硫黄素T对淀粉样β-蛋白质40聚集成核动力学的双重影响
李松 刘夫锋 余林玲 赵彦娇 董晓燕
(天津大学化工学院生物工程系,系统生物工程教育部重点实验室,天津300072)
硫黄素T对淀粉样β-蛋白质40聚集成核动力学的双重影响
李松刘夫锋余林玲赵彦娇董晓燕*
(天津大学化工学院生物工程系,系统生物工程教育部重点实验室,天津300072)
荧光染料硫黄素T常用于淀粉样纤维聚集过程的定性定量检测。虽然有研究表明,某些抑制淀粉样蛋白质聚集的小分子抑制剂会与硫黄素T相互作用,影响其测试结果。但硫黄素T如何影响淀粉样蛋白质的聚集成核动力学尚不清晰。本文以淀粉样β-蛋白质40(Aβ40)为模型,系统研究了硫黄素T对Aβ40聚集成核的影响。研究发现:硫黄素T能够显著改变Aβ40的聚集成核动力学,且影响程度与硫黄素T的浓度密切相关。即在低浓度硫黄素T存在下,Aβ40成核速率的延迟时间先随着硫黄素T浓度的升高而缩短,后随着硫黄素T浓度的升高延迟时间反而延长。但延伸的速率却随硫黄素T浓度的升高而缓慢增大。另外,硫黄素T基本不会影响Aβ40的二级结构和纤维形态。同时,等温滴定微量热实验结果表明,硫黄素T结合Aβ40之间的主要作用力为疏水相互作用。据此,本研究提出硫黄素T对Aβ40聚集成核动力学的双重影响机理。这些结果有助于进一步了解硫黄素T与淀粉样蛋白质的作用特点,为今后硫黄素T在Aβ40聚集成核动力学实验中的使用提供参考。
淀粉样β蛋白质;硫黄素T;荧光动力学分析;成核;分子相互作用
1 lntroduction
Alzheimerʹs disease(AD),the most prevalent neurodegenerative disease,is closely related to the aggregation of amyloid βprotein(Aβ)in brain.Aβ contains 39-43 residues and Aβ40 and Aβ42 are the most prevalent variations1.It is well known that Aβ monomers aggregate into oligomers with conformational transition from randomcoil to β-sheet simultaneously2,3,and then form protofibrils as well as mature fibrils by elongation4.Studying the aggregation kinetics of Aβ has long been and is still important for the development of both diagnostic methods and the effective inhibitors that interfere withAβ aggregation5.
The benzothiazole dye thioflavin T(ThT)[see Fig.S1(Supporting Information)]has a high selectivity for amyloid fibrils and exhibits enhanced fluorescence at about 480 nm when bound to amyloid fibrils6.So,ThT fluorescence analysis has been widely utilized for identification and quantitative determination of amyloid fibrils,thus exploring the fibrillization kinetics of amyloid proteins in vitro7.Many studies have found that ThT significantly biases the ThT fluorescence readings in in situ real-time ThT assays8,9.For example,Hudson et al.10have found that the presence of curcumin at concentrations as low as 0.01 μmol∙L-1was sufficient to interference with the ThT fluorescence assay for Aβ detection.
Up to now,several binding models of ThT on amyloid fibrils have been proposed based on the experimental and theoretical studies11.Of them,dual binding modes of ThT to the protofibrils have been widely approved.In the primary mode,ThT binds to a groove formed by side chains of residues along the fibril axis.The binding is highly directional,with the ThT long axis parallel to the elongation axis of the fibrils.In the secondary mode,ThT binds parallel to the β-strands on the edge or in the middle of a β-sheet. Wu et al.12suggest that the primary mode is notably more favorable than the secondary mode.
In the use of ThT in the studies of amyloid aggregation kinetics,most of previous studies considered that ThT does not affect or only slightly affects the aggregation kinetics of amyloid proteins in vitro13,but some researches indicated that ThT reducesAβ3-42 aggregation and decreases the levels of soluble aggregation-prone oligomeric proteins in vivo14.Recently,DʹAmico et al.15found that in situ presence of ThT promotes Aβ40 aggregation and shortens the lag phase under near-physiological conditions.However,the effect of ThT on the aggregation kinetics of Aβ40 is not fully understood.Hence,we have performed our research to explore the ThT effect on the Aβ40 fibrillization kinetics by using extensive physical,biophysical,and thermodynamics analyses.The effects of ThT on the secondary structure and morphology of Aβ fibrils were probed using circular dichroism(CD)and transmission electron microscopy(TEM),respectively.The molecular interactions between ThT and Aβ40 were studied using isothermal titration calorimetry(ITC).Finally,we explored the molecular mechanisms of the effects of ThT onAβ fibrillization.Our findings are expected to provide new insights into the application of ThT in the study ofAβ aggregation.
2 Materials and methods
2.1Materials
Hexafluoroisopropanol(HFIP),phosphotungstic acid,and ThT were purchased from Sigma(St.Louis,MO,USA).Aβ40 was purchased as lyophilized powder with a purity of>95%from GL Biochem(Shanghai,China).Other chemicals were all of the highest purity available from local sources.
2.2Aβ40 monomer solution preparation
Aβ40 was prepared as described in literature16,17.Briefly,the received Aβ40 was dissolved to 1 mg∙mL-1in HFIP and lyophilized by a vacuum freeze-dryer(Labconco,America)to obtain the lyophilized peptide.Then the lyophilized peptide was dissolved in 20 mmol∙L-1NaOH at 275 or 550 μmol∙L-1,sonicated for 20 min followed by centrifugation(16000 g)for 30 min at 4°C.The upper 75%of the supernatant was carefully taken out and diluted 10-fold in phosphate buffered saline(PBS)solution(10 mmol∙L-1sodium phosphate containing 100 mmol∙L-1NaCl),and finally pH was adjusted to 7.4(while for seed solution preparation,in situ assay and seeded aggregation experiment,0-128 μmol∙L-1different concentrations of ThT was added in this step),leading to a final protein concentration of 25 or 50 μmol∙L-1.This solution was used immediately for following assays.
2.3In situ ThT fluorescence assay(Aβ incubatedwith ThT at the beginning)
The samples containing Aβ40 with different ThT were incubated at 37°C in a 96-well plate.Every 10 min,the plates were shaken for 5 s,and fluorescence(with excitation and emission at 440 and 480 nm,respectively)was monitored using an Infinite M200 PRO Multifunctional Microplate Reader(Tecan,Groedig,Austria).

For better understanding of molecular mechanisms of Aβ40 aggregation mediated by ThT,a sigmoidal function was used to describe the aggregation kinetics18, where t is the incubation time,F(t)is the real-time fluorescence,t1/2is the time at half completion of the whole aggregation process,k is the elongation rate constant,F0is the baseline before aggregation,A is the amplitude.tlagis the lag time.tlagwas defined as the intercept between the baseline and the tangent with the slope from the midpoint of the fitted sigmoidal curve(the slope equals to the k value),could be calculated by,

Three independent ThT fluorescent experiments were performed for both 25 and 50 μmol∙L-1Aβ40.The shown data points in Fig.1a are the average value of the three repeated experiments with the corresponding standard deviation.For clarity,only onerepresentative curve for 25 μmol∙L-1Aβ40 is reported in Fig.S2 (Supporting Information).
2.4Ex situ ThT fluorescent assay(ThT was added after Aβ incubation)
50 μmol∙L-1Aβ40 monomer solution was incubated at 37°C in a 96-well plate.Every 10 min,the plate was shaken for 5 s.At the scheduled time,the solution would be taken out and mixed with PBS plus ThT.Then the mixed samples were transferred to another plate for fluorescence measurement by the Infinite M200 PRO Multifunctional Microplate Reader(Tecan,Groedig,Austria).In above ex situ assays,each experiment was conducted in triplicate and the average value is reported with its standard deviation.
2.5Circular dichroism(CD)
Far-UV CD measurements on samples in PBS were carried out using a Jasco 810 spectrophotometer(Jasco Inc.,Tokyo,Japan)with a 1.0 mm path length quartz curette.The baseline(PBS buffer alone)was subtracted from the corresponding spectrum.
2.6Transmission electron microscopy
Aβ40 samples(10 μL)were dropped on carbon-coated copper grids(300-mesh)for 3 min,and the excess samples were removed. Then the grids were negatively stained with 3.54 μmol∙L-1phosphotungstic acid(pH 7.4)followed by air-drying.After that the samples were examined by a JEM 100 CXII transmission electron microscope system(JEOL Inc.,Tokyo,Japan)at an accelerating voltage of 100 kV.
2.7lsothermal titration calorimetry
ITC experiments were performed using VP isothermal titration calorimeter(MicroCal,Nothampton,America)as reported19,20with properly modifications.After 20 min degassing,50 μmol∙L-1Aβ 40 in PBS were injected into a 1.425 mLsample cell.A10 μLThT solution in the same buffer as theAβ40 solution was injected over 20 s for 25 times at a constant interval of 350 s via a 299 r∙min-1rotating stirrer-syringe into the sample cell.The titrant was injected to the buffer in the sample cell to obtain the heat of dilution in the control experiment.The heat of dilution was subtracted from the experimental result in the final analysis.
The evaluation software,MicroCal Origin,Version 7.0,provided by the manufacturer was used to analyze the titration data. The binding curves were calculated by a single-site binding model21,and fitted dissociation constant(Kd),binding stoichiometry(N),enthalpy change(∆H),entropy change(∆S),and Gibbs free energy(∆G)were obtained.Herein,∆H and∆S represent electrostatic and hydrophobic interactions,respectively.In above ITC study,each experiment was performed three times to get the average value and the corresponding standard deviation.
3 Results and discussion

Fig.1 Effect of the logarithm of ThT concentration(log2cThT)on the lag time(a)and elongation rate constant(b)of Aβ40 in in situ fluorescence assayIncubation of 25 or 50 μmol∙L-1Aβ40 was done in phosphate buffered saline(PBS)plus 0.125-128 μmol∙L-1ThT at 37°C. The solid lines are drawn to guide the eye.
In order to clearly show the effect of ThT onAβ40 fibrillization,the in situ ThT fluorescence kinetics assays were conducted at different ThT concentrations andAβ40 concentration of 25 μmol∙L-1(Fig.S2).As shown in Fig.S2,Aβ40 exhibited a typical nucleation-elongation profile,that is,a steady lag phase followed by an elongation one,and finally a steady state after equilibrium is reached.Similar figure was also obtained for 50 μmol∙L-1Aβ40 and was not shown.In order to explore the effect of ThT on the aggregation kinetics ofAβ40 in detail,the aggregation kinetic data were fitted to Eqs.(1)and(2).The values of tlagand k in in situ assays were obtained and plotted versus ThT concentrations in Fig.1(a)and Fig.1(b),respectively.It is clear that,in 50 μmol∙L-1Aβ40 solutions,the lag time of Aβ40 decreased slowly by incubating with low concentrations of ThT(<32 μmol∙L-1),consistent with previously published study15.At higher concentrations of ThT (>32 μmol∙L-1),however,the lag time increased slightly with increasing ThT concentrations.The similar phenomenon is also observed in 25 μmol∙L-1Aβ40(Fig.1(a)).Therefore,the ThT concentration has significant impacts on the aggregation kinetics ofAβ40.
From Fig.1(a),it is clear that there is a specific concentration,which promotes greatlyAβ40 fibrillization.At 25 and 50 μmol∙L-1Aβ40,for example,the corresponding specific concentrations of ThT are 16 and 32 μmol∙L-1,respectively.Thus,the specific concentration of ThT increases with Aβ40 concentration.Moreover,the specific concentration ratio of ThT relative toAβ40(i.e.,16:25)is the same in bothAβ40 concentrations used herein.It is noted that the highest value of the concentration ratio of ThT to Aβ40 is 11:25 in DʹAmicoʹs study15which is lower than the specific one.Additionally,the ex situ ThT fluorescence kineticsassays were analyzed as the controls and displayed in Fig.S3 (Supporting Information).It is clear that,for the ex situ assay,similar lag phases were obtained at different ThT concentrations (as long as~23 h).Therefore,the differences in lag phases between in situ and ex situ assays further proved that ThT did participate in the nucleation of Aβ40 and had dual effect on fibrillization ofAβ40 in a dose-dependent manner.As shown in Fig.1(b),the elongation rate constant increased quite slowly with the increase of ThT concentration in the whole concentration range for 25 and 50 μmol∙L-1Aβ40.
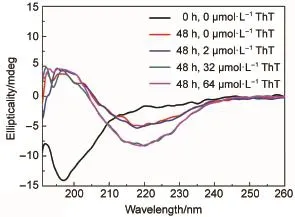
Fig.2CD spectra of 50 μmol∙L-1Aβ40 at different conditionsIncubation of 50 μmol∙L-1Aβ40 was conducted in phosphate buffered saline plus 0-64 μmol∙L-1ThT at 37°C for 0 or 48 h.

Fig.3TEM images ofAβ40 fibrils incubated with different concentrations of ThTFibrils were observed after incubating 50 μmol∙L-1Aβ40 in phosphate buffered saline plus 0 μmol∙L-1(a),0.125 μmol∙L-1(b),2 μmol∙L-1(c),and 32 μmol∙L-1(d)ThT at 37°C for 48 h.
Next,CD and TEM assays were carried out to explore the effects of ThT on the secondary structure and the morphology ofAβ 40 fibrils.As shown in Fig.2,in the absence of ThT,Aβ40 molecules showed typical random coil structures before incubation,indicated by the obvious negative peak at 200 nm.However,after incubated for 48 h,the negative peak at 200 nm was replaced by a negative peak at 216 nm and a positive peak around 198 nm. Thus,Aβ40 had transformed from its initial random coil to a βsheet-rich structure,which is consistent with our previous results22. As comparing their traces in Fig.2,it is clear that Aβ40 molecules incubated with different ThT concentrations from 0.2 to 64 μmol∙L-1for 48 h showed no significant difference in their secondary structures.Thus,ThT molecules cannot affect the conformational transition from its initial random coil to final β-sheet structures. It is noted that the amounts of β-sheet at higher ThT concentrations are higher than those in the absence and presence of lower concentrations(Fig.2).It implied that ThT molecules templatesAβ aggregation-prone conformations,consistent with previous results23.The effect of ThT on the morphologies of Aβ40 fibrils was observed by TEM and shown in Fig.3.It can be seen from Fig.3(a)that,in the absence of ThT,a typical morphology of Aβ40 fibrils was observed after the incubation of 48 h,consistent with the previous published study24.Moreover,in the presence of 0.125-32 μmol∙L-1ThT,similar morphologies of fibrils were also found (Fig.3(b-d)).Therefore,ThT can neither affect the final secondary structure nor change the fibril morphology ofAβ40 fibrils.
Then,ITC experiments were conducted to probe the interactions between ThT and Aβ40.The ITC results for the titration of ThT to Aβ40 monomer are supplied in Fig.S4(Supporting Information),and the thermodynamic parameters derived from the single-site bonding model calculation are summarized in Table 1. It is shown that a weak affinity binding of ThT to Aβ40 was determined with the dissociation constant Kdof~5×10-5mol∙L-1,consistent with previous study11.Besides,the N value of 0.61± 0.06 suggests that one ThT molecule was most-likely bound onto two Aβ40 molecules.It should be noted that N is not the real number of ThT molecules bound to each Aβ40 molecule,but a thermodynamic statistic representing the overall binding effect of ThT molecules toAβ40.
As shown in Table 1,it is clear that the negative value of ΔG means that ThT binds spontaneously ontoAβ40.And,the negative enthalpy(ΔH)and positive entropy(TΔS)mean that ThT bound to Aβ40 is both enthapically and entropically favored.Moreover,the entropy part evidently gives larger contribution to the negative change of Gibbs energy(ΔG)than the enthalpy part does. Therefore,hydrophobic interactions play an important role in the interactions between ThT and Aβ40 although both electrostatic interactions and hydrophobic interactions contributed to the binding of ThT onto Aβ40.From Fig.S1,it is clear that ThT comprises two aromatic rings(i.e.,benzothiazole ring and benzyl ring)and a positive charged dimethylamino group.As we know,Aβ40 also contains many negative charged residues(e.g.,Glu and Asp)and hydrophobic residues(e.g.,Phe,Tyr,Leu,Val,and Ile). It can thus be concluded that ThT molecules bind onto Aβ40 spontaneously through both electrostatic and hydrophobic inter-actions,which was consistent with the results of molecular dynamics simulations25.

Table 1Thermodynamic parameters of the interactions betweenAβ40 and ThTa
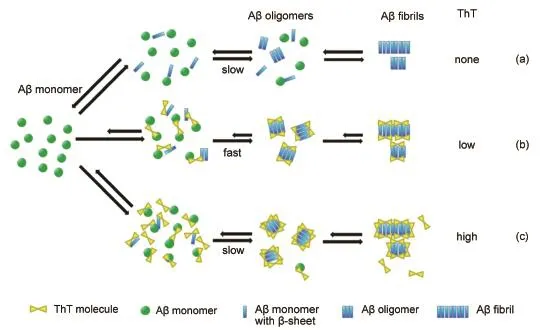
Fig.4Schematic diagram for the possible working mechanisms of the dual effect of ThT onAβ40 fibrillizationDetails are discussed in the text.
Based on the above experimental results,we have proposed a mechanistic model to interpret the working mechanism of the dual effect of ThT on Aβ fibrillization(Fig.4).Aβ fibrillization is a highly complex process that involves sequential formation of various aggregation species(i.e.,oligomers,protofibrils and fibrils)and there exists a balance between them.In the absence of ThT,Aβ monomers with initial random coil structure translate spontaneously into β-sheet rich ones and subsequently self-assembly into different aggregation forms(Fig.4(a)).
At low ThT concentrations,all of ThT molecules spontaneously bound in channels running parallel to the long axis of the Aβ40 aggregates like a wedge(Fig.S5(Supporting Information)),and stabilized the preformed aggregates(Fig.4(b)).So,the stabilized aggregates were worked as the templates to fascinate the contact and interactions between the neighboringAβ40 monomers and the lateral peptide of the preformed aggregates,resulting in the accelerated nucleation rate(Fig.4(b)).At higher ThT concentrations,however,the redundant ThT molecules bound onto the secondary binding site(i.e.,the edge of the β-strands)after the primary binding site was occupied(Fig.S6(Supporting Information)),which would inhibit the contact between the lateral peptide and freeAβ40 monomers and suppressed theAβ40 fibrillization(Fig.4 (c)).Thus,the Aβ40 nucleation rate was slowed down in the presence of high concentrations of ThT(Fig.1(a)).However,the weak binding between ThT and the lateral peptide could hardly affect the elongation process,such as the secondary structure transition(Fig.2)and fibrillar elongation(Fig.1(b)),leading to no significant differences in them at different ThT concentrations.In summary,the effect of ThT onAβ40 fibrillization was the main in the nucleation process rather than in the elongation one.
4 Conclusions
In this work,we focused on influence of ThT on Aβ40 fibrillization kinetics,and the secondary structure and morphology of the fibrils,and explored the thermodynamic binding behavior between ThT and Aβ40.It was found that the nucleation and elongation rates were dependent on the ThT concentrations,while no significant effect on the secondary structure and fibril morphology of fibrils was observed.Comprehensive analyses of the thermodynamic parameters(Kd,ΔH,ΔS,and ΔG)suggested that ThT molecules bound to Aβ40 via weak electrostatic and hydrophobic interactions.Based on the experimental observations,the working mechanism of the dual effect of ThT on Aβ fibrillization was proposed.The work has thus called the attentions need to be paid to the study of amyloid fibrillization using in situ ThT fluorescence assays.Particularly,an in situ ThT fluorescence assay is not recommended for the study on the aggregation process of amyloid,though it is still a useful method in investigating the aggregation mechanism of amyloid proteins as well as developing effective inhibitors of aggregation.
Supporting lnformation:available free of charge via theinternet at http://www.whxb.pku.edu.cn.
References
(1)Hamley,I.W.Chem.Rev.2012,112,5147.doi:10.1021/ cr3000994
(2)Liu,F.F.;Dong,X.Y.;Sun,Y.Acta Phys.-Chim.Sin.2010,26,1643.[刘夫锋,董晓燕,孙彦.物理化学学报,2010,26,1643.]doi:10.3866/PKU.WHXB20100613
(3)Liu,F.F.;Dong,X.Y.;He,L.;Middelberg,A.P.;Sun,Y. J.Phys.Chem.B 2011,115,11879.doi:10.1021/jp202640b
(4)Liu,F.F.;Ji,L.;Dong,X.Y.;Sun,Y.J.Phys.Chem.B 2009,113,11320.doi:Doi 10.1021/Jp905580j
(5)Nguyen,P.;Derreumaux,P.Acc.Chem.Res.2014,47,603. doi:10.1021/ar4002075
(6)Lindberg,D.J.;Wranne,M.S.;Gatty,M.G.;Westerlund,F.;Esbjorner,E.K.Biochem.Biophys.Res.Commun.2015,458,418.doi:10.1016/j.bbrc.2015.01.132
(7)Hsu,J.C.C.;Chen,E.H.L.;Snoeberger,R.C.;Luh,F.Y.;Lim,T.S.;Hsu,C.P.;Chen,R.P.Y.J.Phys.Chem.B 2013,117,3459.doi:10.1021/Jp309331u
(8)Palhano,F.L.;Lee,J.;Grimster,N.P.;Kelly,J.W.J.Am. Chem.Soc.2013,135,7503.doi:10.1021/ja3115696
(9)Hackl,E.V.;Darkwah,J.;Smith,G.;Ermolina,I.Eur.Biophys. J.2015,44,249.doi:10.1007/s00249-015-1019-8
(10)Hudson,S.A.;Ecroyd,H.;Kee,T.W.;Carver,J.A.FEBS J. 2009,276,5960.doi:10.1111/j.1742-4658.2009.07307. xEJB7307
(11)Groenning,M.J.Chem.Biol.2010,3,1.doi:10.1007/s12154-009-0027-527
(12)Wu,C.;Biancalana,M.;Koide,S.;Shea,J.E.J.Mol.Biol. 2009,394,627.doi:10.1016/j.jmb.2009.09.056
(13)Heiser,V.;Scherzinger,E.;Boeddrich,A.;Nordhoff,E.;Lurz, R.;Schugardt,N.;Lehrach,H.;Wanker,E.E.Proc.Natl.Acad. Sci.U.S.A.2000,97,6739.doi:10.1073/pnas.110138997
(14)Alavez,S.;Vantipalli,M.C.;Zucker,D.J.;Klang,I.M.;Lithgow,G.J.Nature 2011,472,226.doi:10.1038/nature09873
(15)D'Amico,M.;Di Carlo,M.G.;Groenning,M.;Militello,V.;Vetri,V.;Leone,M.J.Phys.Chem.Lett.2012,3,1596. doi:10.1021/Jz300412v
(16)Xiong,N.;Dong,X.Y.;Zheng,J.;Liu,F.F.;Sun,Y.ACS Appl. Mater.Inter.2015,7,5650.doi:10.1021/acsami.5b00915
(17)Du,W.J.;Guo,J.J.;Gao,M.T.;Hu,S.Q.;Dong,X.Y.;Han,Y. F.;Liu,F.F.;Jiang,S.Y.;Sun,Y.Sci.Rep.2015,5,7992. doi:10.1038/srep07992
(18)Hellstrand,E.;Boland,B.;Walsh,D.M.;Linse,S.ACS Chem. Neurosci.2010,1,13.doi:10.1021/Cn900015v
(19)Wang,S.H.;Liu,F.F.;Dong,X.Y.;Sun,Y.Biochem.Eng.J. 2012,62,70.doi:10.1016/j.bej.2012.01.005
(20)Wang,S.H.;Liu,F.F.;Dong,X.Y.;Sun,Y.J.Phys.Chem.B 2010,114,11576.doi:10.1021/jp1001435
(21)Robbins,K.J.;Liu,G.;Selmani,V.;Lazo,N.D.Langmuir 2012,28,16490.doi:10.1021/La303677t
(22)Cerda-Costa,N.;Esteras-Chopo,A.;Aviles,F.X.;Serrano,L.;Villegas,V.J.Mol.Biol.2007,366,1351.doi:10.1016/j. jmb.2006.12.007
(23)Schmidt,M.;Rohou,A.;Lasker,K.;Yadav,J.K.;Schiene-Fischer,C.;Fandrich,M.;Grigorieff,N.Proc.Natl.Acad.Sci. U.S.A.2015,112,11858.doi:10.1073/pnas.1503455112
(24)Veloso,A.J.;Dhar,D.;Chow,A.M.;Zhang,B.;Tang,D.W.;Ganesh,H.V.;Mikhaylichenko,S.;Brown,I.R.;Kerman,K. ACS Chem.Neurosci.2013,4,339.doi:10.1021/cn300171c
(25)Di Carlo,M.G.;Minicozzi,V.;Fodera,V.;Militello,V.;Vetri,V.;Morante,S.;Leone,M.Biophys.Chem.2015,206,1. doi:10.1016/j.bpc.2015.06.006
Dual Effect of Thioflavin T on the Nucleation Kinetics of Amyloid β-Protein 40
LI SongLIU Fu-FengYU Lin-LingZHAO Yan-JiaoDONG Xiao-Yan*
(Key Laboratory of Systems Bioengineering of the Ministry of Education,Department of Biochemical Engineering,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,P.R.China)
The fluorescent dye thioflavin T(ThT)is widely used for the qualitative and quantitative detection of amyloid fibrils.However,many small-molecular inhibitors have been shown to compete with ThT in binding the fibrils and therefore greatly affect the ThT fluorescence.The effect of ThT on the aggregation kinetics of amyloid proteins is not yet fully understood.Here,using amyloid β-protein 40(Aβ40)as a model system,we show that ThT significantly alters the aggregation kinetics of Aβ40 in a dose-dependent manner,leading to a decrease-increase trend in the lag time that represents the nucleation rate.Specifically,the lag time decreases as a function of ThT concentration at low ranges,but then begins to increase beyond a specific ThT concentration,which itself increases withAβ40 concentration.By contrast,the elongation rate slowly increases with ThT concentration.As for the secondary structure and morphology of the fibrils,no significant effects of ThT are observed.Isothermal titration calorimetry suggests that the hydrophobic interaction dominates the binding of ThT to Aβ40.Based on these findings,a working mechanism of the dual effects of ThT on Aβ fibrillization is proposed.These results should aid our understanding of the molecular mechanism of ThT binding withAβ and allow practical improvements in the measurement of the nucleation kinetics ofAβ fibrillization.
Amyloid β-protein;Thioflavin T;Fluorescence kinetic assay;Nucleation;Molecular interaction
January 4,2016;Revised:March 21,2016;Published on Web:March 22,2016.
O641
[Article]10.3866/PKU.WHXB201603221www.whxb.pku.edu.cn
*Corresponding author.Email:d_xy@tju.edu.cn;Tel:+86-22-27404981.
The project was supported by the National Natural Science Foundation of China(21236005,21376172,21406160,21576199).
国家自然科学基金(21236005,21376172,21406160,21576199)资助项目
©Editorial office ofActa Physico-Chimica Sinica

