Mg2+掺杂球形LiFePO4/C的制备及电池性能*
杨伟 薛建军 陈胜洲 胡新发 夏信德 林维明
( 1.华南理工大学 化学与化工学院, 广东 广州510640;2.广州鹏辉能源科技股份有限公司, 广东 广州511483;3.广州大学 化学化工学院, 广东 广州510006)
Mg2+掺杂球形LiFePO4/C的制备及电池性能*
杨伟1薛建军2陈胜洲3胡新发2夏信德2林维明1
( 1.华南理工大学 化学与化工学院, 广东 广州510640;2.广州鹏辉能源科技股份有限公司, 广东 广州511483;3.广州大学 化学化工学院, 广东 广州510006)
以柠檬酸为碳源、草酸亚铁为铁源、乙酸镁为镁源,采用喷雾干燥-碳热还原法制备了一系列镁离子掺杂磷酸铁锂(LiMgxFe1-xPO4/C)材料,并研究了镁离子对喷雾干燥-碳热还原法制备的球形磷酸铁锂材料结构和电化学性能的影响.结果表明,实验制得的LiMgxFe1-xPO4/C材料具有规则的球形空心结构和高的比容量;当Mg2+含量较小时其对磷酸铁锂晶体结构不产生影响,其中LiMg0.04Fe0.96PO4/C具有最好的充放电性能,在0.2C、0.5C、1.0C、2.0C、5.0C倍率下首次放电比容量分别为149.0、145.6、141.3、132.6、123.3 mAh/g,1.0C倍率下充放电循环100周后容量保持率大于97.3%.
Mg2+掺杂;球形磷酸铁锂;喷雾干燥-碳热还原;锂离子电池
磷酸铁锂(LiFePO4)作为锂离子电池正极材料,具有安全性高、充放电循环寿命长、原料来源广泛、绿色环保、成本低廉等优点,在电动汽车和固定储能基站等领域有着广阔的市场前景[1- 3].但LiFePO4正极材料的电子导电率小(小于10-9s/cm)及离子扩散系数低(10-16~10-14cm2/s)[4- 5],大大限制了LiFePO4的广泛应用.人们尝试通过多种方法来改善LiFePO4的性能,如制备形状规则均一的LiFePO4纳米颗粒、减小材料的颗粒尺寸以缩短Li+的传输距离、降低传质阻抗[6- 8],或者在磷酸铁锂材料颗粒表面及颗粒之间添加碳材料、纳米金属颗粒等导电剂形成导电结构以提高材料的导电性能[9- 10].Chung等[11]发现在晶体结构中掺杂金属离子可以显著提高LiFePO4电导率后,人们围绕金属离子掺杂改性LiFePO4展开了大量的研究[12- 14].研究发现Mg2+掺杂能够加快Li+在LiFePO4中的扩散速率,提高材料的电子导电率,使电子在材料中的传递更稳定[15].喷雾干燥法制备的球形LiFePO4具有化学组成容易调节、前驱体易于在分子水平上的混合、材料形貌和结构可控、振实密度高等优势,且电极制备工艺简单,受到了研究者的广泛关注[16- 18].因此,文中采用喷雾干燥-碳热还原法制备了一系列球形镁离子掺杂磷酸铁锂(LiMgxFe1-xPO4/C)正极材料,并且考察不同镁掺杂量对磷酸铁锂材料结构和电化学性能的影响,以制备高性能的磷酸铁锂正极材料.
1 实验
1.1材料制备
按摩尔比n(Li)∶n(M)∶n(P)=1∶1∶1(n(M)为Mg和Fe元素的摩尔量总和)分别称取LiOH、FeC2O4·2H2O、Mg(CH3COO)2·4H2O和NH4H2PO4,溶于二次去离子水中,配制1 mol/L(按Mgx+Fe1-x总浓度计算,x=0.00,0.02,0.04,0.08,0.10)溶液,再加入柠檬酸搅拌30 min形成均一的前驱体溶液.将所得前驱体溶液采用YC- 015实验室喷雾干燥器进行喷雾干燥(进风温度200 ℃,进料速度5 mL/min),得到的粉末样品在高纯Ar气保护下750 ℃高温焙烧12 h,制得镁离子掺杂磷酸铁锂正极材料,分别标记为LiFePO4/C、LiMg0.02Fe0.98PO4/C、LiMg0.04-Fe0.96PO4/C、LiMg0.08Fe0.92PO4/C、LiMg0.10Fe0.90PO4/C.1.2材料的物理表征方法
采用北京普析通用公司的XD-3型X射线衍射分析仪(Cu Kα,36 kV,20 mA)对样品进行晶相分析.采用日本Hitachi S- 4800场发射扫描电子显微镜观察LiMgxFe1-xPO4/C的表面形貌.
1.3电池组装与测试
首先将制得的LiMgxFe1-xPO4/C正极材料、乙炔黑、石墨、聚偏氟乙烯(PVDF)以90∶3∶2∶5的质量比混合,以N-甲基吡咯烷酮(NMP)为溶剂,搅拌成固含量为45%的均一正极浆料,并将浆料涂布在20 μm厚的铝箔上,经过辊压、裁切后在真空干燥箱中120 ℃干燥12 h,制得0.14 mm厚的正极片.然后将人造石墨、乙炔黑、羧甲基纤维素钠、丁苯橡胶以94∶2∶1.5∶2.5的质量比混合,以去离子水为溶剂,搅拌成固含量为45%的均一负极浆料,并将浆料涂布在10 μm厚的铜箔上,经过辊压、裁切后在真空干燥箱中105 ℃干燥12 h,制得0.11 mm厚的负极片.最后以1 mol/L的LiPF6溶于碳酸乙烯酯(EC)+碳酸二甲酯(DMC)+炭酸甲乙酯(EMC)(体积比为1∶1∶1)的混和液为电解液,Celgard2400聚丙烯微孔膜为隔膜,在手套箱中高纯氩气气氛下(水含量和氧气含量均小于10-5)卷绕、封装制成052545型软包电池.
采用VoltaLab PGZ301型电化学工作站对未充电的新鲜电池进行交流阻抗测试,扫描频率范围为10-2~105Hz,扰动电压为10 mV.采用新威电池测试系统测试电池充放电性能,充放电制度:以0.1C倍率恒流充电至3.65 V,转恒压充电(截止电流0.05 C),静置30 min后以不同倍率恒流放电至2.50 V.
2 结果与讨论
2.1材料的物理表征结果
图1所示为喷雾干燥制备的镁离子掺杂磷酸铁锂正极材料的X射线衍射(XRD)谱图和标准图谱(JCPDS No.40-1499).为了研究镁离子掺杂对磷酸铁锂材料晶体结构的影响,测试得到了LiFePO4/C、LiMg0.02Fe0.98PO4/C、LiMg0.04Fe0.96PO4/C、LiMg0.08Fe0.92-PO4/C、LiMg0.10Fe0.90PO4/C的XRD谱图(见图1).从图可知,与标准图谱相比,样品LiMg0.02Fe0.98PO4/C、LiMg0.04Fe0.96PO4/C没有含镁杂质峰出现,LiMg0.08-Fe0.92PO4/C、LiMg0.10Fe0.90PO4/C样品出现MgH2P2O7杂质峰,说明镁离子掺杂量较少时,Mg2+均匀分散在LiFePO4材料的晶格中,不改变其晶相结构,而当Mg2+掺杂量过多时,纯相橄榄石结构遭到破坏,LiMg0.10Fe0.90PO4/C的衍射峰强度明显减弱.
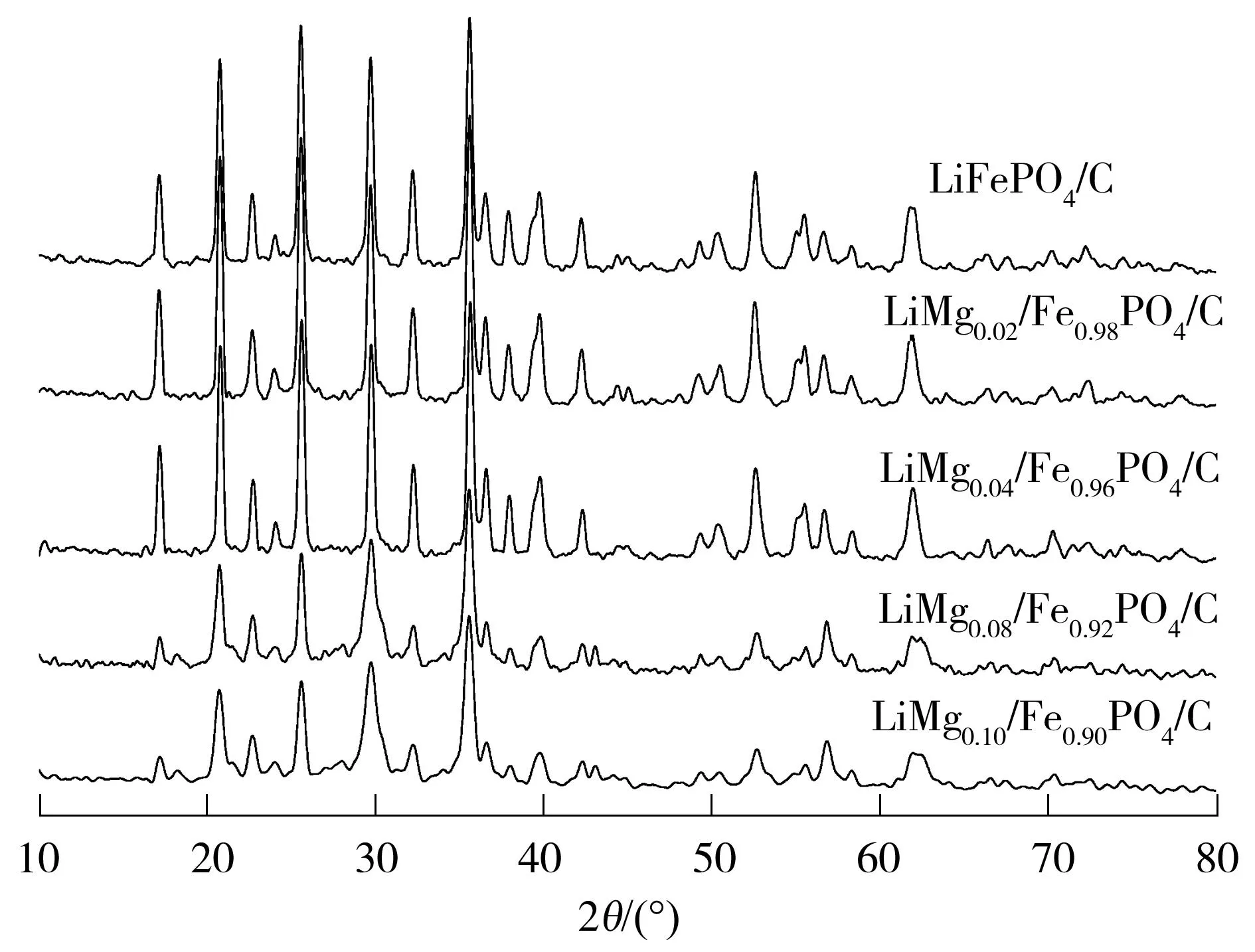
图1喷雾干燥制备的不同Mg2+掺杂量LiMgxFe1-xPO4/C的XRD谱图
Fig.1XRD patterns of LiMgxFe1-xPO4/C with different Mg2+contents prepared via spray drying
图2所示为喷雾干燥制备的镁离子掺杂磷酸铁锂正极材料放大5 000倍的扫描电镜(SEM)照片.从图中可以看出,由于在喷雾干燥过程中液滴内部的水分受热后快速蒸发产生的水蒸气使液滴膨胀,实验制备的LiMgxFe1-xPO4/C形成了二次颗粒构成的空心球形结构,直径为2~8 μm.LiFePO4/C、LiMg0.02Fe0.98PO4/C、LiMg0.04Fe0.96PO4/C的球形结构较为均一、完整,LiMg0.08Fe0.92PO4/C的球形均一性较差,LiMg0.10Fe0.90PO4/C部分空心球破碎,可以看到半球壳状碎片,由此证明合成的材料具有空心球形结构.这可能是因为Mg2+的加入引起前驱体溶液黏度或pH值变化,从而导致生成的球形结构遭到破坏.
2.2LiMgxFe1-xPO4/C材料在不同倍率下的电化学性能
图3给出了喷雾干燥制备的LiMgxFe1-xPO4/C材料在0.1 C倍率下的首次充放电曲线.从图中可以看出,LiMgxFe1-xPO4/C材料的充放电比容量随着Mg元素含量的增加而增加,但Mg含量过大时,材料比容量迅速降低.这可能是由于Mg2+调节了磷酸铁锂的晶格结构,提高了材料的导电性,而当Mg2+过多时,破坏了磷酸铁锂的晶格结构,改变了Li+在固相结构中的扩散路径,不利于Li+嵌入和脱出;另外,磷酸铁锂中出现MgH2P2O7杂相,无电化学活性,导致LiMg0.08Fe0.92PO4/C、LiMg0.10Fe0.90PO4/C的放电比容量较低.

(a)LiFePO4/C

(b)LiMg0.02Fe0.98PO4/C

(c)LiMg0.04Fe0.96PO4/C

(d)LiMg0.08Fe0.92PO4/C

(e)LiMg0.10Fe0.90PO4/C
图2喷雾干燥制备的不同LiMgxFe1-xPO4/C样品的SEM 照片
Fig.2SEM images of different LiMgxFe1-xPO4/C samples prepared via spray drying
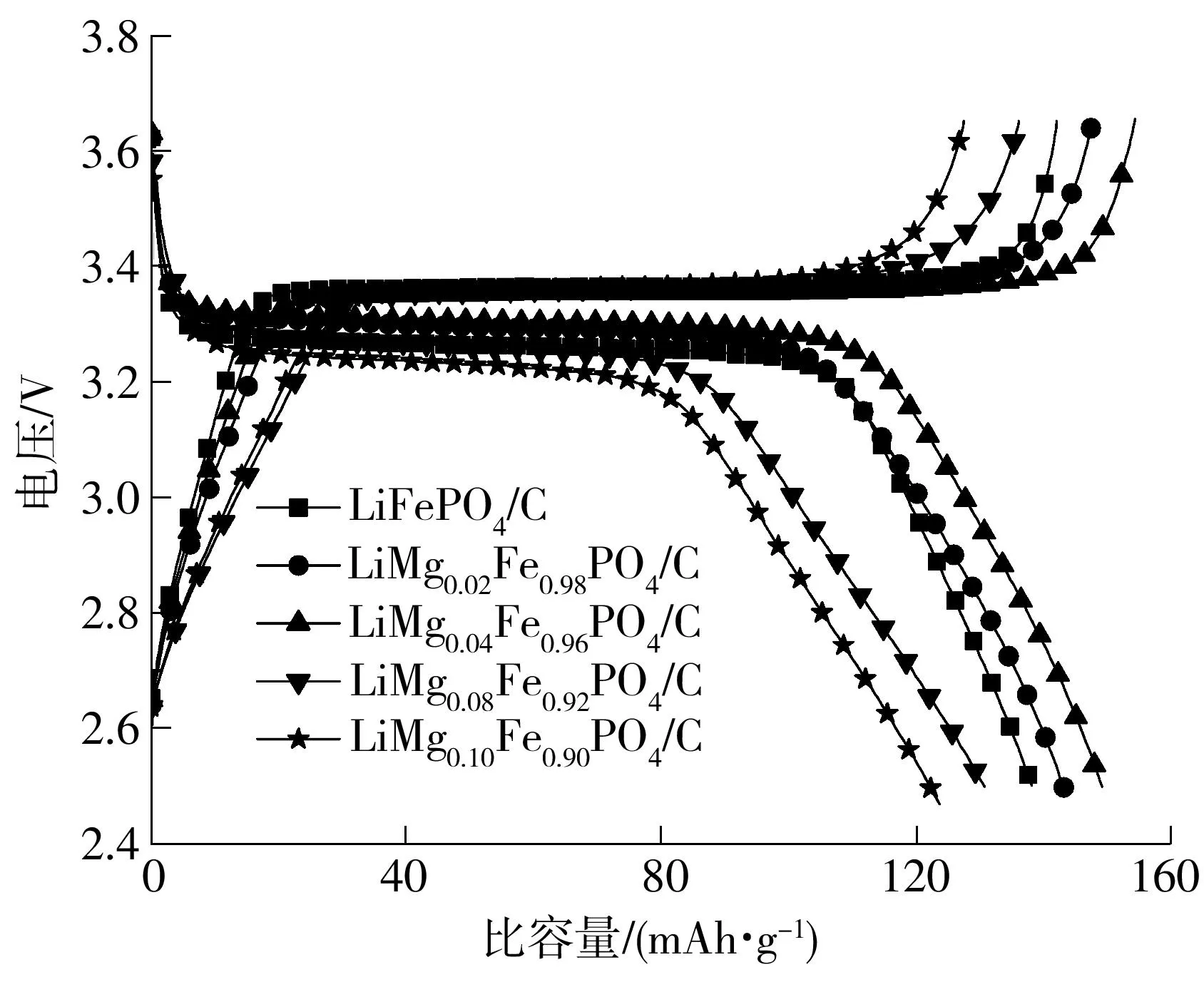
图3喷雾干燥制备的不同LiMgxFe1-xPO4/C材料的0.1C倍率首次充放电曲线
Fig.3Initial charge and discharge curves at 0.1 C of different LiMgxFe1-xPO4/C samples prepared via spray drying
图4为LiMgxFe1-xPO4/C材料在1.0 C倍率下的放电循环曲线.由图可知,在1.0 C倍率下放电时,LiMgxFe1-xPO4/C(x=0.00,0.02,0.04,0.08,0.10)材料的首次放电比容量分别为130.8、134.2、141.3、126.3、119.8 mAh/g,充放电循环100周后容量无明显衰减.LiMg0.04Fe0.96PO4/C材料的首次放电比容量最高,且循环性能较好,充放电循环100周后容量保持率达97.3%.LiMg0.10Fe0.90PO4/C容量衰减严重,充放电循环100周后容量保持率为93.2%.

图4喷雾干燥制备的不同LiMgxFe1-xPO4/C材料在1.0 C倍率下的放电循环曲线
Fig.4Discharge cycling curves at 1.0 C of different LiMgxFe1-x-PO4/C samples prepared via spray drying
图5是LiMgxFe1-xPO4/C(x=0.00,0.02,0.04,0.08,0.10)材料在不同倍率(0.2 C、0.5 C、1.0 C、2.0 C、5.0 C)下的放电循环曲线.由图5可知,所有的LiMgxFe1-xPO4/C样品的放电比容量均随放电倍率的增加而减小,不掺杂Mg2+的纯LiFePO4/C的放电比容量下降最快,掺杂Mg2+的LiMgxFe1-xPO4/C材料的放电比容量则下降较慢,说明Mg2+掺杂有利于提高LiFePO4材料的电子导电率,有效改善高倍率放电性能.LiMg0.04Fe0.96PO4/C材料的放电比容量最高,在不同倍率(0.2 C、0.5 C、1.0 C、2.0 C、5.0 C)下分别为149.0、145.6、141.3、132.6、123.3 mAh/g,其克容量随放电倍率的增加而下降的趋势较弱,而且依次经过0.2 C、0.5 C、1.0 C、2.0 C、5.0 C各循环10周后,再在0.2 C倍率下放电仍能保持初始容量的98.1%,LiMg0.04Fe0.96PO4/C材料具有很好的嵌锂/脱锂活性和稳定性[18].

图5喷雾干燥制备的不同LiMgxFe1-xPO4/C材料在不同倍率下的放电循环曲线
Fig.5Discharge cycling curves of different LiMgxFe1-xPO4/C samples prepared via spray drying at different rates
图6为LiMgxFe1-xPO4/C(x=0.00,0.02,0.04,0.08,0.10)材料的电化学交流阻抗(EIS)谱图.高频区半圆弧反映电荷转移阻抗Rct,低频区的斜线部分反映Li+在材料及电解液中的Warburg扩散阻抗.采用Zview对数据进行拟合,LiMgxFe1-xPO4/C(x=0.00,0.02,0.04,0.08,0.10)的电荷转移阻抗依次分别为38.1、31.6、19.2、41.0、47.9 Ω,随Mg2+掺杂量的增加呈先减小后增加的趋势,LiMg0.04Fe0.96PO4/C具有最低的电荷转移阻抗,这可能是材料具有较好的倍率性能的原因.
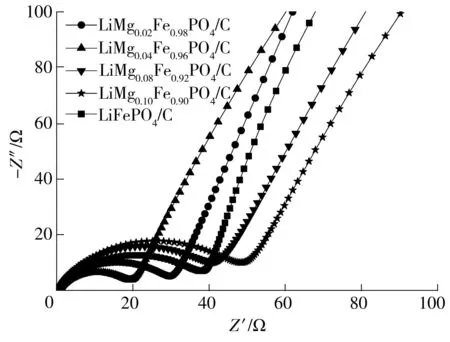
图6喷雾干燥制备的不同LiMgxFe1-xPO4/C材料的交流阻抗谱图
Fig.6EIS spectra of different LiMgxFe1-xPO4/C samples prepared via spray drying
3 结论
采用喷雾干燥-碳热还原法制备了一系列直径约为2~8 μm的空心球形结构Mg2+掺杂磷酸铁锂(LiMgxFe1-xPO4/C)材料,当Mg2+含量较小时其对磷酸铁锂晶体结构不产生影响,Mg2+掺杂能够显著提高喷雾干燥-碳热还原法制备的LiFePO4/C高倍率放电性能,其中LiMg0.04Fe0.96PO4/C具有最好的充放电性能,在0.2 C、0.5 C、1.0 C、2.0 C、5.0 C倍率下首次放电比容量分别为149.0、145.6、141.3、132.6、123.3 mAh/g,1.0C倍率下充放电循环100周后容量保持率大于97.3%.后期研究中应进一步考察LiMgxFe1x-PO4/C材料在低温下的充放电循环性能.
[1]WU X L,JIANG L Y,CAO F F,et al.LiFePO4nanoparticles embedded in a nanoporous carbon matrix:superior cathode material for electrochemical energy-storage devices [J].Advanced Materials,2009,21(25/26):2710- 2714.
[2]YUAN L X,WANG Z H,ZHANG W X,et al.Development and challenges of LiFePO4cathode material for lithium-ion batteries [J].Energy & Environmental Science,2011,4(2):269- 284.
[3]HAUTIER G,JAIN A,ONG S P,et al.Phosphates as lithium-ion battery cathodes:an evaluation based on high-throughput ab initio calculations [J].Chemistry of Materials,2011,23(7):3495- 3508.
[4]DENG S,WANG H,LIU H,et al.Research progress in improving the rate performance of LiFePO4cathode materials [J].Nano-Micro Letters,2014,6(3):209- 226.
[5]WANG Y,HE P,ZHOU H.Olivine LiFePO4:development and future [J].Energy & Environmental Science,2011,4(3):805- 817.
[6]CHANG Z,TANG H,LIU Y,et al.Optimization of synthesis conditions for LiFePO4/C nanocomposites by dimethyl sulfoxide assisted solution-phase method [J].Journal of the Electrochemical Society,2012,159(4):A331- A335.
[7]LI H,ZHOU H.Enhancing the performances of Li-ion bat-teries by carbon-coating:present and future [J].Chemical Communications,2012,48(2):1201- 1217.
[8]NAN C,LU J,LI L,et al.Size and shape control of LiFePO4nanocrystals for better lithium ion battery cathode materials [J].Nano Research,2013,6(7):469- 477.
[9]WANG B,WANG D,WANG Q,et al.Improvement of the electrochemical performance of carbon-coated LiFePO4modified with reduced graphene oxide [J].Journal of Materials Chemistry A,2013,1(1):135- 144.
[10]WANG J,SUN X.Understanding and recent development of carbon coating on LiFePO4cathode materials for lithium-ion batteries [J].Energy & Environmental Science,2012,5(1):5163- 5185.
[11]CHUNG S Y,BLOKING J T,CHIANG Y M.Electronically conductive phospho-olivines as lithium storage electrodes [J].Nature Materials,2002,1(2):123- 128.
[12]HONG J,WANG X L,WANG Q,et al.Structure and electrochemistry of vanadium-modified LiFePO4[J].The Journal of Physical Chemistry C,2012,116(39):20787- 20793.
[13]SHU H,WANG X,WEN W,et al.Effective enhancement of electrochemical properties for LiFePO4/C cathode materials by Na and Ti co-doping [J].Electrochimica Acta,2013,89(1):479- 487.
[14]王震坡,刘文,王悦,等.Mg、Ti离子复合掺杂改性磷酸铁锂正极材料及其电池性能 [J].物理化学学报,2012,28(9):2084- 2090.
WANG Zhen-Po,LIU Wen,WANG Yue,et al.Synthesis and characterization of Mg and Ti ions Co-doped lithium iron phosphate and its lithium-ion batteries [J].Acta Physico Chimica Sinica,2012,28(9):2084- 2090.
[15]ARUMUGAM D,PARUTHIMAL KALAIGNAN G,MA-NISANKAR P.Synthesis and electrochemical characte-rizations of nano-crystalline LiFePO4and Mg-doped LiFePO4cathode materials for rechargeable lithium-ion batteries [J].Journal of Solid State Electrochemistry,2009,13(2):301- 307.[16]KONSTANTINOV K,BEWLAY S,WANG GX,et al.New approach for synthesis of carbon-mixed LiFePO4cathode materials [J].Electrochimica Acta,2004,50(2/3):421- 426.[17]YU F,ZHANG J J,YANG Y F,et al.Preparation and characterization of mesoporous LiFePO4/C microsphere by spray drying assisted template method [J].Journal of Power Sources,2009,189(1):794- 797.
[18]刘全兵,罗传喜,宋慧宇,等.喷雾干燥-碳热还原法制备介孔球形LiFePO4/C正极材料 [J].华南理工大学学报(自然科学版),2011,39(11):27- 32.
LIU Quan-bing,LUO Chuan-xi,SONG Hui-yu,et al.Preparation of mesoporous spherical cathode material LiFePO4/C via spray drying-carbothermal reduction [J].Journal of South China University of Technology (Natural Science Edition),2011,39(11):27- 32.
Preparation and Battery Performance of Spherical LiFePO4/C Doped with Mg2+
YANGWei1XUEJian-jun2CHENSheng-zhou3HUXin-fa2XIAXin-de2LINWei-ming1
(1.School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640,Guangdong,China;2.Guangzhou Great Power Energy & Technology Co., Ltd., Guangzhou 511483, Guangdong, China;3.School of Chemistry and Chemical Engineering, Guangzhou University, Guangzhou 510006, Guangdong, China)
Spherical LiFePO4/C doped with Mg2+(namely LiMgxFe1-xPO4/C) was prepared by means of the spray drying-carbonthermal reduction, with citric acid, ferrous oxalate and magnesium acetate as the carbon source, the iron source and the magnesium source, respectively. Then, the effects of Mg2+doping on the structure and electrochemical performance of the prepared LiFePO4/C material were investigated. The results indicate that (1) LiMgxFe1-xPO4/C possesses a hollow ball-shaped structure and a high specific capacity; (2) the crystal structure of lithium iron phosphate keeps unchanged at a low Mg2+dosage; and (3) LiMg0.04Fe0.96PO4/C possesses the best charge/discharge performance. For instance, its initial discharge capacities are 149.0, 145.6, 141.3, 132.6 and 123.3 mAh/g at the rates of 0.2C, 0.5C, 1.0C, 2.0C and 5.0 C, respectively; and the capacity retention ratio of LiMg0.04Fe0.96PO4/C at 1.0C keeps more than 97.3% after 100 charge-discharge cycles.
Mg2+doping; spherical lithium iron phosphate; spray drying-carbothermal reduction; lithium ion battery
2015- 08- 24
国家自然科学基金资助项目(21376056,21463030);广东省战略性新兴产业发展专项资金新能源汽车产学研项目(2011- 1579)
杨伟(1982-),男,博士,助理研究员,主要从事应用电化学与新能源材料研究.E-mail:wyang608@163.com
1000- 565X(2016)06- 0009- 05
TM 910.4
10.3969/j.issn.1000-565X.2016.06.002
Foundation items: Supported by the National Natural Science Foundation of China(21376056,21463030)

