Feiji Recipe Reverses Lung Cancer Immune Escapeby Inhibiting the Expression of IDO
Zu-Jun Que,Ling Bi,Zhi-Yi Zhou,Lei Zhou,Qing Wang,Bin Luo,Jian-Wen Liu,He-Gen Li*,Jian-Hui Tian,*
1Shanghai Institute of Traditional Chinese Medicine,Institute of TCM Oncology,725 South Wanping Rd,Shanghai 200032,China.2Longhua Hospital,Shanghai University of Traditional Chinese Medicine,National Clinical Research Centre for Traditional Chinese Medicine and Oncology,725 South Wanping Rd,Shanghai 200032,China.3State Key Laboratory of Bioreactor Engineering and Shanghai Key Laboratory of Chemical Biology,School of Pharmacy,East China,University of Science and Technology,#268,130 Meilong Rd,Shanghai,200237,China.
Introduction
Over the past decade,the evasion of immune detection by tumors has been a key focus of research for oncologists and immunologists alike,and in 2011,the ability to evade immune destruction was formally suggested as a hallmark of the cancer cell[1].Indoleamine 2,3-dioxygenase(IDO)has been well characterized as a pivotal enzyme for immune escape of cancer.IDO is involved in many aspects of cancer including the suppression of T and natural killer(NK)cells,the generation and activation of T regulatory cells and myeloid-derived suppressor cells,and the promotion of tumor angiogenesis[2].
In human bodies,IDO has been detected in lung,stomach,spleen,liver,kidney,thymus,epididymis and brain[3].In vivo studies revealed that IDO was expressed constitutively in immune privileged environment and tissue with large area of mucosal surface.The role of IDO in regulating the immune response is thought to underlie its activity in promoting tumorigenesis.Importantly,IDO expression by tumors is inversely correlated with tumor-infiltrating CD8+T cell numbers in several types of malignancies including esophageal, ovarian and endometrial carcinomas,which may contribute to poor clinical outcome[4,5].Inhibition of IDO thus provides an attractive adjuvant strategy in augmenting anticancer and antiviral CD8+ T cell targeting of highly immunogenic antigens[6].IDO inhibitors have entered clinical trials for cancer treatment[7];however,their potential value for lung cancer hasnot been explored.
Herbs and plant-based formulations have been used by practitioners of Traditional Chinese Medicine(TCM)to treat human diseases.Feiji Recipe is a Chinese herbal recipe that has been used in clinical practice for more than 20 years.It has been proven that Feiji Recipe can stabilize the tumor body,obviously reduce the clinical symptoms of advanced non-small cell lung cancer(NSCLC),enhance patients’quality of life, and is effective in recovering immuno-surveillance and intervening immune escape of lung cancer through multi-pathway to enhance the clinical therapeutic efficacy[8].To provide a new model for studying the role of IDO in lung cancer,we develop a lung cancer cell line over-expressing IDO to explore its potential effect in lung cancer tumorigenesis,and whether Feiji Recipe regulates IDO expression.These results aim to clarify the mechanism of Feiji Recipe on reversing immune escape.
Materials and Methods
Reagents
TRIzolTMreagent was purchased from Invitrogen(Carlsbad,CA,USA).DMSO,Concanavalin A(ConA)and 3-[4,5-dimethylthylthiazol-2-yl]-2,5-diphenyl-tetra-zolium bromide(MTT)were purchased from Sigma Chemical Co.(St.Louis,MO,USA).Dulbecco’s Modified Eagle’s Medium(DMEM)was purchased from Gibco BRL(Gibco,NY,USA).Monoclonal anti-IDO and anti-ß-actin antibodies were purchased from Santa Cruz Biotechnology(Santa Cruz,CA,USA).Primers were purchased from Jie Rui Co.(Shanghai,China).
Sample preparation
Feiji Recipe is a Chinese herbal recipe that is consisted of:30 g of Sheng Huang Qi(Radix Astragali);15g of Bei Sha Shen(Radix Glehniae);12g of Mai Men Dong(Radix Ophiopogonis);12g of Tian Dong(Asparagus Cochinchinensis);15g of Fu Ling(Poria);30g of Shi Shang Bai(Selaginella Doederleinii);30g of Shi Jian Chuan(Salvia chinensia Benth);30g of Yu Xing Cao(Houttuynia cordata Thunb);and 24g of Qi Ye Yi Zhi Hua(Parispolyphylla,Houttuynia cordata Thunb)[9].The herbs were boiled with water until the solution of crude drug at 2.5g/mL final density,which were infused from esophagus into stomach of Wistar rats,once a day for 3 d,the dose was about 7 times to adult dose whose body weight was 65kg.After infusion of the drug for 2 h at the last time,rats were given the drug again.1 h later,blood was collected from inferior vena cava,centrifuged at 2000 r/min for 15 min to get serum,which was put at 56℃for 30 min to get rid of other possible biological living substance,then removed bacteria by 0.22μm millipore filtration membrane,sealed up and stored in the-20℃for further use.Meanwhile,rat serum without Feiji Recipe was prepared for the negative control.
Cells
The mouse Lewis lung cancer cell line 2LL was purchased from the Cell Bank of the Chinese Academic of Science and was cultured in DMEM medium(Gibco,NY,USA),supplemented with 10%(v/v)dialyzed heat-inactivated fetal serum (FBS)(Gibco BRL), penicillin (100 units/ml), and streptomycin(100 g/ml,Invitrogen Corp).All cultures were maintained at 37℃in a humidified atmosphere with 5%CO2.
Mice
Male C57BL/6 mice(18±2g,6–8 weeks of age)and male SD rat(200±10g,6–8 weeks of age)were purchased from the Experimental Animal Center of Fudan University(Shanghai,China).License number:SCXK(Shanghai)2012–0002.The animals were housed in SPF standards animal laboratory with 25±2℃,70%humidity and a 12-h light/dark cycle.All of the experimental protocols were approved by the Animal Care and Use Committee of Longhua Hospital of Shanghai University of Traditional Chinese Medicine and written following the ARRIVE guidelines.All experiments were performed in accordance with published National Institutes of Health guidelines.
Plasmids and transfection
The mammalian expression vector for human IDO(pEGFP-IDO)was generously provided by Dr.Qichao Chen(Jinan University,Guangzhou,China)and used to produce 2LL-EGFP-IDO cells.Introduction into 2LL cells was performed by lentiviral transduction by Shanghai Innovation Biotechnology Co.,Ltd.The pEGFP-N1 vector was used as a plasmid control for producing 2LL-EGFPcells.
Total RNA extraction and mRNA detection by RT-PCR
Total RNA from 2LL,2LL-EGFPand 2LL-EGFP-IDO cells was isolated as described previously[10].Total RNA(1µg)was used as a template for the RT reaction by using SuperScript II according to the Reverse Transcription System from Takara Co.(Takara Shuzo,Shiga,Japan).An aliquot(2µl)of RT product was used for PCR amplification.IDO cDNA(269 BP)was amplified using the sense primer 5’-GCTGTTCCTTA-CTGCCAACT-3’and the antisense primer 5’-AGCA-AAGTGTCCCGTTCT-3;and GAPDH sense primer 5’-GGTCGGAGTCAACGGATTTG-3’ and the antisense primer 5’-ATGAGCCCCAGCCTTCTCCAT-3’was also amplified as an internal standard.RT-PCR experiments were repeated 3 times.
Western blot analysis of IDO
Western blotting was conducted as described previously [11].The blots were visualized with enhanced chemiluninescence detection,and imaged on Kodak™X-OMAT film.All western blot experiments were repeated three times.
In vitro cell growth kinetics
2LL,2LL-EGFP and 2LL-EGFP-IDO cells(500 cells each)were seeded onto a 96-well plate and cultured in DMEM medium containing 10%fetal calf serum.Every 24 h,5 mg/ml MTT was added.After removal of MTT,the formazan precipitate was solubilized in DMSO(100µl/well)and measured on a microplate reader(Bio-rad,California,USA)at an absorbance wavelength of 570 nm and reference wavelength of 630 nm,and a growth curve was drawn from the results.
Splenocyte isolation
Fresh lymphocyte suspensions were prepared before each experiment from male C57BL/6 mice as previously described [12]. The mice were exsanguinated,and then spleens were immediately excised.Cells derived from the spleens were sequentially passed through a 100-mesh screen to harvest the mononuclear lymphocytes. The erythrocytes were lysed with Gey’s reagent for 5 min and then washed with sterile PBS.Mononuclear lymphocytes were isolated by buoyant density centrifugation(10 min at 2000 rpm)and resuspended at 1×106 cells/ml in DMEM medium(Gibco Co.)supplemented with 10%(v/v)heat-inactivated bovine serum(Gibco Co.).The cells were seeded in 6 new culture plates at 2×105lymphocytes per well and incubated at 37°C in a humidified atmosphere of 5%CO2for the indicated period.
Co-culture of 2LL-EGFP-IDO cells with T-cells
As previously described[13],1×1042LL-EGFP-IDO cells/per well were plated in a 12-well plate.After the cells attached to the bottom,they were co-cultured with 1×105T-cells in DMEM medium containing 10%FBS,and 200 U/ml human recombinant IL-2(Shuanglu,Beijing,China)at 37°C and 5%CO2for 2d.Cultures of 2LL-EGFP cells with T-cells were performed as controls.After co-incubation,the non-adherent T-cells were collected and analyzed by flow cytometry.
Flow cytometric analysis of immune cellular markers
Flow cytometric analysis of surface phenotypic markers for CD8+T cells was performed as described[3]. Cells were labeled with 1µl anti-mouse fluorescence-labeled anti-CD8 antibody (BD Pharmingen)in 100μl of PBS added 2%FBS for 30 min,as per the manufacturer’s recommendation.Then,the cells were washed in FACS buffer(PBS,pH 7.4,with 1%FBS).Cytometry was performed using Cell Quest software on a FACScan flow cytometer(Becton Dickinson,Mountain View,CA).Suitable negative isotype controls were used to rule out background fluorescence.Data were generated by cytofluorometric analyses of 10,000 events.The percentage of each positive population was determined using quadrant statistics.
Statistical analysis
Statistical analysis was carried out by SPSS 22.0.Each experimental value was expressed as the mean ±standard deviation (SD).Statistical analysis was performed using Origin7.0 software to evaluate the significance of differences between groups considered as*P<0.05;**P<0.01;***P<0.001.All data represent the mean of triplicates.
Results
Establishment of stable IDO-expressing cancer cells
To establish stable IDO-expressing mouse Lewis lung cancer cells,we transfected the expression vector pEGFP-IDO encoding human IDO cDNA into 2LL cells.An empty vector(pEGFP-N1)was also transfected in parallel as for control.The transfected cells were confirmed to be 100% GFP positive,suggesting that the transfection was completed.To confirm IDO expression in 2LL-EGFP-IDO cells,we performed RT-PCR and western blotting of IDO mRNA and protein.A 269bp RT-PCR product was amplified from 2LL-EGFP-IDO cells,but not the parental 2LL cells or the 2LL-EGFP control cells(Figure 1A).Furthermore,IDO protein was detected exclusively in cellular lysates of 2LL-EGFP-IDO cells(Figure 1B).These results verified the exogenous expression of IDO in the 2LL-EGFP-IDO cells.
2LL-EGFP-IDO cells have IDO enzymatic activity
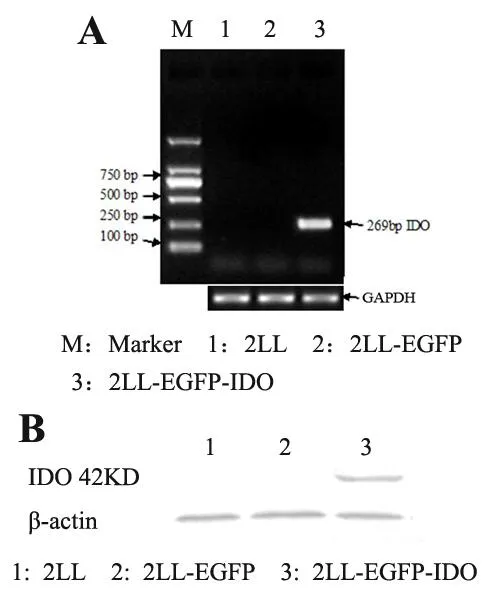
Figure 1.Expression of IDO mRNA and protein in 2LL-EGFP-IDO cells.
To further verify the establishment of a stable IDO cell line,we assessed the IDO enzymatic activity for the 2LL,2LL-EGFPand 2LL-EGFP-IDO cell lines by measuring the concentration of tryptophan and its main catabolites,kynurenines,in the conditioned medium.HPLC results showed conditioned medium from 2LL and 2LL-EGFP displayed a higher concentration of tryptophan and lower kynurenine after 48 hours,suggesting that the cells harbor some constitutive ability to degrade tryptophan in the absence of IDO expression. However, the tryptophan in 2LL-EGFP-IDO cell medium reduced significantly,and the concentration of kynurenines markedly increased(Figure 2).These results suggested that 2LL-EGFP-IDO cells produce IDO activity,which converts tryptophan to kynurenines.
IDO expression does not affect the level of 2LL cell proliferation in vitro
To ensure that stable expression of IDO does not affect the level of 2LL cell proliferation,we performed MTT assays of 2LL-EGFP and 2LL-EGFP-IDO cells as compared to parental 2LL cells.No significant differences were observed in cell proliferation among the three cell lines over the first 72 hours of cell culture(Figure 3).After 72 hours,a slight decrease in the cell viability of 2LL-EGFP-IDO cells was observed,possibly due to the consumption of the essential amino-acid tryptophan in the culture medium;however,this decrease was not statistically signifcant.These results suggested that the transfected cells do not differ significantly in their viability.
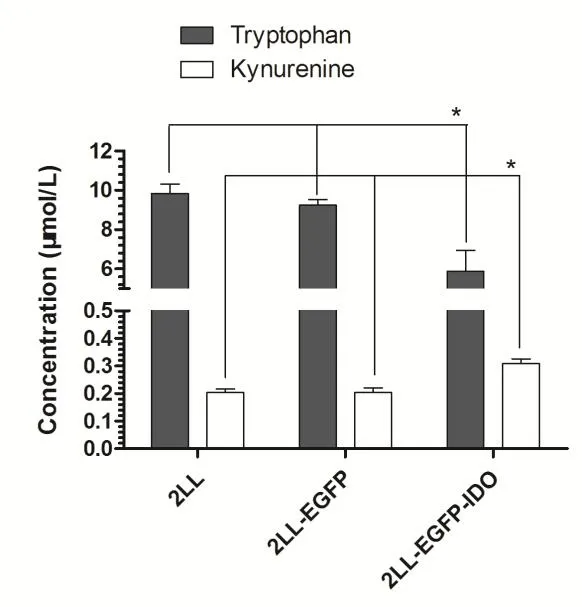
Figure 2.2LL-EGFP-IDO cells possess increased IDO enzyme activity.
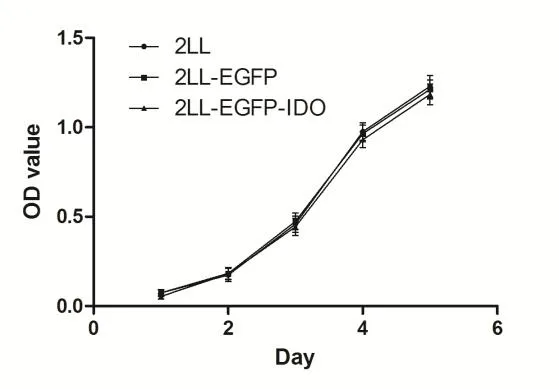
Figure 3 Effect of IDO overexpression on cell proliferation in vitro.
Feiji Recipe up-regulates the percentage of CD8+T cells by suppressing IDO expression
To determine the effect of Feiji Recipe on IDO expression and the percentages of CD8+T-cell subsets,1×105T cells were co-cultured with 1×1042LL-EGFP-IDO cells.After 2 days,the percentage of CD8+T cell was measured by FACS,and the expression of IDO in 2LL-EGFP-IDO cells was measured by western blot.The percentage of CD8+T cells in the 2LL-EGFP-IDO cell co-culture group was significantly lower than that in the 2LL-EGFP cell(Figure 4A).Feiji Recipe up-regulated the proportion of CD8+T cells,and inhibited the expression of IDO(Figure 4B).In conclusion,Feiji Recipe can inhibit IDO expression and reverse CD8+T cells suppression.
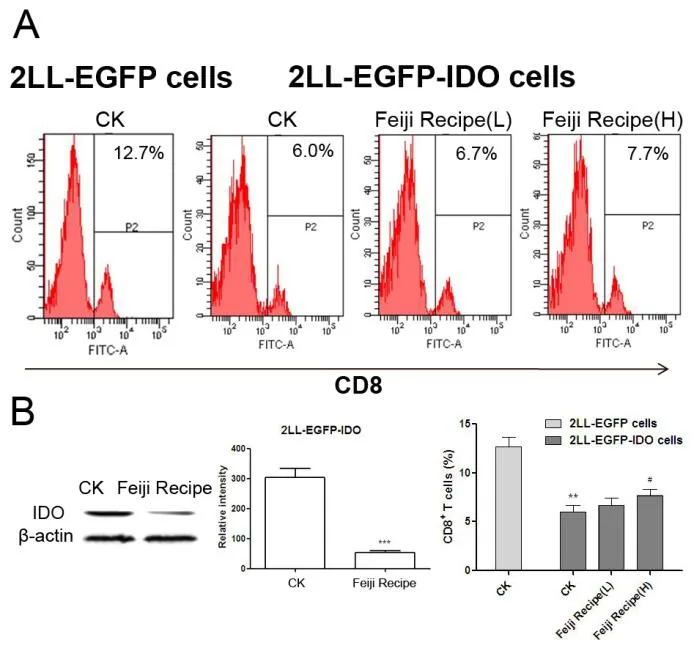
Figure 4.Feiji Recipe suppresses IDO expression,increases the percentage of CD8+T cells.
Discussion
Lung cancer is the leading cause of death among all malignancies,irrespective of sex distribution[14].The overall survival(OS)of lung cancer is still very poor,with a 5-year OS of 15%in patients with NSCLC.Traditional interventions have been developed based on the rationale that cancer cells should be killed to the most extent tyrosine kinase inhibitors such as gefitinib and erlotinib,which rely on EGFR mutations as the origin of the cancer,which limit their application to a small subset of patients with NSCLC[15].However,increasing evidence favors the concept that immune editing that occurs in the micro- and internal environment are critical for carcinogenesis,acquisition of a malignant phenotype and spreading of the cancer[16].IDO plays a key role in the immune escape of lung cancer,and therefore,the regulation of its expression may provide a strategy for enhancing immune editing.
Past clinical research has shown that Feiji Recipe can alleviate the symptoms of patients with advanced NSCLC,inhibit the progression of cancer and alleviate the side effects of chemotherapy [8].Possible mechanism underlying the action of Feiji Recipe is that it regulates the immune escape of lung cancer cells through restoring the body’s immune surveillance function[8].Further study has revealed that Feiji Recipe could exert an inhibitory effect on lung cancer growth and metastasis[17].
In this study,we transfected the human IDO1 gene into the 2LL mouse lung cancer cell line with lentivirus as the vector.Then,we verified the stable IDO expression by gene and protein levels,and IDO enzyme activity.These results are consistent with Norio Yoshidastudy[18].It is well documented that IDO induces the exhaustion of tryptophan and accumulation of kynurenine in the microenvironment of the tumor[19,20].We applied HPLC to confirm that 2LL-EGFP-IDO cells produce high concentration of kynurenine,and reduced level of tryptophan(Figure 3).
IDO inhibits the antitumor immunity of cytotoxic T cells by promoting cell cycle arrest and apoptosis[21].Jingyan Sun’s research showed that IDO induced apoptosis of competent T cells and increased proportion of Treg cells[22].The percentage of CD8+T cells was significantly lower when co-cultured with 2LL-EGFP-IDO cells as compared to that with 2LL-EGFP cells,suggestive of the impaired T cell function induced by IDO(Figure 4).Interestingly,Feiji Recipe can inhibit IDO expression,and reverse CD8+T cells suppression.Vinod P.Balachandran pointed out that Imatinib potentiated anti-tumor T cell responsed through the inhibition of IDO[23].In summary,Feiji Recipe may regulate tumor immuse escape through the inhibition of IDO expression.
The 2LL-EGFP-IDO cell line provides future prospects for exploring the function and mechanism of IDO in the carcinogenesis and spread of lung cancer,either in vitro or in vivo.Using this reliable cell line,the effect of IDO on anticancer immunity within the lung cancer microenvironment may be observed more accurately in the subcutaneous tumor graft model.Furthermore,peripheral immune escape provoked by IDO in lung cancer could also be elicited.This would potentially help in understanding the process of immune escape of lung cancer.In summary,Feiji Recipe is possible to reverse the immune escape of tumor via inhibiting the expression of IDO,therefore preventing the recurrence and metastasis of lung cancer.
Acknowledgments
This Study was supported by National Natural Science Foundation of China(Grant No:81173224,81373621),Shanghai Municipal Committee of Science and Technology (No.11ZR1437000),Longhua Medical Project(D-11)Jian-hui Tian and Specific Research Program from National Clinical Base of TCM(No.JDZX2012125)
Conflict of Interests
The authors have declared that no competing interests exist.
Reference
1.Hanahan D,Weinberg R.Hallmarks of Cancer:The Next Generation.Cell,2011 144(5):646-674.
2.Prendergast G C,Smith C,Thomas S,et al.Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer.Cancer Immunol Immunother,2014 63(7):721-735.
3.Liu P,Xie B L,Cai S H,et al.Expression of indoleamine 2,3-dioxygenase in nasopharyngeal carcinoma impairs the cytolytic function of peripheral blood lymphocytes.Bmc Cancer,2009 9(1):416.
4.Zhang G,Liu W L,Zhang L,et al.Involvement of indoleamine 2,3-dioxygenase in impairing tumor-infiltrating CD8 T-cell functions in esophageal squamous cell carcinoma.Clin Dev Immunol,2011 2011(1):384726.
5.Inaba T,Ino K,Kajiyama H,et al.Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma.Gynecol Oncol,2009,115(2):185-192.
6.Rytelewski M,Meilleur C E,Yekta M A,et al.Suppression of immunodominant antitumor and antiviral CD8+T cell responses by indoleamine 2,3-dioxygenase.Plos One,2014,9(2):e90439.
7.Liu X H,Chen S L,An L I,et al.IDO is a nodal pathogenic driver of lung cancer and metastasis development.Cancer Discov,2012 2(8):722-735.
8.Huang Y S,Shi Z M.Intervention Effect of Feiji Recipe on Immune Escape of Lung Cancer.Chin J Integ Tradit West Med,2007 27(6):501-504.
9.Tian JH,Liu L S,Shi Z M,et al.A Randomized Controlled Pilot Trial of"Feiji Recipe"on Quality of Life of Non-Small Cell Lung Cancer Patients.Am JChin Med,2012 38(1):15-25.
10.Hosseinitabatabaei A,Jalili R B,Li Y,et al.Mechanism underlying defective interferon gamma-induced IDO expression in non-obese diabetic mouse fibroblasts.Plos One,2012 7(5):e37747.
11.Clarke B L,Gebhardt B M,Blalock J E.Mitogen-stimulated lymphocytes release biologically active corticotropin.Endocrinology,1993 132(3):983-988.
12.Wang J,Wu A,Xu Y,et al.M(2)-A induces apoptosis and G(2)-M arrest via inhibiting PI3K/Akt pathway in HL60 cells.Cancer Lett,2009 283(2):193-202.
13.Zheng X,Koropatnick J,Li M,et al.Reinstalling antitumor immunity by inhibiting tumor-derived immunosuppressive molecule IDO through RNA interference.JImmunol,2006 177(8):5639-5646.
14.Siegel R,Desantis C,Jemal A.Colorectal cancer statistics,2014-Siegel-2014-CA:A Cancer Journal for Clinicians-Wiley Online Library.CA Cancer JClin,2014 64(2):104-117.
15.Sechler M,Cizmic A D,Avasarala S,et al.Non-small-cell lung cancer:molecular targeted therapy and personalized medicine–drug resistance, mechanisms, and strategies.Pharmacogenomics Per Med,2013 6(default):25-36.
16.Prendergast G C.Immune escape as a fundamental trait of cancer:focus on IDO.Oncogene,2008,27(28):3889-3900.
17.Tian JH,Shi Z M,Zhou Z Y,et al.Effects of Feiji Formula on lung cancer metastasis in mice.Journal of Chinese Integrative Medicine,2008 6(8):827-829.
18.Yoshida N,Ino K,Ishida Y,et al.Overexpression of indoleamine 2,3-dioxygenase in human endometrial carcinoma cells induces rapid tumor growth in a mouse xenograft model.Clin Cancer Res,2008,14(22):7251-7259.
19.Mellor A L,Munn D H.IDO expression by dendritic cells: tolerance and tryptophan catabolism.Nat Rev Immunol,2004,4(10):762-774.
20.Munn D H,Sharma M D,Mellor A L.Ligation of B7-1/B7-2 by human CD4+T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells.JImmunol,2004 172(7):4100-4010.
21.Löb S,Königsrainer A,Rammensee H G,et al.Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy:can we see the wood for the trees?.Nat Rev Cancer,2009 9(6):445-452.
22.Sun J,Yu J,Li H,et al.Upregulated expression of indoleamine 2,3-dioxygenase in CHO cells induces apoptosis of competent T cells and increases proportion of Treg cells.J Exp Clin Cancer Res,2011 30(6):82.
23.Balachandran V P,Cavnar M J,Zeng S,et al.Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido.Nat Med,2011 17(9):1094-1100.
 Traditional Medicine Research2016年3期
Traditional Medicine Research2016年3期
- Traditional Medicine Research的其它文章
- Explore the medication rules of Chinese medicine of Professor Huang Wenzheng for the treatment of chronic kidney disease based on data mining
- Uncertainty profile for NIR analysis of tanshinone Icontent in tanshinone extract powders
- Computational study of the molecular mechanism of Lonicera japonica organic acidsagainst influenza
- Molecular docking study on the molecular mechanism of rhaponticin for treatment of chronic myelocytic leukemia
- The present treatment and mechanism progress of arsenic trioxide in hematological malignancies
- Traditional Chinese medicine should not beignored during the development of precision medicine with Chinese characteristics
