一步法合成螺双芴及螺氧杂蒽衍生物及其在有机发光二极管中的应用:性能增强及相关的光学现象
关玉巧 宋娟 孙威 章琴 汤超 李雪 冯晓苗 钱妍 陶友田 陈淑芬,* 汪联辉 黄维,*
(1南京邮电大学信息材料与纳米技术研究院,先进生物与化学制造协同创新中心,有机电子与信息显示国家重点实验室培育基地,南京210023;2南京工业大学,先进生物与化学制造协同创新中心,柔性电子重点实验室,南京211816;3南京工程学院机械工程学院,南京211167)
一步法合成螺双芴及螺氧杂蒽衍生物及其在有机发光二极管中的应用:性能增强及相关的光学现象
关玉巧1,#宋娟1,#孙威1章琴1汤超2李雪3冯晓苗1钱妍1陶友田2陈淑芬1,*汪联辉1黄维2,*
(1南京邮电大学信息材料与纳米技术研究院,先进生物与化学制造协同创新中心,有机电子与信息显示国家重点实验室培育基地,南京210023;2南京工业大学,先进生物与化学制造协同创新中心,柔性电子重点实验室,南京211816;3南京工程学院机械工程学院,南京211167)
以芴为原料,以钯为催化剂一步合成了2-(9-苯基芴基)-9,9′螺二芴(PF-SBF)。以PF-SBF作为有机发光二极管的发光及主体材料(FIrpic为磷光客体)时,观察到了不同于PF-SBF及FIrpic发光的红光带。这分别源于PF-SBF分子间的聚集和发光层/传输层诱导的激基复合物。通过选择合适的空穴和电子传输层,有效抑制了激基复合物的发光。同时,PF-SBF和TAPC双主体的结构不仅实现了纯FIrpic和Ir(ppy)3蓝光和绿光,还大幅提升了器件性能。蓝光、绿光器件的最大电流效率和最大亮度分达到16.7、50.5 cd∙A-1和7857 cd∙m-2(11 V)、23390 cd∙m-2(8 V)。另外,除了PF-SBF,利用相似的合成方法,我们也合成了2-(9-苯基芴基)-9,9′螺芴氧杂蒽(PF-SFX),其较大的三线态能级(2.8 eV)较PF-SBF更适合做蓝光主体。以TAPC和PFSFX为双主体的器件最大电流效率提升到了22.6 cd∙A-1。所有实验结果均表明,PF-SBF和PF-SFX是构建高效绿光/蓝光磷光主体材料的有效结构单元。
钯催化;一步法;激基复合物;双主体;蓝光有机发光二极管
The project was supported by the National Key Basic Research Program of China(973)(2015CB932202,2012CB933301),National Natural
Science Foundation of China(61274065,51173081,61136003,BZ2010043,51372119,51172110,21304047,21373114,21003076),Innovation
Team of the Ministry of Education of China(IRT1148),Ministry of Education Humanities and Social Science Research Projects,China
(13YJCZH091),Natural Science Foundation of Jiangsu Province,China(BK20141424),PriorityAcademic Program Development of Jiangsu
Provincial Higher Education Institutions,China(YX030001),Ordinary University Graduate Student Practical Innovation Projects of Jiangsu
Province,China(SJLX15_0390),Pandeng Project of Nanjing University of Posts and Telecommunications,China(NY214085),and Open
Foundation from Jilin University,China(IOSKL2015KF32).
(1Key Laboratory for Organic Electronics and Information Displays and Institute of Advanced Materials,and Jiangsu National Synergetic Innovation Center for Advanced Materials,Nanjing University of Posts and Telecommunications,Nanjing 210023,P.R.China;2Key Laboratory of Flexible Electronics and Institute of Advanced Materials,National Synergistic Innovation Center for Advanced Materials,Nanjing Tech University,Nanjing 211816,P.R.China;3Mechanical Engineering Institute,Nanjing Institute of Technology,Nanjing 211167,P.R.China)
1 lntroduction
Organic light-emitting devices(OLEDs)have attracted great attention because of their potential applications in full-color displays,backlight sources,and solid state lighting1-4.After three decadesʹresearch and development,red and green OLEDs have been rapidly developed with satisfactory efficiency and saturated chromaticity,however,blue OLEDs with high efficiencies are still rather rare due to intrinsically wide band gaps of blue emitting materials and their hosts5-9.Fluorene-based compounds have been one of the most wellknown blue emission materials in the past several years due to their high photoluminescence(PL)quantum efficiencies and good thermal stability10-12.As one type of fluorenes,spirofluorene compounds own advantages of high glass transition temperature,good solubility,and amorphous nature due to a high steric and rigid structure brought by perpendicular arrangement of two π-electron systems.Furthermore,this perpendicular arrangement of the two π-electron systems in spirofluorenes is able to effectively suppress excimer formation which is frequently observed in many solid state fluorescent dyes13,14.Organic spirofluorene materials,e.g.,spirobifluorenes with asymmetric substitution,spiro-substituted spiro fluorene,spirofluorenelinked phenylanthracene,and spirofluorene-linked anthracene,usually emit blue light15-20.Since Tour et al.10successfully introduced spirobifluorene unit into organic electronics in 1996,spirobifluorene and its derivatives are frequently used as blue emitters or hosts of blue light materials to achieve high performances blue OLEDs11.In 2002,Wu and his colleagues12used 2,7-bis[2-(4-tert-butylphenyl)pyrimidine-5-yl-9,9ʹ-spirobifluorene as a host of perylene in a blue OLED and obtained a maximum brightness of 80000 cd∙m-2.Lee et al.21fabricated a simple blue device using 2,7-bis(diphenylphosphoryl)-9,9ʹ-spirobi[fluorene]as a host material and realized a quantum efficiency of as high as 20.3%without any LiF electron injection layer.Recently,Wangʹs team22synthesized 3,6-di(9H-carbazol-9-yl)-9,9ʹ-spirobi[fluorene],with which as a host they exhibited a OLED with a very low turnon voltage of 2.8 V and a high current efficiency of 34.2 cd∙A-1.
In this paper,we synthesized a new spirobi[fluorene]derivative named 2ʹ-(9-phenyl-fluoren-9-yl)-9,9ʹ-spirobi[fluorene](PF-SBF),which contains fluorene-substituted spirobifluorene units,and applied it as a blue emitter or a host of a blue phosphor bis(3,5-difluoro-2-(2-pyridyl)phenyl-(2-carboxypyri-dyl)iridium(III)(FIrpic)in OLEDs.We found an emission in red light band in addition to the PF-SBF and FIrpicʹs intrinsic blue light and removed this red light band through analyzing the origin of this phenomenon and designing the device structure.We finally fabricated a high-performance blue-emission OLED structure with a cohost and a proper electron/hole transport layer.In addition,we replaced the fluorene with a xanthene in spirobifluorene structure of PF-SBF and obtained a new host material 2-(9-phenyl-fluoren-9-yl)spiro[fluorene-9,9ʹ-xanthene](PF-SFX),which owns a wide band gap and is more suitable for a blue light host.Using PF-SFX as a host of FIrpic,we acquired a high luminous efficiency of 22.6 cd∙A-1and a pure chromaticity of(0.15,0.32).
2 Experimental
All the materials used in OLEDs except PF-SBF,PF-SFX,andpoly(3,4-ethyienedioxythiophene):poly(styrenesulfonate)(PEDOT: PSS)were purchased from Hanfeng Chemical with purity of 99% and were directly used without further purification.Noting that PEDOT:PSS was obtained from Heraeus Precious Metals GmbH &Co.KG(Germany).All reagents used in synthesis were purchased from J&K Chemical with purity of>97%.The new materials PF-SBF and PF-SFX we synthesized were purified by column chromatography first,then further purified by recrystallization.The purity of the materials meets the requirement of the device.The ultraviolet-visible(UV-Vis)spectra,room-temperature and low temperature PL spectra of PF-SBF and PF-SFX were respectively measured with an ultraviolet-visible spectrophotometer(Japan,Shimadzu,UV-3600),a spectrofluorophotometer (Japan,Shimadzu,RF-5301PC),and a Hitachi F-4500(Japan)fluorescence spectrophotometer.The cyclic voltammetry(CV)measurementswereperformedwithaCHI660Csystem (Shanghai)in a typical there-electrode cell.The highest occupied molecular orbital(HOMO)energy level was estimated with regard to the reference oxidation energy level of ferrocene(-4.8 eV)and the lowest unoccupied molecular orbital(LUMO)energy level was estimated through the HOMO energy level and the band gap. The thermogravimetry analysis(TGA)of PF-SBF and PF-SFX was performed in a DTG-60 system(Japan,Shimadzu)at a ramping rate of 10°C∙min-1under an argon flow rate of 20 mL∙min-1from room temperature to 600°C.The differential scanning calorimetry(DSC)of these two complexes were performed in a DSC-60Asystem(Japan,Shimadzu)at a ramping rate of 10°C∙min-1under an argon flow rate of 20 mL∙min-1from room temperature to 240°C.The electroluminescence(EL)characteristics including luminance,Commission Internationale de L'Eclairage (CIE)coordinates,and EL spectra were measured with a PR655 spectrometer(America).The luminance-voltage curves were simultaneously measured with a Keithley 2400 voltage-current sourcemeter(America),while the efficiencies were directly calculated from the above measured parameters.
PF-SBF was synthesized via a one-step method23.PtBuPh2(12.1 mg,0.05 mmol,0.1 equiv)and KOtBu(67 mg,0.6 mmol,1.2 equiv)were added into a dried Schlenk tube in an argon-filled glove box,where the Schlenk tube contained a mixture solution of 2-bromo-9,9ʹ-spirobi[fluorene](197 mg,0.5 mmol,1 equiv),9-phenylfluorene(145 mg,0.6 mmol,1.2 equiv),Pd(dba)2(14.3 mg,0.025 mmol,0.05 equiv),and 2 mL toluene.The mixtures were stirred at 100°C for 10 h and then quenched with water.The final reaction mixture was extracted with ethyl ether for 3 times (3×10 mL).The organic layers were combined,dried(Na2SO4),and filtered,with the solvent removed under a reduced pressure. Column chromatography on silica gel(hexane:CH2Cl2,10:1 (volume ratio))afforded the desired product of white solid(279 mg)with a yield of 86%.1H NMR(400 MHz,CDCl3)δ 7.87-7.72 (m,5H),7.64(d,J=8.0 Hz,1H),7.36(m,6H),7.22-7.13(m,6H),7.11-6.95(m,7H),6.92(s,1H),6.80(d,J=7.5 Hz,2H),6.72(d,J=7.6 Hz,1H).13C NMR(100 MHz,CDCl3)δ 151.3,149.2,148.9,148.8,146.0,145.3,141.7,141.3,140.7,140.1,128.0,127.8,127.7,127.7,127.6,127.4,127.1,126.5,126.0, 125.0,123.9,120.1,120.1,119.9,119.5,66.1,65.6.HRMS (MALDI/DHB)(Waters Micromass GCT,Waters Q-Tof Premier,America):calcd for C44H28[M]+556.2189;found 556.2185.
PF-SFX was also synthesized with a similar method with PFSBF.PtBuPh2(12.1 mg,0.05 mmol,0.1 equiv)and tBuOK(67 mg,0.6 mmol,1.2 equiv)added into a dried Schlenk tube in an argon-filled glove box,where the Schlenk tube contained a mixture solution of 2-bromo-9,9ʹ-spiro[fluorene-9,9ʹ-xanthene](205 mg,0.5 mmol,1 equiv),9-phenylfluorene(145 mg,0.6 mmol,1.2 equiv),Pd(dba)2(14.3 mg,0.025 mmol,0.05 equiv),and 2 mL toluene.The reaction conditions,extraction approach and post-treatment process of the product were same with those of PF-SBF.Column chromatography on silica gel(hexane:CH2Cl2,10:1 for volume ratio)afforded the desired product(269 mg)with a yield of 94%.1H NMR(400 MHz,CDCl3)δ 7.79(d,J=7.6 Hz,2H),7.70(d,J=7.5 Hz,1H),7.60(d,J=8.0 Hz,1H),7.44 (d,J=1.4 Hz,1H),7.41-7.34(m,2H),7.33-7.14(m,12H),7.08 (ddd,J=15.7,7.6,2.4 Hz,6H),6.89-6.81(m,2H),6.52(d,J= 7.7 Hz,2H).13C NMR(100 MHz,CDCl3)δ 155.42,153.58,151.67,151.18,146.18,146.01,140.13,139.67,138.06,128.17,128.12,128.07,127.83,127.76,127.66,127.48,127.45,127.20,127.03,126.59,126.08,125.51,125.22,123.16,120.20,119.94,119.49,116.78,65.69,54.54.HRMS(MALDI/DHB)(Waters Micromass GCT,Waters Q-Tof Premier,America):calcd for C44H28O[M]+572.2140;found 572.2140.
3 Results and discussion
PF-SBF was synthesized through palladium-catalyzed crosscoupling of triarylmethyl C―H bonds with aryl halides via one step reaction,with the synthetic route shown in Scheme 1(a).The approach was detailedly described in Experimental details23.It should be noted that the PF-SBFʹs yield is as high as 86%,which is quite high compared to those similar structure materials synthesized by Friedel-Crafts or Suzuki reaction.The UV absorption and PLspectra of PF-SBF show a main absorption peak of 295 nm with a 307 nm shoulder and PL peaks of 334 and 384.5 nm in anhydrous ethanol solution,as shown in Fig.1.The LUMO and HOMO energy levels of PF-SBF were measured by a CV method and its detailed information was described in Supporting Information.The CV curve of PF-SBF is shown in Fig.S1(a)(Supporting Information)and the LUMO and HOMO energy levels are calculated to be-1.9 and-5.6 eV,respectively.As the TGAcurve shown in Fig.2,the decomposition temperature(Td)value is 348°C which corresponds to a 5%weight loss.As indicated in the DSC curve shown in Fig.S2(Supporting Information),no significant signal of glass transition temperature(Tg)is observed. From the above characteristics,we inferred that as-synthesized PFSBF can be used as a deep-blue emitting material or a host for other emitting materials.
We first employed PF-SBF as a blue emission material with a common OLED structure of ITO/MoO3(2 nm)/4,4ʹ,4ʹ-tris[3-methylphenylphenylamino]-triphenylamine(m-MTDATA):MoO3(mass ratio of 3:1,15 nm)/m-MTDATA(25 nm)/tris-(phenyl-pyrazole)-iridium((Ir(ppz)3,10 nm)/PF-SBF(30 nm)/4,7-diphenyl-1,10-phenanthroline(Bphen,30 nm)/LiF(1 nm)/Al(100 nm).This structure was denoted as Structure A.Fig.S3(Supporting Information)shows luminance-voltage-current efficiency(L-V-CE)characteristics and normalized EL spectra of Structure A.Detailed data were summarized in Table S1(Supporting Information). Analysis on these data indicates that both the luminance and the current efficiency in StructureAare quite low when using only PFSBF as an emitting layer(EML).In addition,the normalized EL spectrum has a strong emission in red light band in addition to the PF-SBFʹs intrinsic blue light,which is possibly caused by intermolecular aggregation24.The above information indicates that PFSBF may be more suitable for a host than a blue emitter.
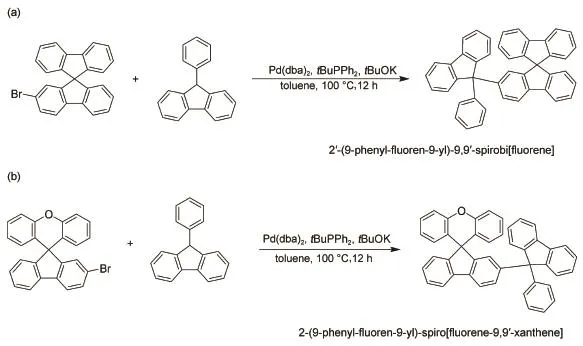
Scheme 1Synthetic routes of PF-SBF(a)and PF-SFX(b)
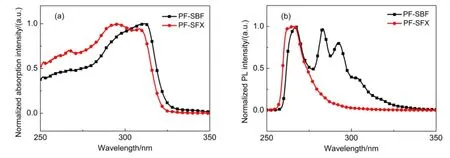
Fig.1 Room-temperature absorption(a)and PL(b)spectra of PF-SBF and PF-SFX in anhydrous ethanol solution
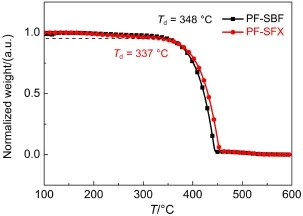
Fig.2TGAcurve s of PF-SBF and PF-SFX
In the following part,PF-SBF was used as the host of the blue phosphor FIrpic with a device structure similar with Structure A except replacing PF-SBF with FIrpic-doped PF-SBF(10%(w)),which was denoted as Structure B.Doping FIrpic not only reduces intermolecular aggregation of PF-SBF,accompanied with the restrainment of the red emitting band,but also realizes energy transfer from PF-SBF to the blue light guest FIrpic with a further improved device efficiency.As shown in Fig.S4(Supporting Information)and Table S1,the performances including turn-on voltage(Von),L and CE have great improvements compared with those of Structure A.It should be noted that when PF-SBF was used as the FIrpic host,the EL spectrum shows a typical emission of FIrpic,indicating an efficient energy transfer from PF-SBF toFIrpic.This point was further approved by the energy level in Fig.3 (a),in which the LUMO and HOMO energy levels of FIrpic(2.8 and 5.5 eV)are located within those(1.9 and 5.6 eV)of the PFSBF host.
Electron withdrawing/donating-inefficient group on PF-SBF implied its poor charge transport ability,which can be calculated with Mott-Gurney equation25
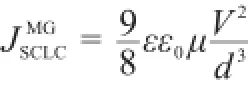
where,ε0is the vacuum permittivity,ε is the relative dielectric constant of PF-SBF,μ is the carrier mobility,V is the voltage drop,and d is the thickness of PF-SBF.
The hole and electron mobilities of PF-SBF are 6.1×10-6and 8.0×10-5cm2∙V-1∙s-1,estimated from the injection current density-voltage characteristics in Fig.S5(Supporting Information). The low mobility values of both holes and electrons lead to poor performances in above devices.
In order to improve device performances as well as suppressing red light band,we fabricated cohost devices named Structure C,in which di-[4-(N,N-ditolyl-amino)-phenyl]cyclohexane(TAPC)owning a fine hole transport ability and PF-SBF were employed as cohost.Structure C still utilized a similar configuration with Structure B,except replacing PF-SBF with PF-SBF:TAPC.The mass ratios of TAPC:PF-SBF were 2:1,1:1,0:1,and 1:0,respectively.Fig.4(a)shows L-V-CE characteristics of Structure C,while the parameters including Von,L,and CE are summarized in Table S2(Supporting Information).From Fig.4(a),we observed that the device employing TAPC and PF-SBF as a cohost exhibits obvious enhancements on brightness and efficiency compared with a TAPC or PF-SBF single host.The best performances occurs at the mass ratio of around 1:1 for the TAPC:PF-SBF cohost,realizing L and CE of 2907 cd∙m-2and 5.4 cd∙A-1.When employing TAPC:PF-SBF as a cohost,it occurs another shoulder on the EL spectra at around 580 nm in addition to the blue emission of FIrpic(Fig.4(b)),while this shoulder is totally suppressed with only PF-SBF,indicating it is different from the aggregation of PFSBF molecules.So in the following part,we investigated the phosphorescence spectra of PF-SBF,the energy levels of hosts and adjacent hole/electron transport layers to explore the origin of the 580 nm shoulder peak.
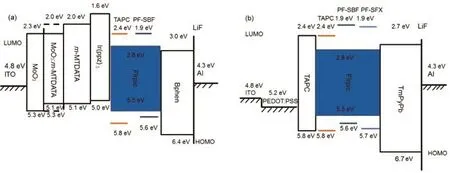
Fig.3Device structures and energy levels of Structures B,C(a)and D(b)ITO:indium tin oxide
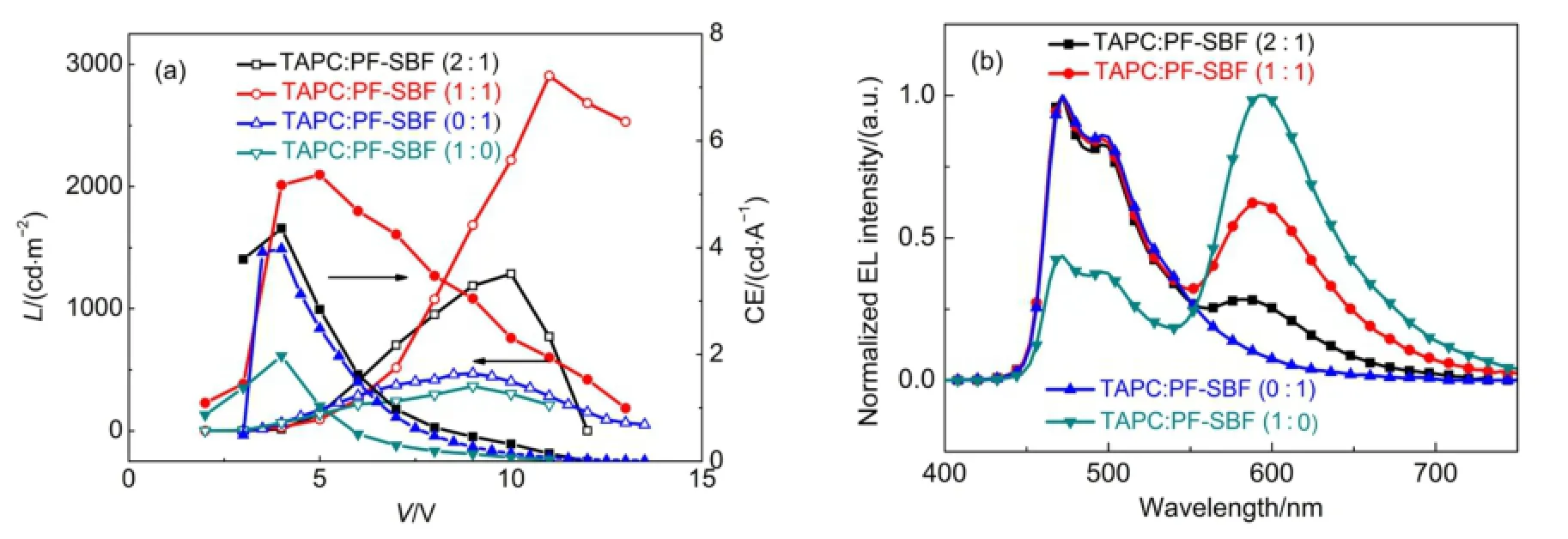
Fig.4L-V-CE characteristics(a)and normalized ELspectra(b)of Structure CHollow symbols represent luminance and solid symbols represent current efficiency.
In common,fluorene usually generates a green emission band totally different from its intrinsic emission,which is normally attributed to fluorenone defects generated at C9 position with a substituent of alkyl group26.For the case of PF-SBF,there are noalkyl groups at any position,which is hard to be oxidized,so that the fluorenone defect will not occur.We also suspected that the 580 nm shoulder peak is from the phosphorescent emission of PFSBF,so we tested PF-SBFʹs phosphorescence spectra at 77 K,as shown in Fig.5,from which we found the phosphorescent emission peak is at 496 and 530 nm.In the vicinity of 580 nm,the phosphorescent emission of PF-SBF has already significantly decayed,indicating that the 580 nm shoulder in the EL spectra is not induced by the PF-SBFʹs phosphorescence.

Fig.5Phosphorescence spectra of PF-SBF and PF-SFX in toluene solvent at 77 K
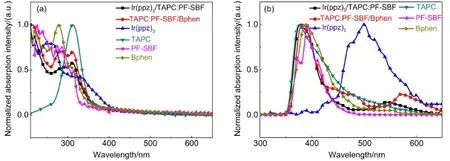
Fig.6Normalized absorption(a)and PL(b)intensities of EMLand its adjacent layers in Structure C
In addition to the molecular aggregation and the intrinsic phosphorescent emission of the host material,the energy mismatch between the hosts and the adjacent electron/hole transport layer may also induce a long-waveband emission,normally called “exciplex”.So we gave energy levels of EML and its adjacent electron and hole transport layers.As shown in Fig.3(b),the LUMO and HOMO energy levels of PF-SBF and TAPC are 1.9/ 5.6 eV and 2.4/5.8 eV,respectively.Alarge LUMO energy barrier of≥0.6 eV between TAPC(or PF-SBF)and Bphen(3.0/6.4 eV)together with a poor electron transport property of TAPC made electrons injection into EML very difficult.Furthermore,a large HOMO energy barrier of more than 0.6 eV as well as poor hole conduction capability of Bphen led to holes accumulation at the Bphen/EML interface,making it easily produce exciplex at this interface27.To further verify the presence of exciplex,we measured the absorption and PL spectra of the TAPC:PF-SBF host and its adjacent layers,respectively,as shown in Fig.6(a).The absorption spectrum of TAPC:PF-SBF/Bphen layer shows no obvious change but its PL spectrum(Fig.6(b))generates a new shoulder at 571.5 nm compared with those of the TAPC,PF-SBF,and Bphen film. And this orange peak in the PL spectrum is consistent with that in the EL spectrum,proving its origin from an exciplex.In addition,the PL spectrum of Ir(ppz)3/TAPC:PF-SBF also produces a redshifted emission compared with the Ir(ppz)3,TAPC or PF-SBF layer,indicating that there also exists an exciplex emission at the interface of Ir(ppz)3and TAPC:PF-SBF.
In order to eliminate the exciplex emission,we replaced the present hole and electron transport layers with TAPC and 1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene(TmPyPb).Amuch lower LUMO energy level of TmPyPb(2.7 eV)reduced the electron injection barrier,while a larger HOMO energy level efficiently confined the holes into the TmPyPb(Fig.3(b)).The new device structure was ITO/PEDOT:PSS/TAPC(10 nm)/TAPC:PF-SBF:FIrpic(mass ratio of 1:1:0.2,30 nm)/TmPyPb/LiF(1 nm)/Al,denoted as Structure D.With optimal PEDOT:PSS and TmPyPb thicknesses of~40-50 nm and 40 nm,the maximum current efficiency and brightness rise up to 16.7 cd∙A-1(6 V)and 7857 cd∙m-2(11 V),as shown in Fig.7(a)and Table 1.The Vonis as low as 3.4 V along with the employment of new electron transport layer and the elimination of Ir(ppz)3.As can be seen from Fig.7(b),a more important point is that the optimized device exhibits a typical FIrpic emission property and effectively gets rid of the exciplex-induced red light band,indicating a proper device structure and an efficient energy transfer from mixed host TAPC:PF-SBF to the EML.
Referring to the low-temperature phosphorescence spectra in Fig.5,we calculated the triplet energy level of PF-SBF to be 2.5 eV,and this value suggests that PF-SBF may be more suitable for a green light host.So we fabricated a green OLED named Structure E in order to make sure of this idea.Structure E still utilized a similar device configuration with Structure D with only TAPC:PF-SBF:FIrpic(10%(w),30 nm)being replaced by TAPC: PF-SBF:tris(2-phenylpyridine)iridium(III)(Ir(ppy)3,8%(w),30 nm).Fig.8 shows L-V-CE characteristics and normalized EL spectra of Structure E,with all parameters including Von,L,and CE being summarized in Table 1.The maximum current efficiency and brightness are as high as 50.5 cd∙A-1(6 V)and 23390 cd∙m-2(8 V),while Vonis as low as 3.0 V.As can be seen in Fig.8(b),thedevice exhibits a typical Ir(ppy)3emission property without any other additional emission band.
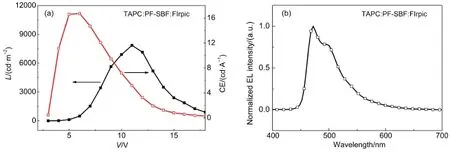
Fig.7L-V-CE characteristics(a)and normalized ELspectrum(b)of Structure D
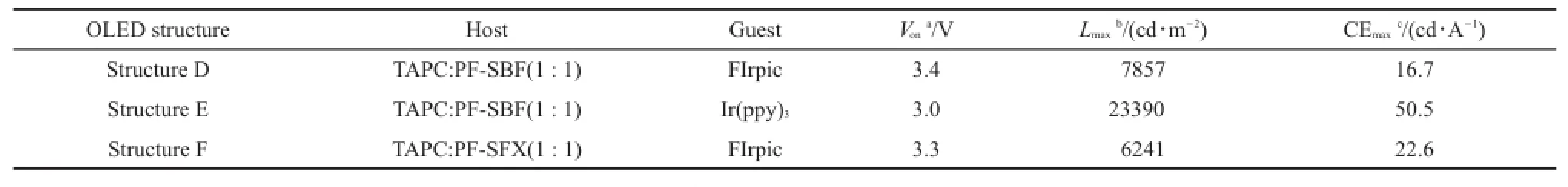
Table 1Summarized OLED performances for Structures D,E,and F
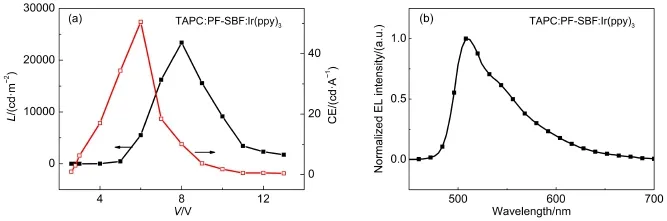
Fig.8L-V-CE characteristics(a)and normalized ELspectrum(b)of Structure E
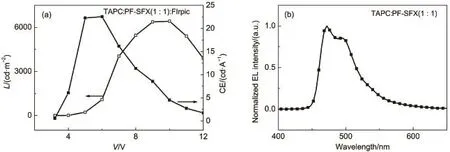
Fig.9L-V-CE characteristics(a)and normalized ELspectrum(b)of Structure F
Above data demonstrated that the narrow band gap PF-SBF is suitable for a green phosphorescent host instead of a blue one,which may be due to the π-π interaction between fluorene substituent and fluorene unit in spirobifluorene structure.We wondered if we replace one fluorene unit in spirobifluorene structure with xanthene,this π-π interaction will be restrained and the corresponding energy band will be broadened.To prove the hypothesis,we newly synthesized PF-SFX,where xanthene replaced fluorene in spirobifluorene structure of PF-SBF,with the synthetic route shown in Scheme 1(b).Comparing with PF-SBF in Fig.1,we observed an obvious blue shift in both absorption and PL spectra of PF-SFX,with a main absorption peak of 295 nm with a 307 nm shoulder and a main PL peak of 384.5 nm with a shoulder of 392.5 nm.From the CV curve of PF-SFX in Fig.S1(b),we calculated the LUMO and HOMO energy levels to be-1.9 and -5.7 eV.In addition,we also calculated its triplet energy level from Fig.5 to be 2.8 eV,demonstrating that PF-SFX is more suitable for a blue host than PF-SBF.Others parameters like Td(337°C),Tg,and the hole/electron mobility(7.3×10-6and 9.2× 10-5cm2∙V-1∙s-1)were also measured(Fig.2 and Fig.S2(Supporting Information))and results indicate that PF-SFX owns a similar property with PF-SBF.Thus we fabricated a device (Structure F)by replacing PF-SBF in Structure D with PF-SFX,with L-V-CE characteristics and normalized EL spectra shown in Fig.9 and Table 1.Structure F exhibits improved current efficiency and brightness of 22.6 cd∙A-1(6 V)and 6421 cd∙m-2(8 V),as well as a slight decline in Vonto 3.3 V.Much better performances in the PF-SFX-based blue OLED powerfully confirm that reducing π-π interaction is helpful to broaden the band gap and enhance the EL performances.
4 Conclusions
In summary,we synthesized a new spirobifluorene derivative PF-SBF and a xanthene derivative PF-SFX with high yields via one-step synthesis.Utilizing PF-SBF as an emitter and a host of blue phosphor FIrpic,we observed a red light band different from the intrinsic blue emission of PF-SBF and FIrpic,which is respectively attributed to intermolecular aggregation of PF-SBF and exciplexes generated at the interfaces of EML and electron transport/blocking layer.The exciplex emission was restrained with a proper hole and electron transport layer.Employing a PFSBF:TAPC cohost,the high-performance blue and green emissions were achieved with maximum current efficiencies of 16.7 and 50.5 cd∙A-1and maximum brightnesses of 7857 and 23390 cd∙m-2for FIrpic and Ir(ppy)3,respectively.Actually,PF-SBF is not suitable for a blue phosphorescent host due to its narrow triplet energy level of only 2.5 eV.Therefore,a new xanthene derivative PF-SFX with a large triplet energy level of 2.8 eV was synthesized via reducing π-π interaction between the fluorene substituent and the fluorene unit in spirobifluorene structure by replacing one fluorene unit in spirobifluorene structure with xanthene.Using PFSFX as the FIrpic host,the luminous efficiency and brightness were significantly improved,reaching 22.6 cd∙A-1and 6421 cd∙m-2.Carrier-transport unit-free in PF-SBF and PF-SFX limits their carrier mobilities and device performances.Our future work will focus on new materialsʹdesign based on PF-SBF and PF-SFX with high carrier transport abilities,and we believe that the device performances will be further improved in the near future.
Supporting lnformation:available free of charge via the internet at http://www.whxb.pku.edu.cn.
References
(1)Xiao,L.X.;Hu,S.Y.;Kong,S.;Chen,Z.J.;Qu,B.;Gong,Q. H.Acta Phys.-Chim.Sin.2011,27,977.[肖立新,胡双元,孔胜,陈志坚,曲波,龚旗煌.物理化学学报,2011,27,977.]doi:10.3866/PKU.WHXB20110325
(2)Kido,J.;Kimura,M.;Nagai,K.Science 1995,267,1332. doi:10.1126/science.267.5202.1332
(3)Tang,P.;Xiao,J.J.;Zheng,C.;Wang,S.;Chen,R.F.Acta Phys.-Chim.Sin.2013,29,667.[汤鹏,肖坚坚,郑超,王石,陈润锋.物理化学学报,2013,29,667.]doi:10.3866/ PKU.WHXB201302062
(4)Chen,S.F.;Deng,L.L.;Xie,J.;Peng,L.;Xie,L.H.;Fan,Q. L.;Huang,W.Adv.Mater.2010,22,5227.doi:10.1002/ adma.201001167
(5)Adachi,C.;Baldo,M.A.;Thompson,M.E.;Forrest,S.R. J.Appl.Phys.2001,90,5048.doi:10.1063/1.1409582
(6)Fan,C.H.;Sun,P.;Su,T.H.;Cheng,C.H.Adv.Mater.2011,23,2981.doi:10.1002/adma.v23.26
(7)Ho,C.L.;Li,H.;Wong,W.Y.J.Organomet.Chem.2014,751,261.doi:10.1016/j.jorganchem.2013.09.035
(8)Lu,P.;Hong,H.;Cai,G.;Djurovich,P.;Weber,W.P.;Thompson,M.E.J.Am.Chem.Soc.2000,122,7480. doi:10.1021/ja000354q
(9)Su,S.J.;Cai,C.;Kido,J.Chem.Mater.2011,23,274. doi:10.1021/cm102975d
(10)Wu,R.L.;Schumm,J.S.;Pearson,D.L.;Tour,J.M.J.Org. Chem.1996,61,6906.doi:10.1021/jo960897b
(11)Xiao,H.;Shen,H.;Lin,Y.;Su,J.;Tian,H.Dyes Pigm.2007,73,224.doi:10.1016/j.dyepig.2005.11.010
(12)Wu,C.C.;Lin,Y.T.;Chiang,H.H.;Cho,T.Y.;Chen,C.W.;Wong,K.T.;Liao,Y.L;Lee,G.H.;Peng,S.M.Appl.Phys. Lett.2002,81,577.doi:10.1063/1.1493669
(13)Kim,K.S.;Jeon,Y.M.;Kim,J.W.;Lee,C.W.;Gong,M.S. Org.Electron.2008,9,797.doi:10.1016/j.orgel.2008.05.013
(14)Tsuzuki,T.;Tokio,S.Appl.Phys.Lett.2009,94,033302. doi:10.1063/1.3073709
(15)Lin,Y.;Chen,Z.K.;Ye,T.L.;Dai,Y.F.;Ma,D.G.;Ma,Z.;Liu,Q.D.;Chen,Y.J.Polym.Sci.,Part A:Polym.Chem.2010,48,292.doi:10.1002/pola.23783
(16)Prelog,V.;Bedeković,D.Helv.Chim.Acta 1979,62,2285. doi:10.1002/hlca.19790620725
(17)Harada,N.;Ono,H.;Nishiwaki,T.;Uda,H.J.Chem.Soc.,Chem.Commun.1991,No.24,1753.doi:10.1039/ C39910001753
(18)Spehr,T.;Siebert,A.;Lieker,T.F.;Salbeck,S.;Rabe,T.;Riedl,T.;Johannes,H.H.;Kowalsky,W.;Wang,J.;Weimann,T.;Hinze,P.Appl.Phys.Lett.2005,87,161103.doi:10.1063/ 1.2105996
(19)Shen,W.J.;Dodda,R.;Wu,C.C.;Wu,F.I.;Liu,T.H.;Chen,H.H.;Chen,C.H.;Shu,C.F.Chem.Mater.2004,16,930.doi: 10.1021/cm0345117
(20)Gebeyehu,D.;Walzer,K.;He,G.;Pfeiffer,M.;Leo,K.;Brandt,J.;Gerhard,A.;Stoessel,P.;Vestweber,H.Synth.Met.2005,148,205.doi:10.1016/j.synthmet.2004.09.024
(21)Jeon,S.O.;Lee,H.S.;Jeon,Y.M.;Kim,J.W.;Lee,C.W.;Gong,M.S.Bull.Korean Chem.Soc.2009,30,863. doi:10.5012/bkcs.2009.30.4.863
(22)Wang,L.;Pan,B.;Zhu,L.P.;Wang,B.;Wang,Y.X.;Liu,Y.K.;Jin,J.J.;Chen,J.S.;Ma,D.G.Dyes Pigm.2015,114,222.doi: 10.1016/j.dyepig.2014.11.011
(23)Cao,X.;Yang,W.;Liu,C.;Wei,F.;Wu,K.;Sun,W.;Song,J.;Xie,L.H.;Huang,W.Org.Lett.2013,15,3102.doi:10.1021/ ol4013052
(24)Huang,J.;Sun,N.;Chen,P.;Tang,R.;Li,Q.;Ma,D.;Li,Z. Chem.Commun.2014,50,2136.doi:10.1039/c3cc49313j
(25)Brutting,W.;Berleb,S.;Mueckl,A.G.Org.Electron.2001,2 (1),1.doi:10.1016/S1566-1199(01)00009-X
(26)Jang,S.E.;Joo,C.W.;Jeon,S.O.;Yook,K.S.;Lee,J.Y.Org. Electron.2010,11,1059.doi:10.1016/j.orgel.2010.03.005
(27)Shin,H.;Lee,S.;Kim,K.H.;Moon,C.K.;Yoo,S.J.;Lee,J. H.;Kim,J.J.Adv.Mater.2014,26,4730.doi:10.1002/adma. v26.27
One-Step Synthesis of Spirobi[fluorene]and Spiro[fluorene-9,9′-xanthene]Derivatives and Their Applications in Organic Light-Emitting Devices:Performance Enhancement and Related Optical Phenomena
GUAN Yu-Qiao1,#SONG Juan1,#SUN Wei1ZHANG Qin1TANG Chao2
LI Xue3FENG Xiao-Miao1QIAN Yan1TAO You-Tian2CHEN Shu-Fen1,*WANG Lian-Hui1HUANG Wei2,*
January 5,2016;Revised:March 22,2016;Published on Web:March 23,2016.
Employing fluorene as substrate,we synthesized a new spirobifluorene derivative,2′-(9-phenylfluoren-9-yl)-9,9′-spirobi[fluorene](PF-SBF),through a one-step palladium-catalyzed cross-coupling reaction. Utilizing PF-SBF as an emitter and as a host of the blue phosphor bis(3,5-difluoro-2-(2-pyridyl)phenyl-(2-carboxypyri-dyl))iridium(III)(FIrpic)in organic light-emitting devices(OLEDs),we observed a red light band different from the intrinsic blue emission of PF-SBF and FIrpic.This was attributed to the intermolecular aggregation of PF-SBF and to exciplexes generated at the interfaces of the emitting layer and the electron transport layer.The exciplex emission was then restrained through a suitable selection of hole and electron transport layer.Employing PF-SBF with di-[4-(N,N-ditolyl-amino)-phenyl]cyclohexane(TAPC)as a cohost,we obtained high-performance blue and green emissions from FIrpic and tris(2-phenylpyridine)iridium(III)(Ir(ppy)3). The maximum current efficiencies and luminances of the blue and green OLEDs were as high as 16.7 and 50.5 cd∙A-1and 7857(at 11 V)and 23390 cd∙m-2(at 8 V),respectively.As an alternative to PF-SBF,we also synthesized a new xanthene derivative,2-(9-phenyl-fluoren-9-yl)spiro[fluorene-9,9′-xanthene](PF-SFX),with a large triplet energy level of 2.8 eV.Using PF-SFX similarly as a host of FIrpic,the current efficiency and luminance were significantly improved to 22.6 cd∙A-1and 6421 cd∙m-2(at 10 V).These results demonstrate the potential of PF-SBF and PF-SFX as new building blocks for high-efficiency green/blue phosphorescent host materials.
Palladium catalysis;One-step method;Exciplex;Cohost;Blue organic light-emitting diode
O649
[Article]10.3866/PKU.WHXB201603232www.whxb.pku.edu.cn
国家重点基础研究发展规划项目(973)(2015CB932202,2012CB933301),国家自然科学基金(61274065,51173081,61136003,BZ2010043,
51372119,51172110,21304047,21373114,21003076),教育部创新团队(IRT1148),教育部人文社会科学基金(13YJCZH091),江苏省自然科学基金(BK20141424),江苏高校优势学科建设工程资助项目(YX030001),江苏省普通高校研究生实践创新项目(SJLX15_0390),南京邮电大学攀登项目(NY214085)及吉林大学开放课题(IOSKL2015KF32)资助
©Editorial office ofActa Physico-Chimica Sinica
*Corresponding authors.CHEN Shu-Fen,Email:iamsfchen@njupt.edu.cn.HUANG Wei,Email:wei-huang@njtech.edu.cn;Tel:+86-25-85866332.#These authors contributed equally to this work.

