FT-IR、XPS和DFT研究水杨酸钠在针铁矿或赤铁矿上的吸附机理
FT-IR、XPS和DFT研究水杨酸钠在针铁矿或赤铁矿上的吸附机理
胡慧萍1王梦1,*丁治英1,*姬广富2
(1中南大学化学化工学院,长沙410083;2中国工程物理研究院流体物理研究所,四川绵阳621900)
采用傅里叶变换红外(FT-IR)光谱、X射线光电子能谱(XPS)以及基于周期平面波的密度泛函理论(DFT)分别研究了水杨酸钠在针铁矿或赤铁矿表面上的吸附结构,并将计算得到的光电子能谱移动(CLS)和电荷转移与实验得到的XPS结果进行对比。FT-IR结果表明,水杨酸钠可能以双齿双核(V)和双齿单核(IV)的形式分别吸附于针铁矿或赤铁矿表面。由DFT计算结果可知,水杨酸钠在针铁矿(101)晶面上形成双齿双核化合物(V)的吸附能为-5.46 eV。而水杨酸钠在针铁矿(101)晶面上形成双齿单核化合物(IV)的吸附能为3.80 eV,因此水杨酸钠在针铁矿上基本不以双齿单核化合物(IV)构型存在。水杨酸钠在赤铁矿(001)晶面上形成双齿单核化合物(IV)时吸附能为-4.07 eV,说明水杨酸钠在赤铁矿(001)晶面上形成了双齿单核化合物(IV)。另外,理论计算的针铁矿(101)晶面上吸附位点铁原子的Fe 2p的CLS值(-0.68 eV)与实验观察到的Fe 2p的CLS值(-0.5 eV)吻合。理论计算的赤铁矿(001)晶面上吸附位点铁原子的Fe 2p的CLS值(-0.80 eV)与实验观察到的Fe 2p的CLS值(-0.8 eV)吻合。因此,水杨酸钠吸附在针铁矿表面时能够通过羧酸基团上一个氧原子和酚羟基上的氧原子与针铁矿(101)表面上的两个铁原子形成双齿双核(V)结构,而在赤铁矿(001)表面上,水杨酸钠中羧酸基团上一个氧原子和酚羟基上的氧原子与赤铁矿(001)表面上的一个铁原子形成了双齿单核(IV)结构。
针铁矿;赤铁矿;水杨酸钠吸附;FT-IR;XPS;DFT计算
www.whxb.pku.edu.cn
1 Introduction
The bauxite ores ordinarily contain from 0.1%to 0.4%(w,mass fraction)organic compounds and occasionally as high as 0.6% (w)1.These organic compounds are comprised of a complex mixture of humates,lignin,and cellulose2.On digestion of this bauxite in the Bayer process,between 50%and 90%of the organic compounds in the bauxite ore may be extracted into the Bayer liquor as dissolved organic compounds which build up to an equilibrium level.The dissolved organic compounds presented in Bayer liquors are mainly aliphatic and aromatic compounds with carboxylic groups and hydroxyl groups(such as sodium formate, sodium acetate,sodium oxalate,sodium salicylate,disodium phthalate and so on).Goethite and hematite,the most common iron-containing minerals in red mud,play an important role in the entrainment,adsorption and precipitation of the dissolved organic species in the Bayer liquor due to their high surface areas and high densities of reactive surface sites.According to previous studies3,4, we have found that the dissolved organic compounds had negative effects on the settling performance of goethite or hematite slurries in the absence of flocculants,and sodium salicylate had a more remarkable negative effect than the sodium formate did.We have also investigated the adsorption mechanism of sodium formate onto the goethite or hematite surface by Fourier transform infrared (FT-IR)spectroscopy,X-ray photoemission spectroscopy(XPS), and periodic plane-wave density functional theory(DFT)calculation methods5,which showed that chemisorptions with different interfacial structures occurred between sodium formate and the goethite or hematite surface.Thus,an effort of molecular-level understanding of interfacial structure-property relationships between sodium salicylate and the goethite or hematite surface was made in this paper.
The interfacial structure of sodium salicylate on the goethite or hematite surface has not been well understood.From a critical perusal of the public literature,the complex that occurred in natural aquatic systems6,7and fluid/rock systems8over a pH range from 2 to 10 between sodium salicylate and the goethite or hematite surface has been widely investigated by batch adsorption experiments and FT-IR measurement9-12,but no consistent conclusion was drawn about the interfacial structures.For example, via FT-IR measurement,the adsorption of sodium salicylate on the goethite or hematite surface was described as a bidentate mononuclear structure involving the phenolic oxygen atom(Ph-O-),one oxygen atom of carboxylic group(COO-),and one surface iron atom of goethite or hematite11,12.In addition,Yost et al.11proposed that the interfacial structure on the goethite surface could be either an electrostatic outer-sphere complex or a very weakly bound bridging bidentate complex around pH 5.5.Meanwhile,Biber and Stumm12proposed an alternate surface complex structure for salicylate adsorption to goethite involving binding one oxygen atom of carboxylate groups(COO-),and hydrogen bonding between surface oxygen atoms of goethite and phenolic functional groups at pH 7.However,the Bayer liquor is extremely alkaline with NaOH concentration range from 2 to 3 mol·L-1,and the interfacial structure of sodium salicylate on the goethite or hematite surface under this condition has not been investigated.
XPS is one of the most extensively used surface analytical techniques due to its high sensitivity of 0.1%(atomic ratio)13and the ability to probe the electronic and geometric structures of solid surface with adsorbed molecules14,15.In this technique,the binding energies of emitted core electrons are determined through their escape kinetic energies.When referred to a given reference,that binding energy shift can be expressed as core level shift(CLS) which reflects the local atomic coordination and the relative oxidation state16.
DFT calculations are suitable for a variety of surface science applications17,and the increased application of DFT modeling to chemical questions about environmental interface reactivity is emerging in literatures:DFT employing a Gaussian-type basis set on molecular clusters containing two iron ions has been used to study the competitive adsorption of salicylate and catechol on goethite surface18.While it captures some aspects of the adsorption process,the small cluster size does not allow realistic modeling of the goethite surface structure.The periodic DFT calculations are valuable for estimating surface complex structures and adsorption energies because they explicitly include details of the mineral surface.Otte et al.19employed DFT calculation using a projected augmented wave to investigate the adsorption of arsenate on the goethite(101)surface.Spin-polarized DFT calculations17were carried out to model analogs of arsenic surface complexes on the hematite(001)surface which is a prevalent growth face.Furthermore,the adsorptions and reactions of SO2on clean and oxygen-precovered Pd(100)were investigated with XPSand DFT calculations,and the adsorbed SO2species were identified by comparing the calculated CLS20with the experimental photoemission.This(incomplete,but demonstrative)summary represents the emerging role of,and growing interest in,DFT modeling applied to chemical questions about environmental interface reactivity.
In this paper,goethite(101)and hematite(001)surfaces which represent the high reactivity properties of goethite and hematite are taken into account.A combination of FT-IR,XPS,and DFT calculations was performed to study the surface structures of sodium salicylate bound to goethite or hematite in the highly caustic liquor.Modeling of the structures,energetics,electronic structures and CLS of sodium salicylate on goethite(101)and hematite(001)surfaces were performed in a self-consistent manner.Possible interfacial structures of sodium salicylate on goethite(101)or hematite(001)surface are obtained.And these CLS of Fe 2p and charge transfer of the adsorption iron sites calculated by DFT with periodic interfacial structures are confronted to the X-ray photoemission experiments.
2 Methods
2.1Experimental details
2.1.1Materials and characterization
Goethite and hematite were prepared according to the procedure of Schwertmann and Cornell21,then dried at 60°C for 24 h.The resulting particles of goethite or hematite were identified by X-ray powder diffraction(XRD)pattern on X-ray diffractometer(D/max 2500,Rigaku Corporation,Cu Kαradiation,Japan).The XRD patterns are presented in Fig.1.The surface area and particle size distribution were measured by adsorption/desorption N2(g)isotherm(Monosorb Autosorb,Quantachrome Instruments Ltd., USA)and laser diffraction(Mastersizer2000,Malvern Instruments Ltd.,UK),respectively.The results are given in Table 1.Sodium hydroxide and sodium salicylate were of analytical grade.
2.1.2Spectral measurements

Fig.1 XRD patterns of samples
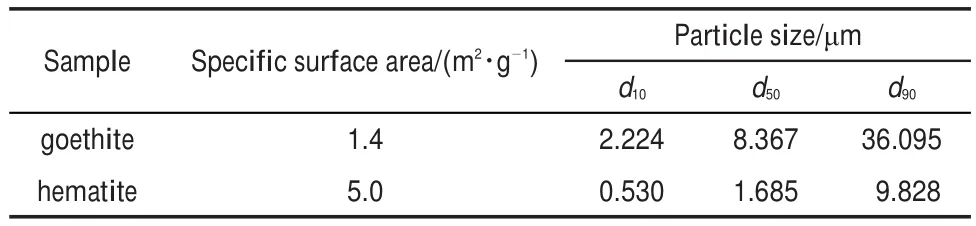
Table 1 Properties of goethite and hematite samples
The technique used for adsorbing sodium salicylate on the goethite or hematite surface was modified from that developed by Jones et al.22.0.25 g goethite or hematite was placed in an airtight conical flask with 50 mL of 0.001 mol·L-1sodium hydroxide solution and sonicated at 60°C for 10 h to obtain a suspension, and the suspension was centrifuged for 30 min at 4000 r·min-1to separate the supernatant and the solid.The supernatant was decanted to obtain a fresh,carbonate-free goethite or hematite solid placed in an airtight conical flask.50 mL sodium salicylate solution in pH 13 with certain concentration was added to the conical flask with 0.25 g of carbonate-free goethite or hematite solid,and the molar ratio for carboxylate groups of sodium salicylate to ferric ion of goethite or hematite was 10:1.After the mixture was sonicated at 60°C for 10 h and equilibrated for 24 h, the mixture was centrifuged at 4000 r·min-1to obtain a solid,and the solid was washed with de-ionized water for one time and dried in a vacuum oven at 60°C for 24 h.The samples of goethite or hematite after the treatment of sodium salicylate(SSa-treated goethite and hematite)were obtained.Goethite or hematite before the treatment of sodium salicylate(untreated goethite or hematite) was prepared in the same manner except that sodium salicylate was not added to the carbonate-free goethite or hematite suspension.
The infrared spectra of the samples were measured by a Nicolet-6700 FT-IR spectrometer(Thermo Scientific Co.,USA).The samples were analyzed on X-ray photoelectron spectrometer (ESCALAB 250XI,Thermo Scientific Co.,USA)utilizing a monochromatic Al KαX-ray at 1486.6 eV.All measurements were carried out at the pressure below 10-8Pa and with a flood gun for charge neutralization.All spectra were charge-referenced so that the unfunctionalized aliphatic C 1s component occurred at 284.8 eV.Curve fittings of Fe 2p spectra were performed using the Avantage software.The reduced chi-squared(χ2)value incorporated in the Avantage was used as a potentially useful guide to assess the fitting quality.
2.2Computational details

Fig.2 Optimized structure of sodium salicylate
The structure of sodium salicylate is shown in Fig.2.Periodic slab model of the goethite surface was created by cleaving thesurface(101)23from the experimental crystal structure of bulk goethite(space group Pnma24,25),which is modeled in a supercell geometry with a vacuum space of 1 nm and extended to(1×3)15. The thickness of the goethite(101)slab amounted to eight Fe layers.The periodic slab model of hematite surface was created by cleaving the surface(001)from the structure of bulk hematite (space group R3c26).The surface(001)of hematite was extended to(1×2)and modeled using a periodic slab consisting of six O layers and twelve Fe layers on which the terminal Featoms contain triply-coordinated oxygen atoms in the subsurface layer,with an excess of 1.5 nm of vacuum separating the periodic images in the direction along the surface normal17.Initial magnetic moments of Fe atoms were assigned so as to obtain an antiferromagnetic goethite and hematite slab in each case.The goethite(101)or hematite(001)surfaces was passivated by water dissociation products to surface dangling Fe and O atoms(as shown in Fig.3).

Fig.3 Truncated side views of the models of the surface slab
According to the previous literature12,carboxylate groups may be adsorbed on inorganic(oxide)surfaces as a monodentate mononuclear(I)structure,a bidentate mononuclear(II)structure or a bidentate binuclear(III)structure,and salicylate groups may be adsorbed on inorganic(oxide)surfaces as a bidentate mononuclear(IV)structure or a bidentate binuclear(V)structure(as shown in Fig.4)
In this study,the free sodium salicylate was added to the goethite(101)surface in a bidentate binuclear(V)structure or a bidentate mononuclear(IV)structure(as shown in Fig.5(a,b)).And the free sodium salicylate was added to the hematite(001)surface in a bidentate mononuclear(IV)structure(as shown in Fig.5(c)).
DFT calculations were carried out with the CASTEP code27,28in Materials Studio 6.0 using density functional theory.Plane wave basis sets were used to solve the Kohn-Sham equations.The generalized gradient approximation(GGA)electron exchange and correlation effects were described using the Perdew Burke Ernzerhof(PBE)29.The on-site Coulomb interaction of 3d electrons,the GGA+U method was applied to the Fe atoms to improve the description of the electronic properties,the suggested values of the Hubbard U were correspondingly 5 eV for goethite19and 2.5 eV for hematite30.For the electronic integration in reciprocal space,the Brillouin zone was sampled according to theMonkhorst-Pack scheme31.The k point was set to gamma for free sodium salacylate and fine for surface structures with or without sodium salicylate.

Fig.4 Mode of complex structures of metal-salicylate compounds
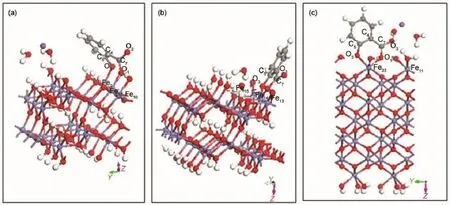
Fig.5 Illustration of the optimized interfacial structures on goethite and hematite
For free sodium salicylate,the ionic cores were described by the norm conserving pseudopotentials32.The wave functions were expanded with an energy cutoff of 750 eV for geometry optimization and vibrational analysis.All positions were relaxed and fully optimized up to a force convergence of 0.3 eV·nm-1.The absence of imaginary frequencies verified that all structures were true minima33.The final structure of the geometry optimization was subjected to a single-point energy calculation with the ultrasoft pseudopotentials34and an energy cutoff of 340 eV for the plane-wave basis set to achieve an accuracy of total energy differences of 2×10-5eV·atom-1.
As to the models of surface slabs,SSa-goethite systems and SSa-hematite system,the optimized parameter was set as the same as that used in the single-point energy calculation for free sodium salicylate.The structures of the adsorbate and the four outer Fe layers of goethite(101)surface or the adsorbate and the two outer Fe layers of hematite(001)surface were relaxed and fully optimized up to a force convergence of 0.5 eV·nm-1,while the inner layers kept fixed at their bulk positions to reproduce the properties of bulk goethite or hematite.
The adsorption energy,Eads,of the adsorbate at goethite or hematite surfaces in(eV)was described as Eq.(1):

where Esurfaceand Eadsorbate-surface(in eV)were the total energies of goethite or hematite slabs before and after the adsorption of the adsorbate.Eadsorbate(eV)was the total energy of a free adsorbate molecule.
For the calculation of CLS of Fe 2p,geometry-optimized SSagoethite systems and SSa-hematite system were used as models of the interfacial structures.We modeled CLS of Fe 2p as total energy differences between the system with a core hole on the excited Fe atom and the unperturbed system.The on-the-fly pseudopotential provided in the Materials Studio software was adopted to describe the excited Fe atom which included a corehole in the 2p level.In a pseudopotential formulation,absolute binding energies were not accessible.However,CLS could be accurately achieved with respect to a given reference.In this work, the reference was taken to be the surface iron atoms located at the same position on goethite(101)or hematite(001)surface.Within these calculations,we arrived at Eq.(2)for the CLS of a single atom in the interfacial structures:

where ECLS(eV)was the core level shift of a certain system.Eionand Egsrepresented the ground state energies of the system with and without a core hole,respectively.
The CLS of Fe 2p and charge transfer of the adsorbed surface iron site calculated by DFT with periodic interfacial structures were confronted to the X-ray photoemission experiments,and accurate interfacial structures were verified on the basis of the consistence between the calculated and experimental results.
3 Results and discussion
3.1FT-IR spectroscopy analysis
The FT-IR spectra of free SSa,untreated goethite,and SSatreated goethite are depicted in Fig.6.The FT-IR spectra of free SSa,untreated hematite,and SSa-treated hematite are depicted in Fig.7.

Fig.6 FT-IR spectra of samples

Fig.7 FT-IR spectra of samples
Several studies11,22have demonstrated that the expected frequency shifts occurred when carboxylic acids or their salts were adsorbed as carboxylates on inorganic(oxide)surfaces.When the carboxyl group of carboxylic acids or their salts is directly involved in the adsorption,it is possible to identify the structures on the basis of the carboxylate asymmetric(νasym)and symmetric(νsym) stretches,and their separation(Δν=νasym-νsym).Δν(adsorbed)and Δν(salt)are correspondingly the separation of the symmetric and asymmetric stretches of the carboxylate group of the adsorbed carboxylate salt and the unadsorbed carboxylate salt.They can beused to identify the adsorbed structures(as shown in Fig.4):when the value of Δν(adsorbed)is smaller than that of Δν(salt),a bidentate mononuclear(II)structure is observed(two oxygen atoms of the carboxylate group form two bonds with one metal atom on the solid surface).However,when the value of Δν(adsorbed)is greater than that of Δν(salt),a monodentate mononuclear(I) structure is seen(only one oxygen atom of the carboxylate group binds with one metal atom on the solid surface).In bidentate binuclear(III)structure(two oxygen atoms of the carboxylate group bind with two metal atoms on the solid surface),Δν(adsorbed) almost equals to Δν(salt).
In addition,sodium salicylate may be adsorbed on inorganic (oxide)surfaces with a bidentate mononuclear(IV)structure or a bidentate binuclear(V)structure(as shown in Fig.4).In the bidentate mononuclear(IV)structure,both the oxygen atom of phenolic group and one oxygen atom of the carboxylate group bind with one surface iron atom.Whereas,when the oxygen atom of phenolic group and one oxygen atom of the carboxylate group bind with two adjacent surface atoms of inorganic(oxide),a bidentate binuclear(V)structure may be formed.
In the FT-IR spectrum of free sodium salicylate(Fig.6(a)),the asymmetric and symmetric stretches of the carboxylate group can be identified as the bands at 1587 and 1378 cm-1.The bands at 1623,1486,and 1469 cm-1are correspondingly assigned to the 8a, 16b,and 16a C―C ring stretching modes of benzene based on the work of Varsányi35.The band at 1295 cm-1is attributed to the bending mode of the phenolic(Ph―O―H)group.The phenolic Ph―O stretching vibration is represented by the band at 1250 cm-1in sodium salicylate.The band frequencies between 1200 and 1000 cm-1are the C―H inner plane bending vibration.
As shown in Fig.6(b),the bands at 1639 and 1381 cm-1are assigned to OH bending vibration bands of adsorbed water and constitutional water of the untreated goethite.As shown in Fig.7 (b),the bands at 1627 and 1384 cm-1are assigned to the vibrations of adsorbed water and constitutional water of the untreated hematite.
In the FT-IR spectra of SSa-treated goethite(Fig.6(c)),the asymmetric(νasym)and symmetric(νsym)stretching vibrations of carboxylate group of adsorbed sodium salicylate are correspondingly at 1572 and 1381 cm-1.In the FT-IR spectra of SSatreated hematite(Fig.7(c)),the asymmetric and symmetric stretches frequencies of the carboxylate group of adsorbed sodium salicylate are at 1571 and 1385 cm-1,respectively.The value of Δν(salt)for unadsorbed sodium salicylate is 208 cm-1,Δν(adsorbed)values for sodium salicylate adsorbed on the goethite or hematite surface are correspondingly 191 and 187 cm-1.
However,according to Yost et al.′s work11,a distinction among the types of Fe-carboxylate complexes cannot be identified readily from their FT-IR spectra based solely on the simple rule of comparing Δν(adsorbed)value with Δν(salt)value when salicylate coordinates with Fe(III).As shown in Fig.7(c),the absence of the bending frequency of the Ph―O―H in adsorbed sodium salicylate on the goethite or hematite surface indicates the deprotonation and coordination of phenolic group with the surface iron atom of goethite or hematite.Furthermore,the bending vibration of phenolic group shifts to 1000-1100 cm-1.Therefore,we assumed that sodium salicylate may be adsorbed on the goethite and hematite surfaces as a bidentate mononuclear(IV)or a bidentate binuclear(V)structure.
3.2X-ray photoelectron spectroscopy analysis
For the analysis of Fe 2p XPS spectra,a Shirley background is used for the Fe 2p1/2and Fe 2p3/2envelopes.The Fe 2p1/2and Fe 2p3/2envelopes are fitted using peaks corresponding to the Gupta and Sen(GS)multiplets,surface structures,and shake-up satellites36.The peak ascribed to surface structures is added with a higher binding energy and a larger full width at half-maximum (FWHM)than the GS multiplets.Asingle large peak representing the satellites due to shake-up is also added.Asingle low intensity peak on the low-binding-energy side of the envelope is added to account for the information of Fe ions with a lower oxidation state than normal oxidation state by the production of defects in neighboring sites,and this peak is referred to as the‘pre-peak'. The fitting of each compound follows the GS predictions well. The Fe 2p spectra and fitting curves for goethite or hematite before and after treatment of sodium salicylate are depicted in Fig.8. Table 2 lists the fitting peaks of the Fe 2p envelopes for goethite or hematite before and after the treatment of sodium salicylate.
As presented in Fig.8 and Table 2,it is observed that all fitting peaks of Fe 2p for goethite or hematite after the treatment of sodium salicylate were correspondingly downshifted relative to untreated goethite or hematite.The peak 2 and surface peaks in the Fe 2p3/2envelope for SSa-treated goethite are correspondingly downshifted to 711.3 and 714.5 eV relative to those(711.8 and 715.0 eV)in the Fe 2p3/2envelope for untreated goethite.That is to say,the peaks in the Fe 2p spectrum for SSa-treated goethite are correspondingly downshifted by 0.5 eV compared with untreated goethite.The peak 2 and surface peaks in the Fe 2p3/2envelope for SSa-treated hematite are correspondingly downshifted to 711.1 and 714.2 eV relative to those(711.9 and 715.0 eV)in the Fe 2p3/2envelope for untreated hematite.That is to say,the peaks in the Fe 2p spectrum for SSa-treated hematite are both downshifted by 0.8 eV compared with untreated hematite.
In this work,the downshift of binding energies of Fe 2p spectra for SSa-treated goethite or hematite will be explained by the atomic potential model37.The atomic potential model assumes that the atomic core potential varies linearly with the electron density of atoms,and the oxidation of one atom results in the increase of the atomic binding energy of the inner electron,whereas the reduction of one atom results in the decrease of the atomic binding energy of the inner electron.After sodium salicylate was adsorbed on the goethite or hematite surface,the binding energies of Fe 2p spectra were correspondingly decreased with respect to untreated goethite or hematite.According to the atomic potential model,the ion atoms may be partially reduced to a lower valence state.The coordination between oxygen atoms of carboxylate or phenolic group from sodium salicylate and surface iron atoms of goethiteor hematite may explain the partial reduction of iron atoms on the surfaces,because these iron atoms accepted electron clouds from oxygen atoms of carboxylate or phenolic group of sodium salicylate.However,the core level shift of the peaks of Fe 2p spectra for SSa-treated hematite was larger than that for SSa-treated goethite,which may be due to the differences of the interfacial structures between SSa-treated goethite and SSa-treated hematite.

Fig.8 Fe 2p XPS spectra and the fitting curves of samples

Table 2 Fitting peaks of Fe 2p spectra for goethite or hematite before and after the treatment of SSa
In the bidentate mononuclear(IV)structure,the oxygen atom of phenolic group and one oxygen atom of the carboxylate group attach and donate electrons to one surface iron atom.Whereas,in a bidentate binuclear(V)structure,the oxygen atom of phenolic group and one oxygen atom of the carboxylate group correspondingly attach and donate electrons to two adjacent surface iron atoms.Thus,the electronic charge densities of the adsorbed iron atoms with a bidentate mononuclear(IV)structure could be higher than those with a bidentate binuclear(V)structure.According to the above mentioned relationship between the change of the binding energies and electronic charge densities of adsorbed atoms on solid surfaces,we can assume that the binding energy of Fe 2p spectrum for adsorbed iron atoms with a bidentate mononuclear(IV)structure could be lower than that with a bidentate binuclear(V)structure.Thus,the decrease of binding energy of adsorbed iron atoms relative to unadsorbed iron atoms would be larger in a bidentate mononuclear(IV)structure than that in a bidentate binuclear(V)structure.
As listed in Table 2,the binding energies of Fe 2p3/2and Fe 2p1/2for SSa-treated goethite were decreased by 0.5 eV relative to the untreated goethite,and the binding energy of Fe 2p3/2and Fe 2p1/2for SSa-treated hematite was decreased by 0.8 eV relative to the untreated hematite,which indicates that the adsorbed soidium salicylate may be correspondingly adsorbed on the goethite and hematite surface as a bidentate binuclear(V)structure and a bidentate mononuclear(IV)structure.
3.3Quantum chemical calculations
In this section,we present the optimized interfacial structure, energetics,electronic structures and the CLS of Fe 2p of the in-terfacial structures of sodium salicylate adsorbed on goethite or hematite surfaces after a quantum chemical calculation of sodium salicylate adsorbed on the periodic surface slab of goethite or hematite was carried out.

Table 3 Interatomic distances and the lattice parameters for optimized bulk goethite or hematite in this work and in references
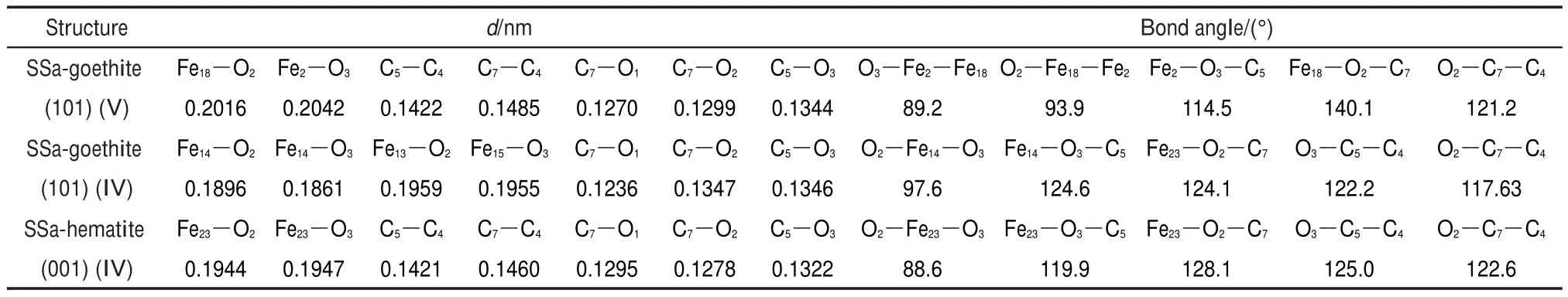
Table 4 Calculated interatomic distances(d)and bond angles for the interfacial structures of SSa adsorbed on goethite(101)or hematite(001)surface
3.3.1Interfacial structures
The OH―OHdistance(0.2925 nm)at iron site of goethite(101) surface matches well the O―OHdistance(0.2702 nm)of the free sodium salicylate,which allows sodium salicylate to form a bidentate binuclear(V)structure(Fig.6(a))on the goethite(101) surface.Moreover,a bidentate mononuclear(IV)structure(Fig.6 (b))on the goethite(101)surface was also modeled in order to further identify the possible structure.While the OH―OHdistances (0.2925 nm)at the iron site of goethite(101)surface are slightly greater than the distance(0.2251 nm)between two oxygen atoms of carboxylate of the free sodium salicylate.Thus,the structure that the two oxygen atoms of the―COO-group are adsorbed with a bidentate mononuclear(II)structure or bidentate binuclear(III) on the goethite(101)surface may not be favorable.
On the hematite(001)surface,the Fe―Fe distance is 0.5035 nm,and the OH―OHdistance at one surface dangling iron atom of the hematite(001)surface passivated by the dissociative water functional groups is 0.2622 nm,which also matches well with the O―OHdistance(0.2702 nm)of the free sodium salicylate,and thus allow sodium salicylate adsorbed as a bidentate mononuclear (IV)structure(Fig.6(c)).However,because the Fe―Fe and the OH―OHdistance at two dangling iron atoms on the hematite(001) surface is 0.5035 nm,which is significantly larger than the O―OHdistance(0.2702 nm)of the free sodium salicylate,the oxygen atom of phenolic group and one oxygen atom of the carboxylate group of sodium salicylate cannot attach to two adjacent surface iron atoms of hematite(001)with a reasonable interatomic distance in the range of chemical bond,which excludes the formation of a bidentate binuclear(V)structure for the adsorbed sodium salicylate on the hematite(001)surface.Moreover,the OH―OHdistances(0.2622 nm)at the iron site of the hematite(001)surface are slightly greater than the distance(0.2251 nm)between two oxygen atoms of carboxylate of the free sodium salicylate.And the structure that the two oxygen atoms of the carboxylate group are adsorbed with a bidentate mononuclear(II)structure or bidentate binuclear(III)on the hematite(001)surface may not be favorable.
Therefore,the bidentate binuclear(V)and bidentate mononuclear(IV)structures on the goethite surface and a bidentate mononuclear(IV)structure on the hematite surface as the starting interfacial structure was verified in detail.Later we will report the significant changes upon structural relaxation.
The interatomic distances and the lattice parameters for bulk goethite or hematite are listed in Table 3.The Fe―O distances in optimized bulk goethite are in the range from 0.1864 to 0.2008 nm,which owns a difference within 0.0128 nm relative to the previously calculated data in the range from 0.1901 to 0.2136 nm for bulk goethite38.The Fe―O distances in optimized bulk hematite are 0.2000 and 0.2024 nm,which are in agreement with the observed data of 0.19511 and 0.21028 nm from the single crystal of hematite39.The results indicate that the calculation method used in this work is reasonable.
The interatomic distances and bond angles for the interfacial structures of sodium salicylate on goethite(101)and hematite (001)surfaces are listed in Table 4.As listed in Table 4,the interatomic distances of Fe18―O2and Fe2―O3are correspondingly 0.2016 and 0.2042 nm in the bidentate binuclear(V)structure of SSa adsorbed on goethite(101)surface,while the interatomic distances of Fe14―O2and Fe14―O3are correspondingly 0.1896 and 0.1861 nm in bidentate mononuclear(IV)structure of SSa ad-sorbed on goethite(101)surface.And the interatomic distances of Fe23―O2and Fe23―O3are correspondingly 0.1944 and 0.1947 nm in the interfacial structures of SSa adsorbed on hematite(001) surface.

Table 5 Calculated adsorption energies(ΔEads)of the sodium salicylate adsorbed on goethite(101)and hematite(001)surfaces

Table 6 Calculated Mulliken charges,charge transfer,and photoemmission CLS of atoms for different structures
3.3.2Adsorption energies of the optimized interfacial
structures
In order to further verify the preferable interfacial structures, we also discuss the adsorption energy of the sodium salicylate on the goethite or hematie surface(Table 5).As listed in Table 5,the bidentate binuclear(V)structure of the sodium salicylate on the goethite(101)surface is favorable with adsorption energy of-5.46 eV,while no adsorption of sodium salicylate on the goethite(101)surface as a bidentate mononuclear(IV)structure occurred with a positive adsorption energy of 3.80 eV.And on the hematite(001)surface,the bidentate mononuclear(IV)structure has the adsorption energy of-4.07 eV,which is a further evidence for the formation of the bidentate mononuclear(IV)structure of the adsorbed sodium salicylate on the hematite(001)surface.
3.3.3Electronic properties and core level shift
The electronic structures and core level shifts of the possible interfacial structures of sodium salicylate adsorbed on goethite (101)and hematite(001)surfaces were calculated,respectively. The calculated Mulliken charges,charge transfer,and CLS values of atoms are listed in Table 6.
First,the calculated Mulliken charge values of free sodium salicylate and the surface complexes are listed in Table 6.Table 6 indicates that the negative charges of free sodium salicylate were mainly clustered on O1and O2atoms of carboxylate group and O3of the ortho phenol group.Hence both oxygen atoms in free sodium salicylate were electron-donating centers and the chemically reactive centers.
After the adsorption of sodium salicylate occurred on the goethite or hematite surface,our calculation results indicate that the charges of oxygen atoms of both the carboxylate group and the ortho phenol group of adsorbed sodium salicylate increased,especially for O1of carboxylate group and O3of the ortho phenol group,and the charges of the surface Fe atom on the adsorbed site decreased.This means that negative charges are transferred from oxygen atoms of sodium salicylate to the surface Fe atom,which results in a lower binding energy of the Fe 2p peaks of the adsorbed surface iron sites than that of unadsorbed surface iron atoms.
On the other hand,we compare the calculated charge transfers and CLS with the experimentally observed core-level shifts of Fe 2p from XPS measurements.We consider that the consistence between the calculated CLS and the experimentally observed CLS is a good indicator of the pertinence of our model.According to the calculated results(listed in Table 6),the calculated CLS of Fe 2p(-0.68 eV)for the adsorbed iron site on goethite(101)surface is consistent with the experimentally observed CLS of Fe 2p(-0.5 eV)for SSa-treated goethite(listed in Table 2).Thus,the goethite (101)surfaces can be predicted to be capable of adsorbing sodium salicylate as a bidentate binuclear(V)structure.On the other hand, our calculated CLS of Fe 2p(-0.80 eV)for the adsorbed iron site on hematite(001)surface is in good agreement with the experimentally observed CLS of Fe 2p(-0.8 eV)for SSa-treated hematite(listed in Table 2).This consistency suggests that our optimized interfacial structure with a bidentate mononuclear(IV) complex for sodium formate-hematite(001)system can be regarded as a reasonable and realistic structure.
4 Conclusions
The adsorption of sodium salicylate on goethite or hematite surface was investigated by FT-IR,XPS,and DFT calculations, respectively.The goemetry optimization by DFT indicates that the sodium salicylate adsorbed on goethite(101)forms a bidentate binuclear(V)structure rather than a bidentate mononuclear(IV) structure.Whereas,the formation of a bidentate mononuclear(IV) structure occurs among one oxygen atom of carboxylate group, one oxygen atom of phenolic group and one iron atom on hematite (001)surface.
The calculated CLS of Fe 2p for the interfacial structure on goethite(101)surface is coincide with the experimental observed CLS of Fe 2p,and the calculated CLS of Fe 2p for the interfacial structure on the hematite(001)surface is in good agreement with the experimentally observed CLS of Fe 2p.Thus,the goethite (101)surface and hematite(001)surface may be predicted to be capable of adsorbing sodium salicylate as bidentate binuclear(V) and bidentate mononuclear(IV)structures,respectively.
References
(1)Swinkels,D.A.;Chouzadjian,K.;Removal of Organics from Bayer Process Streams.US Patent 4836990,1989-6-6.
(2)Power,G.;Loh,J.Hydrometallurgy 2010,105(1-2),1. doi:10.1016/j.hydromet.2010.07.006
(3)Wang,M.;Hu,H.P.;Liu,J.W.;Chen,Q.Y.Journal of Central South University 2016,23,1.[王梦,胡慧萍,刘锦伟,陈启元.中南大学学报,2016,23,1.]
(4)Wang,M.;Hu,H.P.;Liu,J.W.;Chen,Q.Y.Transactions of Nonferrous Metals Society of China 2016,Accepted.[王梦,胡慧萍,刘锦伟,陈启元.有色金属学报,2016,已接受.]
(5)Wang,M.;Hu,H.P.;Chen,Q.Y.;Ji,G.F.FT-IR,XPS and Density Functional Theory Study ofAdsorption Mechanism of Sodium Formate onto Goethite or Hematite.InAlumina& Bauxite;Proceedings of the Minerals,Metals&Materials SocietyAnnual Meeting&Exhibition,Nashville,Tennessee, February 14-18,2016;McGlade,P.T.Ed.;Warrendale,PA 15086 USA,2016.
(6)Tipping,E.Chem.Geol.1981,33(1-4),81.doi:10.1016/0009-2541(81)90086-3
(7)Tipping,E.Geochim.Cosmochim.Ac.1981,45(2),191. doi:10.1016/0016-7037(81)90162-9
(8)Smith,R.E.Geoderma 1993,58(1-2),128.doi:10.1016/0016-7061(93)90091-X
(9)Evanko,C.R.;Dzombak,D.A.Environ.Sci.Technol.1998,32 (19),2846.doi:10.1021/es980256t
(10)Boily,J.F.;Persson,P.;Sjoberg,S.J.Colloid Interface Sci. 2000,227(1),132.doi:10.1006/jcis.2000.6886
(11)Yost,E.C.;Tejedor-Tejedor,M.I.;Anderson,M.A.Environ. Sci.Technol.1990,24(6),822.doi:10.1021/es00076a005
(12)Biber,M.V.;Stumm,W.Environ.Sci.Technol.1994,28(5), 763.doi:10.1021/es00054a004
(13)Zhao,C.Investigation of the Magnetic Properties of NonthiolatedAu Nano-structures Grown by LaserAblation.Ph.D. Dissertation,Virginia Polytechnic Institute and State University, Blacksburg,Virginia,2014.
(14)Zeng,Z.;Ma,X.;Ding,W.;Li,W.Science China Chemistry 2010,53(2),402.doi:10.1007/s11426-010-0086-z
(15)Kubicki,J.D.;Paul,K.W.;Kabalan,L.;Zhu,Q.;Mrozik,M. K.;Aryanpour,M.;Pierre-Louis,A.M.;Strongin,D.R. Langmuir 2012,28(41),14573.doi:10.1021/la303111a
(16)Miceli,G.;Pasquarello,A.Appl.Phys.Lett.2013,102(20), 201607/1.doi:10.1063/1.4807730
(17)Goffinet,C.J.;Mason,S.E.J.Environ.Monitor.2012,14(7), 1860.doi:10.1039/c2em30355h
(18)Yang,Y.;Duan,J.;Jing,C.J.Phys.Chem.C 2013,117(20), 10597.doi:10.1021/jp4027578
(19)Otte,K.;Schmahl,W.W.;Pentcheva,R.J.Phys.Chem.C 2013, 117(30),15571.doi:10.1021/jp400649m
(20)Luckas,N.;Gotterbarm,K.;Streber,R.;Lorenz,M.P.A.; Hoefert,O.;Vines,F.;Papp,C.;Goerling,A.;Steinrueck,H.P. Phys.Chem.Chem.Phys.2011,13(36),16227.doi:10.1039/ c1cp21694e
(21)Schwertmann,U.;Cornell,R.M.Iron Oxides in the Laboratory:Preparation and Characterization;Wiley-VCH: Weinheim,Germany,1991;p 137.
(22)Jones,F.;Farrow,J.B.;van Bronswijk,W.Langmuir 1998,14 (22),6512.doi:10.1021/la971126l
(23)Villalobos,M.;Cheney,M.A.;Alcaraz-Cienfuegos,J.J.Colloid Interface Sci.2009,336(2),412.doi:10.1016/j.jcis.2009.04.052
(24)Paul,K.W.;Kubicki,J.D.;Sparks,D.L.Eur.J.Soil Sci.2007, 58(4),978.doi:10.1111/j.1365-2389.2007.00936.x
(25)Manceau,A.;Nagy,K.L.;Spadini,L.;Ragnarsdottir,K.V. J.Colloid Interface Sci.2000,228(2),306.doi:10.1006/ jcis.2000.6922
(26)Rohrbach,A.;Hafner,J.;Kresse,G.Phys.Rev.B 2004,70(12), 125426.doi:10.1103/PhysRevB.70.125426
(27)Li,Y.;Gao,Y.;Xiao,B.;Min,T.;Fan,Z.;Ma,S.;Xu,L. J.Alloy.Compd.2010,502(1),28.doi:10.1016/j. jallcom.2010.04.184
(28)Refson,K.;Tulip,P.R.;Clark,S.J.Phys.Rev.B 2006,73(15), 155114.doi:10.1103/PhysRevB.73.155114
(29)Perdew,J.P.;Burke,K.;Ernzerhof,M.Phys.Rev.Lett.1996,77 (18),3865.doi:10.1103/PhysRevLett.77.3865
(30)Guo,H.;Barnard,A.S.Phys.Rev.B 2011,83(9),094112. doi:10.1103/PhysRevB.83.094112
(31)Monkhorst,H.J.;Pack,J.D.Phys.Rev.B 1976,13(12),5188. doi:10.1103/PhysRevB.13.5188
(32)Hamann,D.R.;Schlüter,M.;Chiang,C.Phys.Rev.Lett.1979, 43(20),1494.doi:10.1103/PhysRevLett.43.1494
(33)Tavakol,H.J.Mol.Struc.-Theochem 2009,916(1-3),172. doi:10.1016/j.theochem.2009.09.032
(34)Laasonen,K.;Car,R.;Lee,C.;Vanderbilt,D.Phys.Rev.B 1991,43(8),6796.doi:10.1103/PhysRevB.43.6796
(35)Varsányi,G.Normal Vibrations of Benzene and Its Derivatives, In Vibrational Spectra of Benzene Derivatives;Academic Press: New York,1969;pp 141-393.doi:10.1016/B978-0-12-714950-9.50007-7
(36)Grosvenor,A.P.;Kobe,B.A.;Biesinger,M.C.;McIntyre,N.S. Surf.Interface Anal.2004,36(12),1564.doi:10.1002/sia.1984
(37)Cole,R.J.;Gregory,D.A.C.;Weightman,P.Phys.Rev.B 1994, 49(8),5657.doi:10.1103/PhysRevB.49.5657
(38)Blanchard,M.;Balan,E.;Giura,P.;Béneut,K.;Yi,H.;Morin, G.;Pinilla,C.;Lazzeri,M.;Floris,A.Phys.Chem.Miner.2013, 41(4),289.doi:10.1007/s00269-013-0648-7
(39)Sadykov,V.A.;Isupova,L.A.;Tsybulya,S.V.;Cherepanova,S. V.;Litvak,G.S.;Burgina,E.B.;Kustova,G.N.;Kolomiichuk, V.N.;Ivanov,V.P.;Paukshtis,E.A.;Golovin,A.V.; Avvakumov,E.G.J.Solid State Chem.1996,123,191. doi:10.1006/jssc.1996.0168
FT-IR,XPS and DFT Study of the Adsorption Mechanism of Sodium Salicylate onto Goethite or Hematite
HU Hui-Ping1WANG Meng1,*DING Zhi-Ying1,*JI Guang-Fu2
(1College of Chemistry and Chemical Engineering,Central South University,Changsha 410083,P.R.China;2Institute of Fluid Physics,Chinese Academy of Engineering Physics,Mianyang 621900,Sichuan Province,P.R.China)
The adsorption of sodium salicylate on goethite or hematite surfaces was investigated by Fourier transform infrared(FT-IR)spectroscopy,X-ray photoemission spectroscopy(XPS),and periodic plane-wave density functional theory(DFT)calculations.The core level shift(CLS)and charge transfer of the adsorbed surface iron sites calculated by DFT with periodic interfacial structures were compared with the X-ray photoemission experiments.The FT-IR results reveal that the interfacial structure of sodium salicylate adsorbed on goethite or hematite surfaces can be classified as bidentate binuclear(V)or bidentate mononuclear(IV), respectively.The DFT calculated results indicate that the bidentate binuclear(V)structure of sodium salicylate is favorable on the goethite(101)surface,with an adsorption energy of-5.46 eV,while the adsorption of sodium salicylate on the goethite(101)surface as a bidentate mononuclear(IV)structure is not predicted,as it has a positive adsorption energy of 3.80 eV.Conversely,on the hematite(001)surface,the bidentate mononuclear (IV)structure of the adsorbed sodium salicylate has anadsorption energy of-4.07 eV,confirming its favorability. Moreover,the calculated CLS of Fe 2p(-0.68 eV)for the adsorbed iron site on the goethite(101)surface isconsistent with the experimentally observed CLS of Fe 2p(-0.5 eV)for SSa-treated goethite(goethite after the treatment of sodium salicylate).Our calculated CLS of Fe 2p(-0.80 eV)for the adsorbed iron site on the hematite(001)surface is likewise in good agreement with the experimentally observed CLS of Fe 2p(-0.8 eV) for SSa-treated hematite(hematite after the treatment of sodium salicylate).Thus,goethite is predicted to adsorb sodium salicylate as a bidentate binuclear(V)structure via the bonding of one carboxylate oxygen atom and the phenolic oxygen atom of sodium salicylate to two surface iron atoms of goethite(101).Meanwhile,on the hematite surface,the bidentate mononuclear(IV)complex formed via the bonding of one carboxylate oxygen atom and the phenolic oxygen atom of sodium salicylate to one surface iron atom of hematite(001)can be regarded as plausible.
Goethite;Hematite;Sodium salicylate adsorption;FT-IR;XPS;DFT calculation
January 28,2016;Revised:April 22,2016;Published on Web:April 22,2016.
O647
10.3866/PKU.WHXB201604225
*Corresponding authors.WANG Meng,Email:mengwchem@163.com.DING Zhi-Ying,Email:huierding@126.com;Tel:+86-731-88879616. The project was supported by the National Natural Science Foundation of China(51134007,51174231).
国家自然科学基金(51134007,51174231)资助项目
©Editorial office ofActa Physico-Chimica Sinica
[Article]

