Soundscape Dynamics at Anuran Reproductive Sites in Pannonian Biogeographical Region: Effects of Road Noise on Vocal Activity
András WEIPERTH, Ed SMITH, Szilvia SIMIGLA, Miklòs PUKYand Yezhong TANG
1MTA Centre for Ecological Research, Danube Research Institute, H-1113 Budapest, Karolina út 29, Hungary
2Department of Psychology, University of Maryland, College Park, MD 20742, USA
3Chengdu Institute of Biology, Chinese Academy of Sciences, # 9 of Section 4, South Renmin Road, Chengdu 610041,Sichuan, China
Soundscape Dynamics at Anuran Reproductive Sites in Pannonian Biogeographical Region: Effects of Road Noise on Vocal Activity
András WEIPERTH1, Ed SMITH2*, Szilvia SIMIGLA1, Miklòs PUKY1and Yezhong TANG3*
1MTA Centre for Ecological Research, Danube Research Institute, H-1113 Budapest, Karolina út 29, Hungary
2Department of Psychology, University of Maryland, College Park, MD 20742, USA
3Chengdu Institute of Biology, Chinese Academy of Sciences, # 9 of Section 4, South Renmin Road, Chengdu 610041,Sichuan, China
The emerging field of soundscape ecology views ecosystems in terms of biophony, geophony and anthrophony. Soundscape ecology considers the effects of sound on fauna, and this research focuses on anuran breeding lek soundscapes. The sensitivity of anuran breeding leks to acoustic disturbances makes breeding leks an important venue for a comparative soundscape study. We made long-term (> 24 h) sound recordings in three representative wetlands and short-term (< 30 min) recordings in ten sites in the Pannonian Biogeographical Region of Hungary and around the Hungary and Slovakia border. Long-term soundscapes of the fl oodplain stretch, where there is relatively minor anthrophonical disturbance, showed an obvious circadian change in sound intensities. The site with moderate sound contamination exhibited a disturbed pattern of circadian sound variation, while the site with heavy traffi c noise displayed an apparently random temporal soundscape. At different amphibian breeding sites during mating season, our short-term recordings were dominated by anuran calls, bird songs and wind noises, while insect calls and rain were present to a lesser degree. Our study indicates that vehicle traffi c noise is a severe imposition to the natural soundscape,and suggests that soundscape monitoring can provide a reliable and sensitive index of environmental change for both short-term and long-term periods.
soundscape monitoring, anuran breeding site, biophony, geophony, anthrophony
1. Introduction
Soundscape ecology, the science of sound in the landscape, is an emerging field which encompasses the causes and consequences of biological (biophony),geophysical (geophony), and human-produced(anthrophony) sounds (Pijanowski et al., 2011) to understand coupled animal and human dynamics across different scales of distance and time. It is an integrative framework that aims to describe how climate, land transformation, biodiversity patterns, and humanactivities interact through time to form dynamic acoustic landscapes. Monitoring and studying soundscapes may illuminate physical mechanisms for ecological processes and identify courses of landscape change accurately,sensitively, and economically.
Furthermore, the landscape has been reconceived as a dynamic system composed of matter, structured energy,information and meaning (Cosgrove, 2003; Farina, 2010),thus expanding upon the more classical, geographicalecological oriented perspective (Risser et al., 1984;Forman and Godron, 1986; Pickett and Cadenasso, 1995;Wu and Hobbs, 2002; Turner, 2005). In detail, sound produced in the landscape derives from various sources including human, weather, geophysical, and bioacoustic sources (Francis et al., 2011). Soundscape ecology overlaps with landscape ecology since some ecological processes occurring within landscapes can be tightly linked to and refl ected in patterns of soundcape (Formanand Godron, 1981; Urban et al., 1987; Turner, 1989;Turner et al., 2001; Farina, 2006).
In bioacoustics, four animal taxa are well known for intense acoustic emissions: birds, most anurans, some insects, and a few mammals. Most of these animals produce intense sounds during their breeding seasons to attract potential mates and to repel rivals, and they are usually silent at other times. Those sounds are generally the main components of the local soundscape in areas such as ponds and leks (Runkle et al., 1994; Catchpole and Slater, 2003; Farina et al., 2011; Wang et al., 2012). The severe consequences of anthropogenic noise on wildlife have been shown recently over a diverse array of taxa (Barber et al., 2010). Population density of frogs is negatively related to road traffi c which could be attributed partially to the reducement in attractiveness of vocal display by traffic noise since anuran chorus behavior might be affected by man-made acoustic interference either directly through modulating call rates of the chorus participants or indirectly, through suppressing calling behavior of one set of species which in turn stimulated calling in other species (Sun and Narins, 2005;Cunnington and Fahrig, 2010). Nevertheless, ecological changes in response to noise at broad-scales have not yet been examined or tracked over time. In the present study,we compare soundscapes of three wetlands where frogs reproduce in differing levels of anthropogenic noise, and report acoustic analyses of soundscapes at numerous sites around the Pannonian Biogeographical Region.
2. Materials and Methods
2.1 Acoustic recording Study areas include fl oodplains,wetlands and ponds in the Pannonian Biogeographical Region in Hungary and Slovakia where the fire bellied toad (Bombina bombina) is the dominant species in most sampling sites from fl oodplains along the River Ipoly to water habitats near Lake Balaton during our recording time. The common spadefoot toad (Pelobates fuscus),European green toad (Bufotes viridis), European tree frog(Hyla arborea) and water frogs (Pelophylax esculentus)are common anuran species while some of them advertised vocally earlier or later than the period when we recorded. Long-term recordings were made at two sites along the River Ipoly (Hugyag and Hont), northeast Hungary and one site at Lake Balaton (Balatonederics) in order to investigate the temporal changes in soundscapes in the term of circadian period. Short-term recordings were made at seven places in total with some places containing two ponds (i.e. sites) (Budapest, Hont, Hugyag, Ipolydamázsd, Ipolyság/Hont, Ipolyszög,Letkés) (Figure 1) with the purpose of surveying changes among different studied sites.
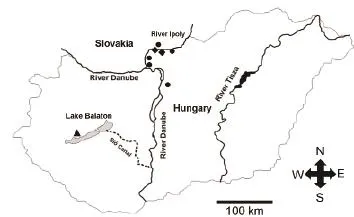
Figure 1 Acoustic recording sites in the Pannonian Biogeographical Region, triangle: only long-term recording (Balatonederics), circle:only short-term recordings (Budapest, Ipolydamázsd, Ipolyság/ Hont, Ipolyszög, Letkés), rhombus: long- and short-term recordings.
Long-term acoustic recordings were made with a portable recorder (Sony, Japan) placed in a nearby tree and oriented to the center of the wetland. The recording volume was fi xed at the level of 25 and the soundscapes were recorded continuously for more than 24 hours. The frequency response of the Sony recorder was from 100 Hz to 16 000 Hz with the sample rate was set to 44 000 Hz. Fifteen-minute short-term recordings were made with the Marantz PMD670/U1B recorder (USA) connected to an AE3300 microphone (Audio-Technica, USA) at each of ten sites. The recording sensitivity, via the gain knob,was set specifically for each recording to optimize the signal noise ratio. The frequency response of the Marantz recorder was fl at to 20 000 Hz, +/- 0.25 dB. Gain knob settings, address, time, temperature, relative humidity and GPS information were recorded at each site.
All recordings were completed in June 1-15, 2013 with each site recorded 1-2 times. Five anuran species' calls were recorded in the Pannonian Biogeographical Region:B. bombina, H. arborea, P. esculentus, P. ridibundus,and P. lessonae, the latter two species contributing only slightly to the soundscapes.
2.2 Data analyses For measuring the intensity, creating the sonogram and analyzing the acoustic component,long-term recordings were fi rst segmented manually into sixty-minute sections. Then, the fi rst fi ve-minute of each one-hour segment was analyzed further. Because the soundscape was changing quite slowly, the five-minute segments represented the complete hour to a large extent. PRAAT (an open-source program released by Universityof Amsterdam) was used to measure relative sound intensities in dB and to create sonograms. For intensity measurement, the “To Intensity” function was used with“Down to Intensity Tier” and “Down to Table Of Real”operations. These relative intensity data were saved as txt files which in turn were loaded into MS Excel in order to calculate means for each segment. PRAAT was also used to create sonograms using the “Analyse Spectrum” function with a Hanning window length of 30 milliseconds. Sonograms were displayed conventionally --as two-dimensional figures (x-axis: time; y-axis:frequency) with warmer hues indicating frequencyspecifi c energy. Sounds were identifi ed by experimenters through visual inspection of the sonograms and listening to the recordings.
For each site, SPL was derived from the short-term recordings. The constant sensitivity of the microphone and the variable sensitivity of the recorder were determined in a calibration step, and then the SPL at each site was determined in a measurement step. In the calibration step,a 1-KHz tone was played into the microphone at 71.3 dB SPL unweighted, measured by Bruel & Kajer 2250G Integrating Sound Level Meter. Reference recordings were made for a range of recorder gain knob settings(“gain settings”) of 3 through 7, in steps of 0.5, and digital RMS levels were computed for each reference recording. Gain settings were plotted against the RMS levels, as shown in Figure 2. An interpolating polynomial (made with Matlab) was fi tted to the data to allow computation of digital RMS levels corresponding to the reference 71.3 dB tone for any gain knob setting between 3.0 and 7.5(Rossing, 1990).

Figure 2 Plots of recorded root mean square (RMS) in volts to knob settings of the recorder used to compute SPL of site soundscapes.
In the measurement step, representative segments of the site recordings were chosen, and average RMS levels were computed for each segment. For each segment, the linear ratio of the segment RMS level to the RMS level of the reference tone recording was computed, taking into account the gain setting used at each recording site. The site-specifi c unweighted SPL levels were then computed by converting the linear ratios to dB levels, and adding these dB levels to the original SPL measured during the calibration step (71.3 dB). A-weighted SPL levels were computed in exactly the same way, except that the sitespecifi c segments were fi ltered with an A-weighting fi lter(in Matlab) before the corresponding digital RMS levels were computed.
3. Results
3.1 Different circadian patterns of soundscap Circadian soundscape variations were examined at three wetland sites: the Hugyag site, the Hont site, and the Balaton site. The Hugyag site (N 48°05'874"; E 19°26'533"; H 147 m) is located in a remote border area with almost no anthropogenic noise. Similarly, the Hont site (N 48°03'494"; E 18°58'264"; H 119 m) is located along the floodplain of River Ipoly, near the border between Hungary and Slovakia but, unlike at Hugyag, it is only 120 meters away from highway E77, so there is heavy traffic noise. The Balaton site (N 46°48'231"; E 17° 24'226"; H 110 m) is situated along Lake Balaton at Balatonederics, and has a medium level of traffi c noise.
The soundscape in Hugyag varied daily in intensity from 50 to 80 dB. The peak sound intensity occurred precisely at 21:30, but no obvious intensity valley could be seen. Low intensities started at 4:30 and lasted to 13:30, with variations between 50.4 and 62.5 dB ( 3A). The relative sound intensities in Hont varied irregularly between 70 and 90 dB (Figure 3B). The fact that the minimal intensity in Hont was higher than that in Hugyag was attributed to the difference in high background of traffic noise. At Lake Balaton a 24-hour variation in sound intensities could be found even with masking of the traffi c noise (Figure 3C). Because of the moderate traffi c noise, the lowest sound intensity at Balaton was higher than that at Hugyag but lower than that at Hont.
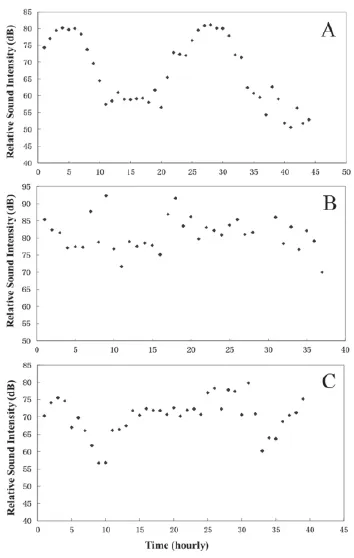
Figure 3 Circadian changes in sound intensity from three wetlands where anurans reproduce. A. Evident variation in pattern of sine wave in Hugyag; B. Irregular pattern of alteration in Hont; C. A pattern with regular wave disturbed by random noises in Lake Balaton.
3.2 Temporal changes in biological components The dominant species of anuran communities in the Pannonian Biogeographical Region is Bombina bombina,a poisonous toad. At Hugyag this species produced advertisement calls nocturnally and diurnally, while circadian variations in intensity peaked around 20:00. The main call energy was concentrated at 470 Hz (Figure 4A). The species of Hyla arborea contributed largely to the soundscape from 21:00 to 1:00, displacing B. bombina as the loudest call, with dominant frequency around 2600 Hz (Figure 4B). At Lake Balaton Pelophylax esculentus and P. ridibundus called simultaneously and formed a chorus consisting of two frog species and traffi c cars. Interestingly, vocal activities of the two Pelophylax species were evoked frequently by the traffic noise(Figure 4C). Crickets and rain contributed some energy to the soundscape at the Balaton site. Soundscape at Hont was consisted mainly of bird songs which were masked largely with traffic noises while P. ridibundus produced calls around and after the midnight, contributing slightly to the overall soundscape.
3.3 Varied structures of soundscape at different sites Soundscapes at the ten sites in the Pannonian Biogeographical Region show variation in mean intensity,temperature, relative humidity and major components(Table 1). For the anuran breeding sites, the principal bioacoustic sources were some anuran species, while birds, insects, and other frogs were minor sound sources(Figure 4D). Other acoustic sources were direct wind and wind in plants. We found no correlations among biological sounds and environmental elements such as temperature and relative humidity (for homogeneity test for variance and binary regression, p values > 0.05); this was probably due to differences in species composition at each site.
4. Discussion
In spatial dimension, a diverse array of sounds produced by mammals, birds, amphibians, and insects might be the main components of the soundscape in forests,grasslands and wetlands (Marler and Slabbekoorn, 2004),while the urban soundscapes are composed of sounds generated by vehicles, machines and other humanproduced sounds (Botteldooren et al., 2004; Raimbault and Dubois, 2005). Abiological parts of the soundscape include gushing rivers flowing over terrain, rain falling through canopies, and wind (Swanson et al., 1988). Time scales of the soundscape vary daily, seasonally and annually in habitats (Tang et al., 2001; Wang et al.,2012), reflecting circadian, reproductive periods, and habitat and/or climate changes, respectively. Soundscapes change dramatically as environments change, and animal vocalizations account for most of these changes. In addition, long-term ecological changes in landscape, e.g.,those accompanying desertization, global climate change,construction of transportation thoroughfares and other human activities, are reflected by soundscape changes too.
It is well known that most anurans, if not all, compete for mate selection through vocalization (Kelley, 2004). Wetlands and/or ponds are sites for lekking by anurans,where acoustic communication, competition, mating, egg laying, and tadpole development occur. There is usually high acoustic background noise, since other animals make sounds around these sites. Circadian and seasonal changes in anuran vocalization can be expected in order to mitigate the interference effects of bio-noise.
Many frog-eating waders forage at the sites where we set up our study from 9:00 to 19:00. Bombina species secrete poison which protects them from birds, thusallowing this toad to make advertisement calls day and night with a slight decrease during bird predation. H. arborea, P. esculentus and P. ridibundus produced calls most intensively after midnight when birds are at rest. Why these two Pelophylax species overlap their calling times, and the breadth of sounds that are observed to elicit calling from the species are both enigmas. At the sites, calling was often initiated by traffi c noise, and we could cause males to start calling by orally mimicking their calls (unpublished data). Many frog species vocalize in the form of chorus in which one male's calls generally stimulate other males to produce calls (Ryan et al.,1981; Fang et al., 2013). It is likely that male frogs are easily inducible behaviorally with less discrimination of stimulation structures while females are usually fastidious, and thus males can be evoked to call by many sounds including the traffi c noise.

Table 1 Sound intensities and acoustic components of the soundscape recorded at different times from different sites within the Pannonian region.

Figure 4 Waveforms and spectrograms of advertisement calls from three anuran species. A. Calls of Bombina bombina; B. Calls of Hyla arborea and B. bombina; C. Calls of Pelophylax esculentus and P. ridibundus; D. Sound recorded from a small pond to show acoustic components. To clearly depict these different vocalizations, sonogram frequencies are from 0 to 5k Hz for A, 0 to 10k Hz for B and C, and 0 to 8.5k Hz for D.
Man-made noise from vehicles and machines might vary in a circadian rhythm, but not with a seasonal period. No site of nature reserves in the continental US is free from this man-made noise (Barber et al., 2011), and the same is most probably true for Europe. An international road, busy with trucks day and night, crosses the border between Hungary and Slovakia near the Hont site. A national road, occupied with relatively few cars at night,runs along Lake Balaton, while no roads exist near the Hugyag site. Soundscapes at these three sites exhibit different temporal patterns, mostly correlated with the traffi c noise. Our study indicates a large infl uence of noise contamination on the anuran bioacoustic components in the soundscape, which would mask the auditory signals of anurans (Bee and Swanson, 2007). A parallel situation exists for birds (Francis et al., 2011).
It has been demonstrated that anthrophony infl uenced negatively reproductive successes of vocal speices through masking acoustic signals for sexual displays(Sun and Narins, 2005; Lengagne, 2008; Cunnington and Fahrig, 2010; Halfwerk et al., 2011). In addition,migratory birds showed a change in ability to gain body condition during migratory stopover when stayed along a“phantom road” in spite of the noise (Ware et al., 2015). Some vocal animals could, however, adjust their call parameters in avoidance of the anthrophonic noise by upshifting frequency in bird (Slabbekoorn and Peet, 2003)and frog (Parris et al., 2009), enhancing intensity, i.e. Lombard effect in bird (Brumm, 2004) and prolonging call duration in frog (Love and Bee, 2010). In contrast,Hyla males were incapable of adjusting their temporal or frequency call structures to increase efficiency of the vocal communication in the noise environment(Lengagne, 2008). The further study should be necessary to investigate the soundscape by measuring acoustic parameters as many as possible not just intensity.
Acknowledgement This work was supported fi nancially by the Program of Exchange Visit between Chinese Academy of Sciences and Hungarian Academy of Sciences and the National Natural Science Foundation of China (NSFC 31272304 to TYZ).
References
Barber J. R., Crooks K. R , Fristrup K. M. 2010. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol Evol, 25: 180-189
Barber J. P., Burdett C. L., Reed S. E., Warner K. A.,Formichella C., Crooks K. R., Theobald D. M., Fristrup K. M. 2011. Anthropogenic noise exposure in protected natural areas: estimating the scale of ecological consequences. Landscape Ecol, 26: 1281-1295
Bee M. A., Swanson E. M. 2007. Auditory masking of anuran advertisement calls by road traffi c noise. Anim Behav, 74: 1765-1776
Botteldooren D., Coensel B. D., Meur T. D. 2004. The temporal structure of the urban soundscape. J Sound Vib, 292: 105-123
Brumm H. 2004. The impact of environmental noise on song amplitude in a territorial bird. J Anim Ecol, 73: 434-440
Catchpole C. K., Slater P. J. B. 2003. Bird song: Biological themes and variations. 2nd Edition. Cambradge Press
Cosgrove D. 2003. Landscape: ecology and semiosis. In: Palang H,Fry G (eds), Landscape interfaces: cultural heritage in changing landscapes. Kluwer, Dordrecht, 15-20
Cunnington G. M., Fahrig L. 2010. Plasticity in the vocalizations of anurans in response to traffi c noise. Acta Oecol, 36(5): 463-470
Fang G. Z., Jiang F., Yang P., Cui J. G., Brauth S. E., Tang Y. Z. 2013. Male vocal competition is dynamic and strongly affected by social contexts in music frogs. Anim Cogn, 17(2): 483-494
Farina A. 2006. Principles and methods in landscape ecology. Springer, NY
Farina A. 2010. Ecology, cognition and landscape. Springer,Dordrecht
Farina A., Lattanzi E., Malavasi R., Pieretti N., Piccioli L. 2011. Avian soundscapes and cognitive landscapes: Theory, application and ecological perspectives. Landscape Ecol, 26: 1257-1267
Forman R. T. T., Godron M. 1981. Patches and structural components for a landscape ecology. BioScience, 31: 733-740
Forman R. T. T., Godron M. 1986. Landscape ecology. John Wiley, New York
Francis C. D., Paritsis J., Ortega C. P., Cruz A. 2011. Landscape patterns of avian habitat use and nesting success resulting from chronic gas well compressor noise in NW New Mexico, USA. Landscape Ecol. doi:10.1007/s10980-011-9609-z
Halfwerk W., Holleman L. J. M., Lessells C. M., Lessells M.,Slabbekoorn H. 2011. Negative impact of traffi c noise on avian reproductive success. Journal of Applied Ecology, 48(1): 210-219.
Slabbekoorn H., Peet M. 2003. Ecology: Birds sing at a higher pitch in urban noise. Nature, 424(6946): 267
Lengagne T. 2008. Traffi c noise affects communication behaviour in a breeding anuran, Hyla arborea. Biol Conserv, 141(8): 2023-2031
Love, E. K., Bee, M. A. 2010. An experimental test of noisedependent voice amplitude regulation in Cope's grey treefrog,Hyla chrysoscelis. Anim behav, 80(3): 509-515
Kelley D. B. 2004. Vocal communication in frogs. Curr Opin Neurobiol, 14: 751-757
Marler P., Slabbekoorn H. 2004. Nature's music: the science of birdsong. Elsevier Academic Press, San Diego, USA
Parris K. M., Velik-Lord M., North J. M. A. 2009. Frogs Call at a Higher Pitch in Traffi c Noise. Ecol Soc, 14(1): 124-124
Pickett S. T. A , Cadenasso M. L. 1995. Landscape ecology: spatial heterogeneity in ecological systems. Science, 269: 331-334
Pijanowski B. C., Farina A., Gage S. H., Dumyahn S. L., Krause B. L. 2011. What is soundscape ecology? An introduction and overview of an emerging new science. Landscape Ecol, 26:1213-1232
Raimbault M., Dubois D. 2005. Urban soundscapes: experiences and knowledge. Cities, 22(5): 339-350
Risser P. G., Karr J. R., Forman R. T. T. 1984. Landscape ecology: Directions and approaches. Illinois Natural History Survey Special Publication 2, Champaign
Rossing T. D. 1990. The Science of Sound. 2nd Edition. Addison-Wesley Publishing Company, 92-93
Runkle L. S., Wells K. D., Robb C. C., Lance S. L. 1994. Individual, nightly, and seasonal variation in calling behavior of the gray tree frog, Hyla versicolor: implications for energy expenditure. Behav Ecol, 5: 318-325
Ryan M. J., Tuttle M. D., Taft L. K. 1981. The costs and benefi ts of frog chorusing behavior. Behav Ecol Sociobiol, 8(4): 273-278
Swanson F. J., Kratz T. K., Caine N., Woodmansee R. G. 1988. Landform effects on ecosystem patterns and processes. BioScience, 38(2): 92-98
Sun J. W., Narins P. M. 2005. Anthropogenic sounds differentially affect amphibian call rate. Biol Conserv, 121(3): 419-427
Tang Y. Z., Zhuang L. Z., Wang Z. W. 2001. Advertisement Calls and Their Relation to Reproductive Cycles in Gekko gecko(Reptilia, Lacertilia). Copeia, 1: 248-253
Turner M. G. 1989. Landscape ecology: the effect of pattern on process. Annu Rev Ecol Syst, 20: 171-197
Turner M. G., Gardner R. H., O'Neill R. V. 2001. Landscape ecology in theory and practice: pattern and process. Springer Press, New York
Turner M. G. 2005. Landscape ecology: what is the state of the science? Annu Rev Ecol Syst, 36: 319-344
Urban D. L., O'Neill R. V., Shugart H. H. 1987. Landscape ecology. BioScience, 37: 119-127
Wang, J. C., Cui J. G., Shi H. T., Brauth S. E., Tang Y. Z. 2012. Effects of Body Size and Environmental Factors on the Acoustic Structure and Temporal Rhythm of Calls in Rhacophorus dennysi. Asian Herpetol Res, 3: 205-212
Ware H. E., Mcclure C. J. W., Carlisle J. D., Barber J. R. 2015. A phantom road experiment reveals traffi c noise is an invisible source of habitat degradation. P Natl Acad Sci USA, 39: 12105-12109
Wu J., Hobbs R. 2002. Key issues and research priorities in landscape ecology: an idiosyncratic synthesis. Landscape Ecol,17: 355-365
*Corresponding authors: Dr. Ed SMITH, from Department of Psychology, University of Maryland, USA, with his research focusing on bioacoustics; Prof. Yezhong TANG, from Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China, with his research focusing on neurobiology of amphibians and reptiles.
E-mail: fastcared@yahoo.com (E. SMITH); tangyz@cib.ac.cn (Y. Z. TANG)
22 September 2015 Accepted: 25 December 2015
 Asian Herpetological Research2016年1期
Asian Herpetological Research2016年1期
- Asian Herpetological Research的其它文章
- First Record of the Poorly Known Skink Sphenomorphus oligolepis(Boulenger, 1914) (Reptilia: Squamata: Scincidae) from Seram Island, Maluku Province, Indonesia
- The Breeding Ecology of a Critically Endangered Salamander, Hynobius amjiensis (Caudata: Hynobiidae), Endemic to Eastern China
- Catalogue of the Type Specimens of Amphibians and Reptiles in the Herpetological Museum of the Chengdu Institute of Biology,Chinese Academy of Sciences: V. Viperidae (Reptilia, Serpentes)
- Effects of Predation by Invasive Western Mosquitofi sh (Gambusia affi nis) on Survival of Eggs, Embryos and Tadpoles of Pelophylax nigromaculatus and Duttaphrynus melanostictus in South China
- No Evidence for Signifi cant Effect of Body Size and Age on Male Mating Success in the Spot-legged Treefrog
- Effects of Pesticide Exposure on Embryonic Development and Hatchling Traits of Turtles
