. Behav Ecol Sociobiol, 40(5): 329-336
Li Z. Y., Jie Y. 2002. Invasive alien species in China. Beijing,China: China Forestry Publishing House, 88 pp (In Chinese)
车子过了坪地,到了富地岗,进入了一片空旷地带,没有人家,没有路灯,漆黑漆黑地,静得怕人。夜风一吹,密密树林里发出沙沙响声,仿佛要向车子扑过来。女孩说,一听到这声音我就怕,要不是深惠公路在修路,打死我也不走这个道。
Lowe S., Browne M., Boudjelas S. 2000. 100 of the world's worst invasive alien species — A selection from the global invasive species Database. Invasive Species Specialist Group, World Conservation Union
Marquis O., Millery A., Guittonneau S., Miaud C. 2006. Toxicity of PAHs and jelly protection of eggs in the common frog Rana temporaria. Amphibia-Reptilia, 27(3): 472-475
McCoy M. W., Touchon J. C., Landberg T., Warkentin K. M.,Vonesh J.R. 2012. Prey responses to predator chemical cues:disentangling the importance of the number and biomass of prey consumed. PLoS ONE, 7(10): e47495
Méndez-Narváez J., Flechas S. V., Amézquita A. 2015. Foam nests provide context-dependent thermal insulation to embryos of three Leptodactylid frogs. Physiol Biochem Zool, 88(3):246-253
Nomura1 F., Prado V. H. M., Silva F. R., Borges R. E., Dias N. Y. N., Rossa-Feres D. C. 2011. Are you experienced? Predator type and predator experience trade-offs in relation to tadpole mortality rates. J Zool, 144(2): 144-150
Nunes A. L., Richter-Boix A. 2013. Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia, 171(1): 115-127
Ovaska K., Davis T. M., Flamarique I. N. 2011. Hatching success and larval survival of the frogs Hyla regilla and Rana auroraunder ambient and artifi cially enhanced solar ultraviolet radiation. Canad J Zool, 75(7): 1081-1088
Pyke G. H. 2005. A review of the biology of Gambusia affi nis and G. holbrooki. Rev Fish Biol Fisher, 15: 339-365
Pyke G. H. 2008. Plague minnow or mosquitofi sh? A review of the biology and impacts of introduced Gambusia species. Annu Rev Ecol Evol Syst, 39(1): 171-191
Salo P., Korpimäki E., Banks P. B., Nordström M., Dickman C. R. 2007. Alien predators are more dangerous than native predators to prey populations. P Roy Soc Lond B, 274(1615):1237-1243
Segev O., Mangel M., Blaustein L. 2009. Deleterious effects by mosquitofish (Gambusia affinis) on the endangered fire salamander (Salamandra infraimmaculata). Anim Conserv,12(1): 29-37
Shulse C. D., Semlitsch R. D. 2014. Western mosquitofish(Gambusia affi nis) bolster the prevalence and severity of tadpole tail injuries in experimental wetlands. Hydrobiologia, 723(1):131-144
Smith G. R., Boyd A., Dayer C. B., Winter K. E. 2007. Behavioral responses of American toad and bullfrog tadpoles to the presence of cues from the invasive fish, Gambusia affinis. Biol Invas,58(5): 743-748
Touchon J. C., Wojdak J. M. 2014. Plastic hatching timing by red-eyed treefrog embryos interacts with larval predator identity and sublethal predation to affect prey morphology but not performance. PLoS ONE, 9(6): e100623
Zeiber R. A., Sutton T. M., Fisher B. E. 2008. Western mosquitofi sh predation on native amphibian eggs and larvae. J Freshw Ecol, 23(4): 663-671
Zhao J. Y., Yang Y., Xi X. Q., Zhang C. B., Sun S. C. 2014. Artifi cial warming facilitates growth but not survival of plateau frog (Rana kukunoris) tadpoles in presence of gape-limited predatory beetles. PLoS ONE, 9(6): e98252
*
Prof. Zhihua LIN, from Lishui University,Zhejiang, China, with his research focusing on physiological ecology of amphibians and reptiles.
E-mail: zhlin1015@126.com
28 June 2015 Accepted: 3 November 2015
 Asian Herpetological Research2016年1期
Asian Herpetological Research2016年1期
- Asian Herpetological Research的其它文章
- First Record of the Poorly Known Skink Sphenomorphus oligolepis(Boulenger, 1914) (Reptilia: Squamata: Scincidae) from Seram Island, Maluku Province, Indonesia
- The Breeding Ecology of a Critically Endangered Salamander, Hynobius amjiensis (Caudata: Hynobiidae), Endemic to Eastern China
- Catalogue of the Type Specimens of Amphibians and Reptiles in the Herpetological Museum of the Chengdu Institute of Biology,Chinese Academy of Sciences: V. Viperidae (Reptilia, Serpentes)
- No Evidence for Signifi cant Effect of Body Size and Age on Male Mating Success in the Spot-legged Treefrog
- Effects of Pesticide Exposure on Embryonic Development and Hatchling Traits of Turtles
- Soundscape Dynamics at Anuran Reproductive Sites in Pannonian Biogeographical Region: Effects of Road Noise on Vocal Activity
