No Evidence for Signifi cant Effect of Body Size and Age on Male Mating Success in the Spot-legged Treefrog
Jianping YU, Cheng CHEN, Long JIN, Li ZHAO and Wenbo LIAO
Key Laboratory of Southwest China Wildlife Resources Conservation (Ministry of Education), China West Normal University, Nanchong 637009, Sichuan, China
No Evidence for Signifi cant Effect of Body Size and Age on Male Mating Success in the Spot-legged Treefrog
Jianping YU, Cheng CHEN, Long JIN, Li ZHAO and Wenbo LIAO*
Key Laboratory of Southwest China Wildlife Resources Conservation (Ministry of Education), China West Normal University, Nanchong 637009, Sichuan, China
In anurans, body size and age of individuals generally affect male mating success. To test whether body size and age have effects on male mating success in the foam-nesting treefrog Polypedates megacephalus, a species widely distributed in China, we analyzed differences in body size and age between mated and unmated males for three populations using a Generalized Linear Mixed Model (GLMM). The results showed that mated males did not exhibit larger body size and older age than unmated males, suggesting that large and/or old male individuals did not have greater mating success than small and/or young males. Moreover, we also found a non-significant size-assortative mating pattern for all populations. Our fi ndings suggest that body size and age of the foam-nesting treefrog do not affect male mating success.
age, body size, male mating success, Polypedates megacephalus, size-assortative
1. Introduction
Sexual selection theory suggests that parental reproductive investment determines which sex is choosy with respect to mate choice and which sex contests (Darwin, 1871). A majority of studies on sexual selection have shown that females are generally the choosier sex and that males compete for mates (Emlen and Oring, 1977; Liao and Lu,2009a; Sih et al., 2014). As a result, sexual selection can promote male mating success and/or female reproductive success (Andersson, 1994).
In amphibians, numerous studies on sexual selection reveal that relatively high values of traits in males (i.e. body size, forearm length and thumb-pad width) will be preferred by females (Bell, 2010; Liao and Lu 2011a,b). As the most important trait, body size is linked to the males' physical strength and the ability to compete for females (Halliday, 1983). Size-dependent mating success,in which large males have higher mating opportunity than small males, is generally interpreted as the consequenceof male-male competition (Cothran, 2008; Chen and Lu,2011; Liao and Lu, 2011b). This is a general pattern in the animal kingdom because large males exhibit more dominance than small males when they compete for access to females or maintain resources necessary for females (Clutton-Brock et al., 1992; Awata et al., 2006;Liao and Lu, 2011b). Moreover, body size also serves as a basis for female mate choice not only because large males may be older and consequently have demonstrated their ability to survive (Halliday, 1983; Wilbur et al.,1978; Wellborn and Cothran, 2007; Liao and Lu, 2011a),but also because females may gain indirect benefi ts from being choosy by passing on good genes to offspring or sons of large males (Andersson, 1994). In some anuran species, male mating success is also correlated with age because old individuals have better survival rate and more successful egg fertilization than do young ones (Byrne and Whiting, 2008; Liao and Lu, 2011a; Somashekar and Krishna, 2011; Kierl and Johnston, 2015).
The spot-legged treefrog (Polypedates megacephalus)is widely distributed in China, at altitudes ranging from 520 m to 2200 m (Fei and Ye, 2001). Breeding activity ranges from mid-April to late July. It is a lekking species where males gather at ponds to wait for females. Females are only present at these ponds during the night formating. Amplectant pairs gather at the edge of the pond and then release foam above the pond's water surface. Immediately, other males came to join the pair, forming an amplexing group of 2 to 5 males. There is no evidence for scramble competition, combats, pushing etc. between males in nests. Except for the knowledge presented above, there is no detailed information about the effect of body size and age on male mating success in the species. The present study is a combination of field collection and skeletochronology to analyze the effect of both body size and age on male mating success in the spot-legged treefrog. More specifi cally, our aims were (1): to analyze the correlation of body size between amplectant males and amplectant females to confi rm size-assortative mating pattern for all populations; (2) to compare the body size between mated and unmated males to gain insight into the relationship between male mating success and body size; (3) to test the relationship between male mating success and age by comparing the age between mated and unmated males.
2. Material and Methods
2.1 Study area We collected three P. megacephalus populations located at different altitudes in Guizhou province in western China during the successive breeding seasons (2014 and 2015). The low-altitude site was located at 449 m in Fanjing mountains (108°44.67' E,27°46.33' N) where the treefrogs reproduce in a farm (5.0 × 3.6 × 2.8 m3; L × W × H) surrounded by farmhouse. The middle-altitude site was located at 680 m in Shangzhong town (108°43.38' E, 27°23.38' N) where the treefrogs reproduce in a farm (3.0 × 4.2 × 3.8 m3; L × W × H) near a small pigfarm. The high-altitude site was located at 1300 m in Leigong mountains (108°10.27' E,26°22.73' N) where the treefrogs reproduce in two natural ponds (2.0 × 1.5 × 1.2 m3, 12.0 × 4.0 × 0.3 m3; L × W × H) surrounded by farmhouse.
2.2 Samplings We conducted fi eld observations for all populations during the breeding season in 2014 and 2015. We recorded the amplectant males and unamplectant males in the pond. The amplectant and unamplectant males were distinguished by the condition where males attend to the group spawning. Within each breeding site we captured all males and females that spend the nights at the pond after laying eggs of all females. For the three breeding sites, the females produce eggs exceeding twenty days. We collected a total of 209 tree frogs(Leigong: 58 males and 20 females; Shangzhong: 48 males and 21 females; Fanjing: 41 males and 21 females). We confi rmed all individuals as adults though observation of secondary sexual traits (presence of nuptial thumb pads in adult males and the infl ated abdomen carrying the developing eggs of adult females). We measured body size (snout-vent length: SVL in mm) of each individual to the nearest 0.1 mm using a vernier calipers. The second phalange of the longest hind fi nger from the right hindlimb of each treefrog was removed and stored in 4% neutral buffered formalin for age determination (see below). Each treefrog was released at the capture site.
2.3 Age determination We used skeletochronology which counts the number of lines of arrested growth(LAGs) in stained cross sections of the phalangeal bones to determine age of each individual (see Castanet and Smirina, 1990). An improved method of paraffin sectioning and Harris's haematoxylin staining to produce histological sections was used for aging adult females and males. The method has been successfully used in many Chinese anurans (Liao and Lu, 2010; Liao and Lu, 2012;Li et al., 2013; Huang et al., 2013). Cross-sections (13 μm thick) of the phalanx with the smallest medullar cavity was used to mount on glass slides. Then we recorded the number of lines of LAGs, taken from mid-diaphyseal sections, using a Motic BA300 digital camera mounted on a Moticam2006 light microscope at×400 magnification. We also analyzed LAG endosteal resorption and double lines following the methods of Castanet and Smirina(1990). Of 209 adult specimens, 207 (145 males and 62 females) exhibited clear LAGs in their bone sections.
2.4 Data analysis All analyses were performed by using the SPSS 21.0 statistical package. Body size was log10-transformed. To assess size-assortative mating pattern for the three populations, we analyzed the correlation of body size between males and females using a Pearson correlation analysis. To test differences in mean body size between paired and unpaired males across altitudes,we used a Generalized Linear Mixed Model (GLMM)with log10(SVL) or age as the dependent variable, mating status as a fi xed factor, and population as a random effect,altitude as covariate. We ran a GLMM with age and altitude as covariate to test differences in body size of paired and unpaired males after removing the effects of age.
3. Results
The breeding season differed significantly across the three breeding sites (Leigong: 18-30 April; Fanjing: 1-12 May; Shangzhong: 14-24 May). Reproduction lastedfour weeks. Sexual activity started at sunset (20:00),with males usually arriving at the breeding sites fi rst, and fi nished when the last males departed (02:30-03:30). The whole process of oviposition lasted 30-80 minutes. We gathered 124 treefrogs (62 pairs) in amplexus. Females had significantly larger body size than males (Table 1). Comparison of SVL of mated pairs revealed a nonsignifi cant size-assortative mating patterns by body size for the three populations (Figure 1; Leigong: r = -0.024,n = 20, P = 0.918; Shangzhong: r = -0.073, n = 21, P = 0.752; Fanjing: r = 0.274, n = 21, P = 0.229).
The GLMM revealed that there was also nonsignifi cant difference in average body size between mated and unmated males (F1,0.435= 0.667, P = 0.669; Table 1)and among altitudes across populations (altitude: F1,4.598= 1.375, P = 0.298; population: Z = 0.207, P = 0.836). Likewise, average age did not differ between mated and unmated males (GLMM: F1,142= 0.409, P = 0.523; Table 1)and among altitudes (F2,142= 1.843, P = 0.177). There was also non-signifi cant difference in body size between mated and unmated males (GLMM: F1,32.269= 0.698, P = 0.410) when controlling the effects of age and altitude on body size (age: F1,138.686= 23.400, P < 0.001; altitude:F1,32.269= 0.698, P = 0.410, P = 0.121; population: Z = 0.667, P = 0.505).
4. Discussion
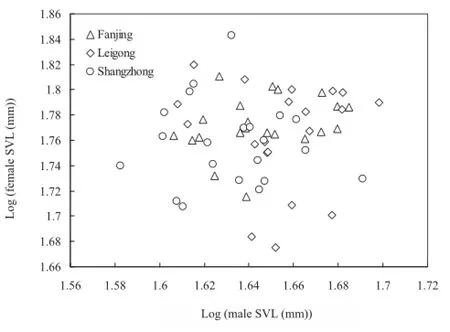
Figure 1 A non-significant size-assortative mating pattern in Polypedates megacephalus for the three populations.
Our results uncover a non-significant size-assortative mating patterns all P. megacephalus populations. Furthermore, mean body size and age do not differ between mated and unmated males in P. megacephalus,suggesting that large or old males do not exhibit higher mating success than do small or young males. Sizeassortative mating pattern can increase fertilization rate by cloacal apposition during spawning (Halliday, 1983). Previous studies have indicated that size-assortative mating in several anurans improves the apposition of female and male cloacae while spawning (Bufo bufo,Davies and Halliday, 1977; Triprion petasatus, Lee and Crump, 1981; Hyla elegans, Hyla labialis, Gutiérrez and Lüddecke, 2002; Agalychnis callidryas and A. moreletii, Briggs, 2008; B. andrewsi, Liao and Lu, 2012). For the treefrog, fertilization rate was not improved by the apposition of female and male cloacae, but group spawning. Moreover, size-assortative mating pattern may be the outcome of competition among males for females (Arak, 1983). However, we did not fi nd a positive correlation of body size between mated males and mated females for 62 pairs. This pattern may attribute to weak male-male competition for females in P. megacephalus when 2-5 males gather at foam nests.
Difference in body size was non-significant between mated and unmated males in P. megacephalus even when controlling for the effect of age and altitude which suggests that large males did not exhibit higher opportunity to obtain mates. This is inconsistent with the prediction that sexual selection act on body size in the related foam-nest species due to female mate choice depending on male calls: larger males have higher callfrequencies, and females can exploit this information(i.e. R. schlegelii, Fukuyama, 1991; R. arboreus, Kusano et al., 1991; R. omeimontis, Liao and Lu, 2011a). For most anurans, body size cannot be a good indicator of male competing advantages, thus, large body size did not reflect a male's ability to compete for a female (Davies and Halliday, 1977; Gutiérrez and Lüddecke, 2002; Liao and Lu, 2009b; Liao et al., 2015). In this study, body size cannot be a good indicator of male competing advantages because intensive sperm competition in group spawning trade off male-male competition. Similar results have been observed in other frogs and toads (Halliday 1983;Liao and Lu, 2011a).
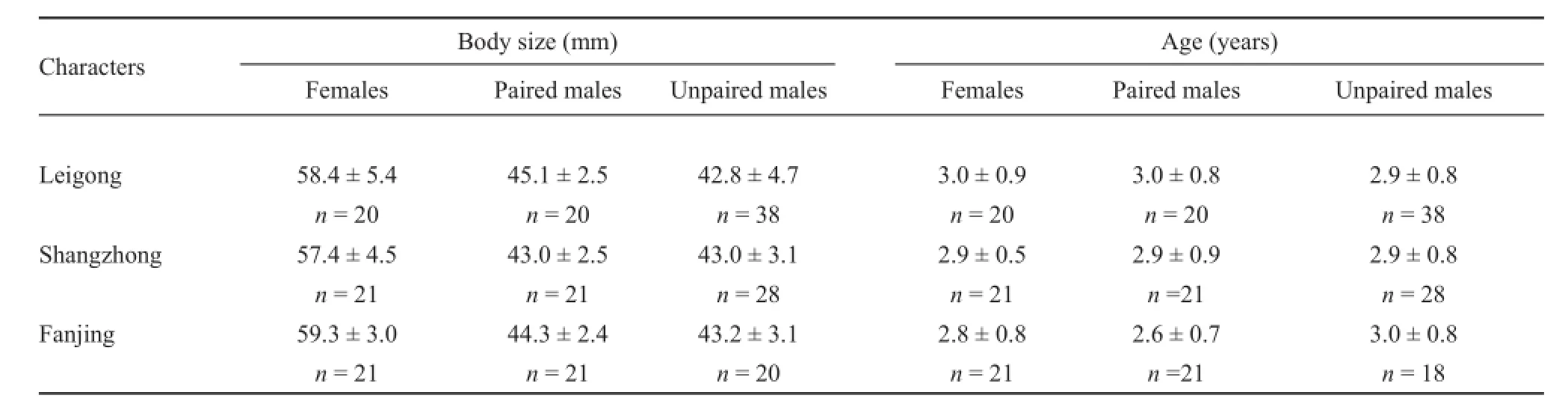
Table 1 Means and standard deviations of body size, age and sampling for three populations of Polypedates megacephalus.
Male attributes that it is important for consideration in females include controlling resources and genetic quality(Howard, 1978; Sih et al., 2014). For anurans, strong competitive ability in old males shows a high genetic quality (Wilbur et al., 1978; Trivers, 1972). In the study,we did not find difference in mean age between mated and unmated males in P. megacephalus, suggesting that old males did not exhibit higher mating success. This is inconsistent with the hypothesis in frogs that females mating with old males would gain indirect genetic benefits in terms of improved offspring fitness (Wilbur et al., 1978; Howard, 1984; Liao and Lu, 2011a).
Age shows a positive correlation with male advertisement calls with a high rate or long duration,thus, female preference for males with high rate or long duration equates with choosing old males (Welch et al.,1998; Liao and Lu, 2011a). In the study, we did not fi nd females preference for old males, suggesting that there may not be indirect genetic benefi ts for females choosing males with long calls. Hence, further investigation is warranted to analyze the relationships between male call rates, female preferences and male age.
Acknowledgements We thank the Sichuan Province Outstanding Youth Academic Technology Leaders Program (2013JQ0016), the Students Science and Technology Innovation Fund of China (201510638016)and Sichuan Province Department of Education Innovation Team Project (14TD0015; 15TD0019) for providing for fi nancial support. All experiments involving the sacrifice of these live animals were approved by the Animal Ethics Committee at China West Normal University.
References
Andersson M. 1994. Sexual Selection. Princeton University Press,Princeton
Arak A. 1983. Male-male competition and mate choice in anuran amphibians. In: Bateson P. (ed). Mate Choice. pp.181-210. Cambridge: Cambridge University Press
Awata S., Takeuchi H., Kohda M. 2006. The effect of body size on mating system and parental roles in a biparental cichlid fish (Julidochromis transcriptus): A preliminary laboratory experiment. J Ethol, 24: 125-132
Bell M. B. V. 2010. Sex and age infl uence responses to changes in the cost of cooperative care in a social carnivore. Beha Ecol, 21:1118-1123
Briggs V. S. 2008. Mating patterns of Red-Eyed Treefrogs,Agalychnis callidryas and A. moreletii. Ethol, 114: 489-498
Byrne P. G., Whiting M. J. 2008. Simultaneous polyandry increases fertilization success in an African foam-nesting treefrog. Anim Behav, 76: 1157-1164
Castanet J., Smirina E. M. 1990. Introduction to the skeletochronological method in amphibians and reptiles. Ann Des Sci Nat Com Zool, 11: 191-196
Chen W., Lu X. 2011. Sex recognition and mate choice in male Rana kukunoris. Herpetol J, 21: 141-144
Clutton-Brock T. H., Price O. F., MacColl A. D. C. 1992. Mate retention, harassment, and the evolution of ungulate leks. Behav Ecol, 3: 234-242
Cothran R. D. 2008. The mechanistic basis of a large male mating advantage in two freshwater amphipod species. Ethology, 114:1145-1153
Darwin C. 1871. The Descent of Man and Selection in Relation to Sex. John Murray, London, UK.
Davies N. B., Halliday T. R. 1977. Optimal mate selection in the toad Bufo bufo. Nature, 269: 56-58
Emlen S. T., Oring L. W. 1977. Ecology, sexual selection, and the evolution of mating systems. Science, 197(4300): 215-223
Fei L., Ye C. Y. 2001. The Colour Handbook of the Amphibians of Sichuan. China Forestry Publishing House
Fukuyama K. 1991. Spawning behaviour and male mating tactics of a foam-nesting treefrog, Rhacophorus schlegelii(Rhacophoridae, Amphibia). Anim Behav, 42: 193-199
Gutiérrez G., Lüddecke H. 2002. Mating pattern and hatching success in a population of the Andean frog, Hyla labialis. Amphibia-Reptilia, 23: 281-292
Halliday T. 1983. The study of mate choice. In: P. Bateson (ed). Mate Choice. pp. 3-32. Cambridge University Press, Cambridge
Howard R. D. 1978. The evolution of mating strategies in bullfrogs,Rana catesbeiana. Evolution, 32: 850-871
Howard R. D. 1984. Alternative mating behaviours of young male bullfrogs. Am Zool 24: 397-406
Huang Y., Zhu H. Q., Liao Y. M., Jin L., Liao W. B. 2013. Age structure, size and growth of a high-altitude bell toad in subtropical montane in southwestern China. Herpetol J, 23:229-232
Kierl N. C., Johnston C. E. 2015. The relationship between breeding coloration and mating success in male pygmy. Envir Biol Fish, 98: 301-306
Kusano T., Toda M., Fukuyama K. 1991. Testes size and breeding systems in Japanese anurans with special reference to large testes size in the treefrog Rhacophorus arboreus (Amphibia,Rhacophoridae). Behav Ecol Sociobiol, 29: 27-31
Lee J. C., Crump M. L. 1981. Morphological correlates of malemating success in Triprion petasatus and Hyla marmorata. Oecologia, 50: 153-157
Li S. T., Wu X., Li D. Y., Lou S. L., Mi Z. P., Liao W. B. 2013. Body size variation of Odorous Frog (Odorrana grahami) across altitudinal gradients. Herpetol J, 23: 187-192
Liao W. B., Liu W. C., Merilä J. 2015. Andrew meets Rensch:Sexual size dimorphism and the inverse of Rensch's rule in Andrew's toad (Bufo andrewsi). Oecologia, 177: 389-399
Liao W. B., Lu X. 2009a. Male mate choice in the Andrew's toad Bufo andrewsi: a preference for larger females. J Ethol, 27:413-417
Liao W. B., Lu X. 2009b. Sex recognition by male Andrew's toad Bufo andrewsi in a subtropical montane region. Beha Proc, 82:100-103
Liao W. B., Lu X. 2010. Age structure and body size of the Chuanxi Tree Frog Hyla annectans chuanxiensis from two different elevations in Sichuan (China). Zool Anz, 248: 255-263
Liao W. B., Lu X. 2011a. Proximate mechanisms leading to large male-mating advantage in the Andrew's toad, Bufo andrewsi. Behaviour, 148: 1087-1102
Liao W. B., Lu X. 2011b. Male mating success in the Omei treefrog(Rhacophorus omeimontis): the infl uence of body size and age. Belg J Zool, 141 (2): 3-10
Liao W. B., Lu X. 2012. Variation in mating patterns in the Andrew's toad Bufo andrewsi along an elevational gradient in southwestern China. Ethol Ecol Evol, 24: 174-186
Sih A., Chang A. T., Wey T. W. 2014. Effects of behavioural type,social skill and the social environment on male mating success in water striders. Anim Behav, 94: 9-17
Somashekar K., Krishna M. S. 2011. Evidence of female preference for older males in Drosophila bipectinata. Zool Stud,50: 1-15
Trivers R. L. 1972. Parental investment and sexual selection. In:B. Campbell (ed). Sexual Selection and the Descent of Man 1871-1971, pp. 136-179. Aldine, Chicago
Welch A. M., Semlitsch R. D., Gerhardt H. C. 1998. Call duration as an indicator of genetic quality in male gray tree frogs. Science, 280: 1928-1930
Wellborn G. A., Cothran R. D. 2007. Evolution and ecology of mating behavior in freshwater amphipods. In: E. Duffy and M. Thiel (eds). Evolutionary Ecology of Social and Sexual Systems:Crustaceans as Model Organisms, pp. 147-167.Cambridge University Press, Cambridge
Wilbur H. M., Rubenstein D. I., Fairchild L. 1978. Sexual selection in toads: the roles of female choice and male body size. Evolution, 32: 264-270
*
Prof. Wenbo LIAO, from Key Laboratory of Southwest China Wildlife Resources Conservation (Ministry of Education), China West Normal University, Nanchong, China, with his research focusing on evolutionary ecology in frogs.
E-mail: Liaobo_0_0@126.com
15 October 2015 Accepted: 20 February 2016
 Asian Herpetological Research2016年1期
Asian Herpetological Research2016年1期
- Asian Herpetological Research的其它文章
- First Record of the Poorly Known Skink Sphenomorphus oligolepis(Boulenger, 1914) (Reptilia: Squamata: Scincidae) from Seram Island, Maluku Province, Indonesia
- The Breeding Ecology of a Critically Endangered Salamander, Hynobius amjiensis (Caudata: Hynobiidae), Endemic to Eastern China
- Catalogue of the Type Specimens of Amphibians and Reptiles in the Herpetological Museum of the Chengdu Institute of Biology,Chinese Academy of Sciences: V. Viperidae (Reptilia, Serpentes)
- Effects of Predation by Invasive Western Mosquitofi sh (Gambusia affi nis) on Survival of Eggs, Embryos and Tadpoles of Pelophylax nigromaculatus and Duttaphrynus melanostictus in South China
- Effects of Pesticide Exposure on Embryonic Development and Hatchling Traits of Turtles
- Soundscape Dynamics at Anuran Reproductive Sites in Pannonian Biogeographical Region: Effects of Road Noise on Vocal Activity
