磁性AgBr/Ag3PO4/ZnFe2O4复合催化剂的制备及光催化性能
孟英爽 安 逸 郭 谦 葛 明,3,*
(1华北理工大学化学工程学院,河北唐山063009;2华北理工大学以升创新教育基地,河北唐山063009;3河北省环境光电催化材料重点实验室,河北唐山063009)
磁性AgBr/Ag3PO4/ZnFe2O4复合催化剂的制备及光催化性能
孟英爽1安逸2郭谦1葛明1,3,*
(1华北理工大学化学工程学院,河北唐山063009;2华北理工大学以升创新教育基地,河北唐山063009;3河北省环境光电催化材料重点实验室,河北唐山063009)
水热法结合原位沉淀法成功制备新型磁性溴化银/磷酸银/铁酸锌(AgBr/Ag3PO4/ZnFe2O4)复合催化剂,并通过X射线衍射、能量色散X射线、场发射扫描电子显微镜、透射电子显微镜和紫外-可见漫反射光谱对其晶相结构、组成、形貌及吸光性能进行了表征。在可见光照射下,所制备的AgBr/Ag3PO4/ZnFe2O4复合催化剂光催化降解罗丹明B(RhB)的活性优于Ag3PO4/ZnFe2O4、AgBr/ZnFe2O4和P25 TiO2。在酸性和碱性溶液中,AgBr/Ag3PO4/ZnFe2O4光催化剂呈现出优良光催化性能。在AgBr/Ag3PO4/ZnFe2O4体系中,光催化降解RhB的速率随着反应体系温度的升高而增大,由阿伦尼乌斯方程计算获得反应体系活化能为31.9 kJ·mol-1。AgBr/Ag3PO4/ZnFe2O4复合材料优异的可见光催化活性归因于光生电荷的有效分离,所产生的超氧自由基和空穴是RhB降解的主要活性物种。
AgBr/Ag3PO4/ZnFe2O4;磁性;光催化;可见光;机理
www.whxb.pku.edu.cn
1 Introduction
Many toxic organic compounds in water are recalcitrantly polluting due to their slow degradation by microorganisms. Heterogeneous photocatalysis becomes an advanced oxidation approach to removing hazardous organic compounds in water1,2. Currently,TiO2is the most frequently used photocatalyst3. However,its large band gap(~3.2 eV)invokes ultra-violet(UV) light to achieve a high photocatalytic activity,which significantly hinders its efficient utilization of solar energy.Therefore,it is highly desirable to develop highly efficient visible-light-driven photocatalysts using sunlight.
In recent years,Ag-based catalysts with high visible-light photocatalytic activities have received increasing attention4. Among the reported Ag-based photocatalysts,silver orthophosphate(Ag3PO4)turns out to be the highest in quantum efficiency and has been widely used for organic pollutants degradation under visible light irradiation5,6.Our previous studies have confirmed that Ag3PO4possesses an excellent photocatalytic performance for dyes degradation under visible light7,8.Nonetheless,it is undeniable that the consumption of a large amount of noble metal silver prevents its practical application.Therefore,Ag3PO4-based composite photocatalysts have been developed to reduce Ag consumption without sacrificing their high photocatalytic performances9-11.
Spinel zinc ferrite(ZnFe2O4)is a visible-light driven photocatalyst with high stability12.Moreover,ZnFe2O4can be feasibly recycled by an external magnet because it is one of the most interesting ferrites13.Recently,many efforts have been devoted to combining ZnFe2O4with other semiconductors to yield recyclable and high-performance composite catalysts.For instance,Chen et al.14employed a solvothermal-liquid phase deposition method to synthesize a Ag3PO4/ZnFe2O4composite photocatalyst with an enhanced photocatalytic activity for 2,4-dichlorophenol degradation.Ge et al.15reported a magnetic Ag3PO4/ZnFe2O4photocatalyst with a high photocatalytic efficiency for dye degradation using visible light.Li et al.16synthesized a magnetically separable ZnFe2O4-ZnO-Ag3PO4hollow nanospheres with photocatalytic activity superior to Ag3PO4/ZnFe2O4.According to previous reports,the AgBr/Ag3PO4composite photocatalysts is much better in photocatalysis than Ag3PO417,18.We anticipate that the hybrid AgBr/Ag3PO4/ZnFe2O4catalyst could be an excellent photocatalytic material based on the facts that the presence of ZnFe2O4in AgBr/Ag3PO4/ZnFe2O4composite reduces the consumption of AgBr/Ag3PO4,and the composite catalyst can be recycled by an external magnet15,16.
Herein,a novel magnetic AgBr/Ag3PO4/ZnFe2O4composite photocatalyst was synthesized via the hydrothermal and in situ precipitation method.Its photocatalytic performance was verified with the photodegradation of rhodamine B(RhB)under the illumination of visible light.The pH-and temperature-dependent experiments were carried out to explore in detail the photocatalysis,recycling and possible photodegradation mechanism of the AgBr/Ag3PO4/ZnFe2O4system.
2 Materials and methods
2.1Preparation of catalysts
All reagents were of analytical grade and used as received from Tianjin Guangfu Chemical Co.ZnFe2O4particles were synthesized via a simple hydrothermal route15.0.001 mol Zn(NO3)2·6H2O(AR) and 0.002 mol FeCl3·6H2O(AR)were dissolved in 30 mLdistilled water,and then 5 mL NaOH solution(3 mol·L-1,AR)was added under stirring.The mixture was transferred to a 50 mL Teflonlined autoclave and thermally treated at 180°C for 15 h.The precipitate was collected by centrifugation,washed with distilled water and pure ethanol for several times,and then calcined at 500°C for 3 h.
The synthetic procedure of the AgBr/Ag3PO4/ZnFe2O4composite catalyst was as follows:100 mLdeionized water and 50 mL absolute ethanol were mixed,then 0.002 mol ZnFe2O4sample was added and sonicated for 20 min before 0.003 mol AgNO3(AR) was added and magnetically stirred for 30 min.0.001 mol Na2HPO4·12H2O(AR)was dissolved in 30 mL deionized water, and the prepared soluton was added dropwise into the ZnFe2O4suspension under magnetic stirring.After 30 min of stirring,20 mL NaBr solution(0.05 mol·L-1,AR)was added dropwise into above-prepared suspension.After stirred for another 30 min,the precipitate was collected by centrifugation,washed with distilled water and pure ethanol for several times,and then dried at 80°C for 2 h.
For comparison,the Ag3PO4/ZnFe2O4or AgBr/ZnFe2O4catalyst was prepared in the absence of either NaBr or Na2HPO4.AgBr/ Ag3PO4could be obtained without addition of the ZnFe2O4nanoparticles.Pure Ag3PO4and AgBr were prepared by a direct ion-exchange method.P25 TiO2was purchased from Degussa AG, Germany.
2.2Characterization
The phase and purity of the as-prepared catalysts were analyzed by X-ray diffraction(XRD)(Rigaku D/Max-2500 X-ray diffractometer,Japan)using Cu Kαradiation,λ=0.154056 nm.The size and shape of the samples were characterized by field emission scanning electron microscope(FESEM,HITACHI S-4800,Japan) associated with energy dispersive X-ray spectroscopy(EDS) (Thermo Fisher,Noran 7,USA)and transmission electron microscopy(TEM,JEOL 2100,Japan).The UV-Vis diffuse reflectance spectroscopy(DRS)was achieved by a UV-Vis spectrophotometer(Shimadzu,UV-3600,Japan).The photoluminescence spectra of the samples were measured with a fluorescence spectrophotometer(FLsp920,Edinburgh).The magnetic measurement instrument(MPMS-XL-7,Quantum Design,USA)was employed to detect the magnetic property of the AgBr/Ag3PO4/ZnFe2O4catalyst at room temperature.
2.3Photocatalytic tests
The photocatalytic activities of the as-prepared photocatalysts for RhB degradation were investigated under visible light illumination.The photodegradation experiments were carried out in a simple photocatalytic reactor8,and the reaction temperature was controlled to be 25±1°C.The catalyst(0.10 g)was added into the RhB solution(10 mg·L-1,100 mL),followed by magnetical stirring in dark for 30 min.During the light irradiation,3 mL of the suspension was taken out at given time intervals and separated by centrifugation.The residual RhB concentration was calculated from the absorbance at 553 nm,as measured by a visible spectrophotometer(722s,China).Nitric acid and sodium hydroxide were used to adjust the initial pH of the RhB solution.The active species generated in the photocatalytic process were detected using in situ capture experiments.
3 Results and discussion
3.1Sample characterization
The structure and purity of the as-synthesized samples were analyzed by XRD,as shown in Fig.1.All peaks in Figs.1a,1b,and 1c can be indexed to ZnFe2O4(JCPDS No.22-1012)19,Ag3PO4(JCPDS No.06-0505)7,and AgBr(JCPDS No.06-0438)20.A comparison of Figs.1a and 1b leads to that the sample(Fig.1d) contains two crystalline phases and is indeed the Ag3PO4/ZnFe2O4composite.The as-prepared sample(Fig.1e)exhibits the coexistence of ZnFe2O4,Ag3PO4and AgBr phases,indicating that the AgBr/Ag3PO4/ZnFe2O4composite catalyst was obtained via the hydrothermal and in situ precipitation method.Similarly,theAgBr/ ZnFe2O4composite catalyst is also successfully prepared(Figs.1a, 1c and 1f).The chemical composition of the AgBr/Ag3PO4/Zn-Fe2O4catalyst was verified by EDS(Fig.2).The detectable elements are O,P,Fe,Zn,Br and Ag(C comes from the conductive tape),further confirming the existence ofAgBr,Ag3PO4and Zn-Fe2O4.
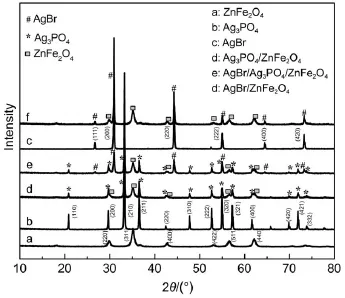
Fig.1 XRD patterns of the as-prepared samples

Fig.2 EDS spectrum of theAgBr/Ag3PO4/ZnFe2O4composite catalyst
Figs.3a,3b and 3c are the FESEM images of the as-synthesized samples.The pure ZnFe2O4sample is composed of nanoparticles (Fig.3a).As shown in Figs.3b and 3c,the ZnFe2O4nanoparticles are loaded at the surfaces of the Ag3PO4and AgBr particles,as compared with Fig.3a.According to Fig.3d,the AgBr/Ag3PO4/ ZnFe2O4sample depicts an irregular shape and varing size.The TEM image of the AgBr/Ag3PO4/ZnFe2O4sample is given in Fig.3e.Obviously,the ZnFe2O4nanoparticles are loaded on the AgBr/Ag3PO4composite particles.Unfortunately,the Ag3PO4and AgBr particles cannot be distinguished by either the low magnification TEM image or the HRTEM image which cannot detect AgBr21.
The optical absorption properties of the as-synthesized samples were measured using a UV-Vis spectrophotometer.The UV-Vis diffuse reflectance spectra of the as-synthesized ZnFe2O4,Ag3PO4, AgBr,Ag3PO4/ZnFe2O4,AgBr/ZnFe2O4and AgBr/Ag3PO4/ZnFe2O4catalysts are displayed in Fig.4.Clearly,all samples exhibit absorption in the visible range(Fig.4).The band gap energy of a semiconductor can be calculated by the following formula22:

where α,h,ν,A and Egare absorption coefficient,planck constant,light frequency,a constant and band gap energy,correspondingly. The value of n is determined from the type of optical transition of a semiconductor(n=1 for direct transition and n=4 for indirect transition).For ZnFe2O4,Ag3PO4and AgBr,n corresponds to 1,1 and 417,23.Thus,the Egestimated by extrapolation of the plots (Fig.5a)of(αhν)2versus hν are 1.85 and 2.42 eV for ZnFe2O4and Ag3PO4,respectively.The Egof AgBr is determined from a plot of (αhν)1/2versus hν about 2.35 eV(Fig.5b).
The valence and conduction band edge potential of a semiconductor can be estimated according to the empirical equations shown below24:

where EVBand ECBare the valence and conduction band edge potentials,respectively;X is the electronegativity of the semiconductor;Eeis the energy of free electrons on the hydrogen scale (about 4.5 eV(vs NHE)).The X values for ZnFe2O4,Ag3PO4and AgBr are 5.05,5.96 and 5.81 eV17,23.As a result,the EVBof Zn-Fe2O4,Ag3PO4and AgBr are calculated to be 1.48,2.67 and 2.49 eV(vs NHE),and their corresponding ECBare-0.37,0.25 and 0.14 eV(vs NHE).
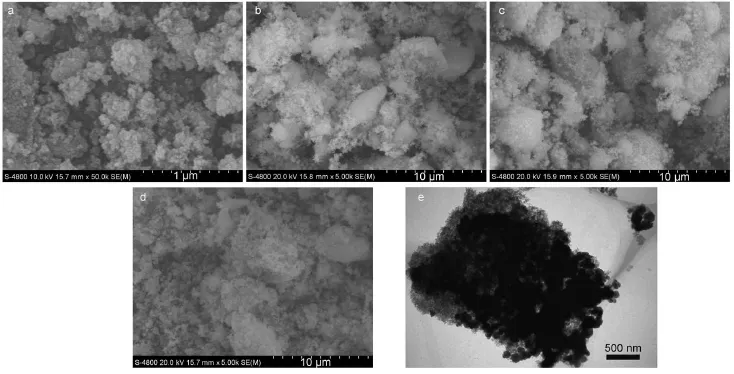
Fig.3 FESEM images of the as-obtained samples
The magnetic property of the as-prepared AgBr/Ag3PO4/Zn-Fe2O4sample was investigated using a magnetic measurement instrument at room temperature.Fig.6 displays the magnetic hysteresis of the AgBr/Ag3PO4/ZnFe2O4catalyst under an applied magnetic field of±2 T.The saturation magnetization(Ms)of the AgBr/Ag3PO4/ZnFe2O4composite is determined to be 2.6 emu·g-1, which is lower than pure ZnFe2O415.The AgBr/Ag3PO4/ZnFe2O4photocatalyst could be separated and recycled from solution by applying an external magnetic field(inset in Fig.6),which is an important advantage for a magnetic photocatalyst.
3.2Photocatalytic activity
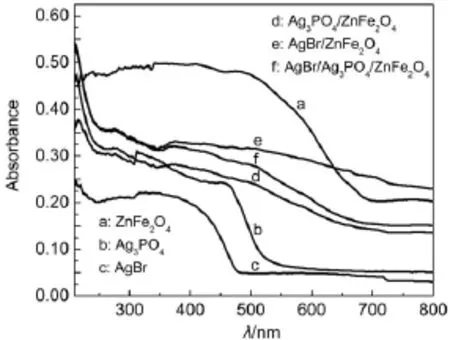
Fig.4 UV-Vis diffuse reflectance spectra of the as-synthesized catalysts

Fig.5 Corresponding band gap energy of ZnFe2O4,Ag3PO4andAgBr

Fig.6 Hysteresis loop ofAgBr/Ag3PO4/ZnFe2O4catalyst at room temperature
The visible-light-driven photocatalytic activities of the assynthesized catalysts were investigated by the photodegradation of RhB in water.Previous studies indicate that the Ag3PO4/Zn-Fe2O4photocatalysts exhibit an excellent photocatalytic activity for organic pollutants degradation under visible light illumination14,15.In this work,the as-prepared Ag3PO4/ZnFe2O4catalyst shows a high photocatalytic performance under visible light,and the AgBr/ZnFe2O4composite also has an excellent photocatalytic capability(Fig.7).After irradiation for 90 min,RhB is nearly 100%degraded over the Ag3PO4/ZnFe2O4or AgBr/ZnFe2O4catalyst(Fig.7).The AgBr/Ag3PO4/ZnFe2O4catalyst possesses a higher photodegradation rate for RhB than Ag3PO4/ZnFe2O4and AgBr/ZnFe2O4(Fig.7).As shown in Fig.S1(Supporting Information),the photoluminescence(PL)emission intensity ofAgBr/ ZnFe2O4orAgBr/Ag3PO4/ZnFe2O4is weaker than that ofAg3PO4/ ZnFe2O4,suggesting that the AgBr/ZnFe2O4or AgBr/Ag3PO4/ ZnFe2O4catalyst has a higher separation rate of electrons and holes,compared with Ag3PO4/ZnFe2O410.According to the PL results(Fig.S1,Supporting Information),the photocatalytic activity of AgBr/ZnFe2O4should be higher than AgBr/Ag3PO4/Zn-Fe2O4,which is not true.This is because the particle size ofAgBr/ Ag3PO4/ZnFe2O4is smaller than that ofAgBr/ZnFe2O4(Figs.3c and 3d),leading to that the AgBr/Ag3PO4/ZnFe2O4catalyst possesses much more active sites.Moreover,the presence of Ag3PO4could adsorb more RhB molecules to promote the photocatalytic activity8.In addition,the as-obtained AgBr/Ag3PO4/ZnFe2O4photocatalyst shows a superior photocatalytic activity for RhB degradation compared with P25 TiO2under visible light illumination (Fig.7).

Fig.7 Photodegradation of RhB by the different catalysts using visible light(pH 7,temperature 25°C)

Fig.8 Photodegradation of RhB overAgBr/Ag3PO4/ZnFe2O4catalyst using visible light at different pH values(temperature 25°C)
Dye wastewater from textile,cosmetics,printing industries and so on often has a wide range of pH and temperature values. Therefore,the RhB photodegradation over the AgBr/Ag3PO4/ ZnFe2O4composite catalyst was scrutinized at different pH (temperature)conditions under visible light irradiation.As shown in Fig.8,the AgBr/Ag3PO4/ZnFe2O4catalyst has a high photocatalytic activity for RhB degradation at various pH values,and the photocatalytic performance of AgBr/Ag3PO4/ZnFe2O4catalyst decreases with increasing pH.In our previous study,the photocatalytic ability of Ag3PO4/ZnFe2O4catalyst for RhB degradation is greatly suppressed in the acidic solution(pH=4)15.In this work, the AgBr/Ag3PO4/ZnFe2O4catalyst in the acidic condition shows a superior photocatalytic activity,and RhB was bearly completely degraded after 60 min at pH 4(Fig.8).This result is attributed to fact that the presence ofAgBr could protectAg3PO4to some extent in the acidic condition25.In addition,the photocatalytic activity of AgBr/Ag3PO4at pH 4 or 10 is lower than that of AgBr/Ag3PO4/ ZnFe2O4(Fig.8 and Fig.S2),indicating that the presence of Zn-Fe2O4could promote the photocatalytic activity of AgBr/Ag3PO4in both acid and basic conditions.This result further reveals that the AgBr/Ag3PO4/ZnFe2O4composite is a good photocatalytic material.
Fig.9a compares the photocatalytic activity of AgBr/Ag3PO4/ ZnFe2O4catalyst for RhB degradation at various temperatures.As can be seen,the photodegradation rate for RhB slightly increases with temperature(Fig.9a).The Langmuir-Hinshelwood model is well established for photocatalysis at low concentrations of pollutants26.The equation is listed as below:

where C0and C are the concentrations of the reactant at time 0 and t,respectively,and k is the apparent reaction rate constant.The k value can be obtained from plot slope of ln(C0/C)versus time.At 298,308,313,and 318 K,the k value was 0.0299,0.0364,0.0552 and 0.0654 min-1,correspondingly.The activation energy(Ea)of the reaction on AgBr/Ag3PO4/ZnFe2O4was determined by the slope of-lnk against 1/T(Fig.9b)according to the Arrhenius equation,and was determined as 31.9 kJ·mol-1.
Further experiments were conducted to recycle the AgBr/ Ag3PO4/ZnFe2O4catalyst for RhB degradation under visible light irradiation.After every 60-min interval of the photocatalytic reaction,concentrated RhB solution was injected,and the catalyst separated by an external magnetic field was washed and put back into the reactor.The RhB degradation efficiency during each run is shown in Fig.10.

Fig.9 (a)Photodegradation of RhB byAgBr/Ag3PO4/ZnFe2O4at different temperature(pH 7);(b)Arrhenius plot based on the effect of temperature
For the first cycle,the AgBr/Ag3PO4/ZnFe2O4catalyst has an excellent photocatalytic performance,which is consistent with the above result(Fig.7).For the second run,the AgBr/Ag3PO4/Zn-Fe2O4composite possesses a lower capacity of removing RhB compared with the first run.This result is due to the formation of metallic Ag which could block the active sites7.Fig.S3(Supporting Information)displays the XRD patterns of the AgBr/ Ag3PO4/ZnFe2O4catalyst before and after the first cycle.Obviously,the Ag peak appears.However,it is worth noticing that the RhB degradation efficiency in the third,fourth and fifth runs tends to be stable compared with the result in the second run.This result is due to fact that the metallic Ag generated in the early stage could prevent the photocorrosion ofAgBr orAg3PO427.
3.3Possible photodegradation mechanism in the
AgBr/Ag3PO4/ZnFe2O4system
Trapping experiments were carried out to detect the reactive oxygen species in the AgBr/Ag3PO4/ZnFe2O4system.Isopropanol, benzoquinone and ammonium oxalate were introduced into the reaction suspension as scavengers for·OH,O2-·and h+,correspondingly7,28.As shown in Fig.11,no obvious inhibition for RhB degradation can be observed when isopropanol is present,indicating that the OH·radicals are not the main reactive oxygen species.However,in the AgBr/Ag3PO4/ZnFe2O4system,the RhB photodegradation rate greatly decreases when benzoquinone or ammonium oxalate is added(Fig.11).The schematic diagram of the possible electron-hole separation in the AgBr/Ag3PO4/ZnFe2O4catalyst is given in Fig.12.Under visible light illumination,the photoinduced electrons could be collected on the conduction band (CB)ofAg3PO4,which can react with O2molecules to generate O2-·radicals,leading to the degradation of RhB29.Meanwhile,the holes accumulated on the valence band(VB)of ZnFe2O4are insufficient to oxidize the adsorbed H2O to OH·radicals14,but could directly attack the adsorbed dye molecules.Based on the above arguments, one may deduce that O2-·and h+are the main reactive oxygen species for RhB degradation in AgBr/Ag3PO4/ZnFe2O4system(Fig.12).

Fig.10 Cycling test in the degradation of RhB byAgBr/Ag3PO4/ ZnFe2O4under visible light irradiation
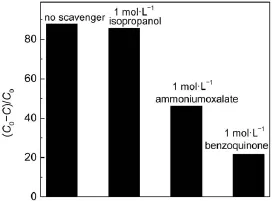
Fig.11 Photodegradation of RhB overAgBr/Ag3PO4/ZnFe2O4catalyst in the different solution
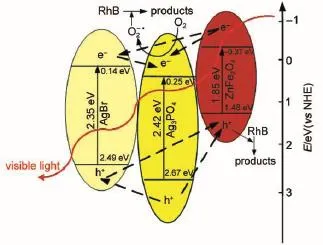
Fig.12 Schematic diagram of the possible electron-hole pairs separation inAgBr/Ag3PO4/ZnFe2O4catalyst
4 Conclusions
The magnetic AgBr/Ag3PO4/ZnFe2O4composite catalyst prepared via the hydrothermal and in situ precipitation route possessed a higher photocatalytic activity for RhB degradation than Ag3PO4/ZnFe2O4,AgBr/ZnFe2O4and P25 TiO2under visible light illumination.Such a composite photocatalyst showed an excellent photocatalytic performance in both acid and basic solutions.Its photodegradation rate for RhB increased with temperature.The light-produced superoxide radicals and holes in this composite catalyst were attributed to be the main active species for the RhB degradation.The AgBr/Ag3PO4/ZnFe2O4photocatalyst could be feasibly recycled by an external magnetic field.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
References
(1)Kubacka,A.;Fernández-García,M.;Colón,G.Chem.Rev. 2012,112,1555.doi:10.1021/cr100454n
(2)Alfano,O.M.;Bahnemann,D.;Cassano,A.E.;Dillert,R.; Goslich,R.Catal.Today 2000,58,199.doi:10.1016/S0920-5861(00)00252-2
(3)Chong,M.N.;Jin,B.;Chow,C.W.K.;Saint,C.Wat.Res. 2010,44,2997.doi:10.1016/j.watres.2010.02.039
(4)Li,G.P.;Wang,Y.X.;Mao,L.Q.RSC Adv.2014,4,53649. doi:10.1039/C4RA08044K
(5)Yi,Z.G.;Ye,J.H.;Kikugawa,N.;Kako,T.;Ouyang,S.X.; Stuart-Williams,H.;Yang,H.;Cao,J.Y.;Luo,W.J.;Li,Z.S.; Liu,Y.;Withers,R.L.Nat.Mater.2010,9,559.doi:10.1038/ nmat2780
(6)Martin,D.J.;Liu,G.G.;Moniz,S.J.A.;Bi,Y.P.;Beale,A.M.; Ye,J.H.;Tang,J.W.Chem.Soc.Rev.2015,44,7808. doi:10.1039/C5CS00380F
(7)Ge,M.;Zhu,N.;Zhao,Y.P.;Li,J.;Liu,L.Ind.Eng.Chem.Res. 2012,51,5167.doi:10.1021/ie202864n
(8)Ge,M.Chin.J.Catal.2014,35,1410.[葛明.催化学报, 2014,35,1410.]doi:10.1016/S1872-2067(14)60079-6
(9)Ge,M.;Tan,M.M.;Cui,G.H.Acta Phys.-Chim.Sin.2014,30, 2107.[葛明,谭勉勉,崔广华.物理化学学报,2014,30, 2107.]doi:10.3866/PKU.WHXB201409041
(10)Dong,C.;Wu,K.L.;Li,M.R.;Liu,L.;Wei,X.W.Catal. Commun.2014,46,32.doi:10.1016/j.catcom.2013.11.018
(11)Hong,X.T.;Wu,X.H.;Zhang,Q.Y.;Xiao,M.F.;Yang,G.L.; Qiu,M.R.;Han,G.C.Appl.Surf.Sci.2012,258,4801. doi:10.1016/j.apsusc.2012.01.102
(12)Fu,Y.S.;Wang,X.Ind.Eng.Chem.Res.2011,50,7210. doi:10.1021/ie200162a
(13)Hochepied,J.F.;Bonvilie,P.;Pileni,M.P.J.Phys.Chem.B 2000,104,905.doi:10.1021/jp991626i
(14)Chen,X.J.;Dai,Y.Z.;Huang,W.K.Mater.Lett.2015,145, 125.doi:10.1016/j.matlet.2015.01.097
(15)Ge,M.;Chen,Y.Y.;Liu,M.L.;Li,M.J.Envi.Chem.Eng. 2015,3,2809.doi:10.1016/j.jece.2015.10.011
(16)Li,J.Q.;Liu,Z.X.;Zhu,Z.F.J.Alloy.Compd.2015,636,229. doi:10.1016/j.jallcom.2015.02.176
(17)Cao,J.;Luo,B.D.;Lin,H.L.;Xu,B.Y.;Chen,S.F.J.Hazard. Mater.2012,217-218,107.doi:10.1016/j.jhazmat.2012.03.002
(18)Wang,B.;Gu,X.Q.;Zhao,Y.L.;Qiang,Y.H.Appl.Surf.Sci. 2013,283,396.doi:10.1016/j.apsusc.2013.06.121
(19)Su,M.H.;He,C.;Sharma,V.K.;Asi,M.A.;Xia,D.H.;Li,X. Z.;Deng,H.Q.;Xiong,Y.J.Hazard.Mater.2012,211-212, 95.doi:10.1016/j.jhazmat.2011.10.006
(20)Wu,Y.;Song,L.M.;Zhang,S.J.;Wu,X.Q.;Zhang,S.N.; Tian,H.F.;Ye,J.Y.Catal.Commun.2013,37,14.doi:10.1016/ j.catcom.2013.03.027
(21)Jiang,J.;Li,H.;Zhang,L.Z.Chem.-A Eur.J.2012,18,6360. doi:10.1002/chem.201102606
(22)Butler,M.A.J.Appl.Phys.1977,48,1914.doi:10.1063/ 1.323948
(23)Chen,X.J.;Dai,Y.Z.;Liu,T.H.;Guo,J.;Wang,X.Y.;Li,F.F. J.Mol.Catal.A:Chem.2015,409,198.doi:10.1016/j. molcata.2015.08.021
(24)Zhang,X.;Zhang,L.Z.;Xie,T.F.;Wang,D.J.J.Phys.Chem. C 2009,113,7371.doi:10.1021/jp900812d
(25)Jia,C.;Xie,X.W.;Ge,M.;Zhao,Y.Q.;Li,Z.L.;Zhang,H.; Cui,G.H.Mat.Sci.Semicon.Proc.2015,36,71.doi:10.1016/j. mssp.2015.02.086
(26)Sakkas,V.A.;Arabatzis,I.M.;Konstantinou,I.K.;Dimou,A. D.;Albanis,T.A.;Falaras,P.Appl.Catal.B:Environ.2004,49, 195.doi:10.1016/j.apcatb.2003.12.008
(27)Liu,Y.P.;Fang,L.;Lu,H.D.;Li,Y.W.;Hu,C.Z.;Yu,H.G. Appl.Catal.B:Environ.2012,115-116,245.doi:10.1016/j. apcatb.2011.12.038
(28)Katsumata,H.;Taniguchi,M.;Kaneco,S.;Suzuki,T.Catal. Commun.2013,34,30.doi:10.1016/j.catcom.2013.01.012
(29)Yang,X.F.;Cui,H.Y.;Li,Y.;Qin,J.L.;Zhang,R.X.;Tang,H. ACS Catal.2013,3,363.doi:10.1021/cs3008126
Synthesis and Photocatalytic Performance of a Magnetic AgBr/Ag3PO4/ZnFe2O4Composite Catalyst
MENG Ying-Shuang1AN Yi2GUO Qian1GE Ming1,3,*
(1College of Chemical Engineering,North China University of Science and Technology,Tangshan 063009,Hebei Province,P.R. China;2Yisheng Innovation Education Base,North China University of Science and Technology,Tangshan 063009, Hebei Province,P.R.China;3Hebei Key Laboratory of Photocatalytic and Electrocatalytic Materials for Environment, Tangshan 063009,Hebei Province,P.R.China)
Hydrothermal processing in conjunction with in situ precipitation were successfully applied to synthesize the magnetic composite catalyst silver bromide/silver phosphate/zinc ferrite(AgBr/Ag3PO4/ZnFe2O4). The phase structure,composition,morphology,and optical property of this material were subsequently assessed by X-ray diffraction,energy dispersive X-ray spectroscopy,field emission scanning electron microscopy, transmission electron microscopy,and UV-Vis diffuse reflectance spectroscopy.Under visible light illumination, the as-prepared AgBr/Ag3PO4/ZnFe2O4photocatalyst exhibited superior photocatalytic performance during rhodamine B(RhB)degradation compared withAg3PO4/ZnFe2O4,AgBr/ZnFe2O4,and P25 TiO2.This new catalyst also showed excellent photocatalytic activity in both acidic and basic solutions.The RhB photodegradation rate was slightly increased at higher temperatures,and the activation energy for this reaction was determined to be 31.9 kJ·mol-1according to theArrhenius equation.The high performance of theAgBr/Ag3PO4/ZnFe2O4catalyst can be attributed to efficient photo-induced charge separation,and the generation of superoxide radicals and holes that are responsible for RhB degradation.
AgBr/Ag3PO4/ZnFe2O4;Magnetism;Photocatalysis;Visible light;Mechanism
February 23,2016;Revised:May 6,2016;Published on Web:May 8,2016.
O644;O649
10.3866/PKU.WHXB201605081
*Corresponding author.Email:geminggena@163.com.Tel:+86-31-52592574.
The project was supported by the Natural Science Foundation of Hebei Province,China(B2014209182),Youth Foundation of Hebei Education
Department,China(QN2014045)and College Students′Innovative Entrepreneurial Training Plan Program of North China University of Science and Technology,China(X2015117).
河北省自然科学基金(B2014209182),河北省教育厅青年基金(QN2014045)和华北理工大学大学生创新创业训练计划项目(X2015117)资助
©Editorial office ofActa Physico-Chimica Sinica
[Article]

