Synthesis of Octadecyltrichlorosilane-modifi ed Amphiphilic Silica Nanoparticle for Latent Fingerprint Development
HUANG Wei, LI XiaoJun,*, WANG Hongfei(. Institute of Forensic Science, Ministry of Public Security, Beijing 00038, China; . Department of Forensic Science, People's Public Security University of China, Beijing 00038, China)
Synthesis of Octadecyltrichlorosilane-modifi ed Amphiphilic Silica Nanoparticle for Latent Fingerprint Development
HUANG Wei1, LI XiaoJun1,*, WANG Hongfei2(1. Institute of Forensic Science, Ministry of Public Security, Beijing 100038, China; 2. Department of Forensic Science, People's Public Security University of China, Beijing 100038, China)
ABSTRACT:Motivated by the demand for the development of latent fingerprint with nanotechnology, a new type of nanometer-sized amphiphilic silica particles was synthesized by sol-gel method and modifi ed using tetraethyl orthosilicate (TEOS) and octadecyltrichlorosilane (OTS). The prepared particles were characterized by various methods to show that their structure and properties were adequate for fingerprint development in the forensic science field. When compared to other silica particles, the modifi ed powder appeared highly lipophilic and monodisperse. The effects of synthesis conditions and size of silica sphere were also discussed. The high-quality fi ngerprints on smooth nonporous objects were developed using the new type of particles, indicating that a new sensitive latent fi ngerprint developing assay was obtained. The prepared particle exhibits such attractive features as time saving, simple procedures, convenient operation and reasonable price.
KEY WORDS:latent fi ngerprint; silica nanoparticle; lipophilic modifi cation; OTS
1 Introduction
For decades, fi ngerprint has remained one of the most important forensic evidence because it builds a direct link between a person and a crime scene or a related object. When unprotected hands touch a surface or an object, the ridge pattern on the fi nger tip of the individual,or “fi ngerprint”, is transferred to the surface through the deposition of sebaceous and eccrine secretions. Normally, fi ngerprints are not visible and are called “latent fi ngerprint”, requiring techniques to make it visualized. Large numbers of techniques[1-4]have been applied in fi ngerprint development area. They are mainly characterized by developing effi ciency, based on the composition of the materials, the surface of the object (porousness,wetness), the age of fingerprint, and others. The available techniques involve various approaches either using dusting agent that adheres to the “sticky” secretions deposited on the object's surface[5-8], or chemical developer that generates chemical interaction with the deposited materials on the fi ngerprint to produce visual color[9-11]. For those agent techniques, powder composites are usually preferred due to their detectable sensitivity and their ability to obtain the nature of the pattern or the color of the support on which the fi ngerprints deposit. Numerous powders with different characteristics such as fluorescent[12], non-f luorescent[13], magne tic[14], non-magnetic and colored[15], are commercially available. During the past 20 years, nanoparticles or nanostructured materials have shown to be the promising alternative to conventional techniques. Nanoparticles have size-dependent properties due to their nano-size range, difference from atomic size particles or bulk material. Generally, the nanostructured ones driven by golden nanoparticles[16,17]can be distinguished from quantum dots and silica nanoparticles. Each of them possesses its advantages and drawbacks in terms of effi ciency and sensitivity according to the nature of the object's surface, the latent materials and other conditions. Though semiconductor quantum dots (QDs e.g., CdSe, CdTe, ZnS, HgTe, InP, GaAs or InAs) have become a hot field of forensic science research over the last decade[18-23], silica nanoparticles differ from QDs in a sense that they are optically inert on their own. Meanwhile, one of its advantages is that the reactions are conducted at low temperature, permitting organic and inorganic species to coexist within the same matrix.
Among the various modes of nanoparticles, materials based on silica (SiO2) obtained by the sol-gel process appear to be an excellent choice for function modifi cation in chemical development area[24,25]. These particles have better surface area, higher chemical and thermal stability,and active surfaces due to silanol groups (Si-OH), providing further potential for further development[26,27]. Sil-ica matrix can be modifi ed in several ways, one of which is post-modifi cations of a silica matrix by covalent bonding of the carboxyl groups of lipophilic or hydrophilic[28]. Amphi philic silica nanoparticles are very important for forensic fingerprint detection since the ridge deposits often consist of organic molecules, such as long carbon chain fatty acids, amino acids as well as inorganic salts and a multitude of unknown contaminants[29]. As such,this method can be used to obtain modifi ed silica spheres and produce a new class of building blocks. It could be interesting to study how this nanoparticle develops fi ngerprints, and then eventually improves the detecting effi ciency of latent fi ngerprints[30,31].
In this article, a simple and cost-effective self-assembly approach is presented to prepare silica nanoparticle using tetraethyl orthosilicate (TEOS) and octadecyltrichlorosilane (OTS). Several work-up methods were utilized to characterize the new nanoparticles. The effects of different TEOS:OTS weight ratios and particle diameters on detecting latent fi ngerprints were tested.
2 Materials and Methods
2.1 Reagents
TEOS and OTS were purchased from Alfa Aesar (Shanghai, China). Ethanol, ammonia and n-hexane were from Beijing Chemical Reagents Company (Beijing,China). All the chemicals were of analytical grade and were used as received without further purification. Deionized water was used throughout the research work.
2.2 Synthesis of Amphiphilic Silica Nanoparticle
Monodisperse silicon dioxide spherical particles were obtained in situ by mixing TEOS with ethanol and deionized water. Ammonia was used as a catalyst during the hydrolysis and condensation processes. OTS was used as a surface modifi er to obtain amphiphilic silica particles. More specifically, the following procedure was used to obtain amphiphilic silica nanoparticle. In the first step,ethanol, deionized water and ammonia (25%) were added into a three-necked fl ask in sequence, and stirred for about 30 min at room temperature. Different quantities of TEOS were then added (0.5, 1 and 2 g) to obtain particles with different diameters. The solution was stirred for 8 hours and then washed 5 times using high-speed centrifugation at 5500 r/min (TDL-5-A, Shanghai Anting Scientifi c Instrument Factory, Shanghai, China). Finally,the monodisperse hydrophilic silicon dioxide spherical particles were dried in a vacuum oven (202-l, Shanghai Yiheng Instruments Co., Ltd., Shanghai, China) for 24 hours. Particles with three average diameters of 250, 550 and 700 nm were obtained. In the second step, the monodisperse hydrophilic silicon dioxide spherical particles were modified by reducing 1 g of silica powder in 100 mL n-hexane. The silica particles were dispersed 30 min using an ultrasonic oscillator. 0.01, 0.02, 0.1 and 0.5 g of OTS were added to obtain particles with the following SiO2: OTS weight ratios: 100:1, 50:1, 10:1 and 2:1. The solution was stirred for 12 hours. The amphiphilic silica nanoparticles were washed twice using high-speed centrifugation at 5500 r/min and dried in a vacuum oven for 24 hours.
2.3 Characterization
The morphology of the amphiphilic silica nanoparticles was examined by field-emission gun scanning electron microscope (FEG-SEM) of a Hitachi S-4800 type (Tokyo, Japan) equipped with a high-resolution secondary electron detector (in lens detector), operated at 10.0 kV and a point-to-point resolution of 2 nm. The optical properties of the silica nanoparticles were characterized using Fourier transform infra-red spectrometer (Perkin-Elmer Corporation, Waltham, USA). Spectra were recorded using the 514.5 nm line of a heliumneon laser at a power of 150 W and spectral resolution of 1 cm-1. The X-ray photo electron spectrum (XPS) of the amphiphilic silica nanoparticles was obtained using an ESCALAB250Xi (Thermo Fisher Scientifi c Ltd.,Waltham, USA) hemispherical electron analyzer, and an aluminum anode as the X-ray source. The X-ray source was operated at 12 keV and 15 mA. Binding energies set at 103.4 eV were corrected using the reference Si2p line from silica, X-ray powder diffraction (XRD) analysis was performed on a Rigaku D/max-2500 diffractometer (Rigaku Corporation, Tokyo, Japan) equipped with Cu Kα radiation (λ=1.54178Å) operating in continuous scan mode at 2° min-1. The measurements were collected at room temperature with a scan range between 3° and 60°.
2.4 Protocol of Latent Fingerprint Detection
The performance of amphiphilic silica nanoparticle powder for the detection of latent fi nger impressions was measured using a series of 300 latent fingerprints. The fingerprints were obtained from 10 donors, who were asked to wash their hands thoroughly with soap. Each one of the 10 fi ngers from each donor was gently rubbed across the donor's forehead and deposited on clean microscopy glass slides. This process was repeated 3 times for each donor, resulting in 3 sets of 100 latent prints. The sample sets were kept at room condition for 1, 3 and 7 days before processed with the silica nanoparticle powder.
Detection of the latent fi ngerprint was performed using the different particles obtained as described above (i.e., particles with different diameters and different SiO2: OTS weight ratios). Brushing was done using squirrel hair fi ngerprint brushes. Fingerprint images were captured using a DCS4 fi ngerprint enhancement system (Foster & Freeman Ltd., Evesham, UK).
3 Results and Discussion
3.1 Mechanism of Amphiphilic Silica Particles Preparation
During the preparation of the monodisperse hydrophilic silica nanoparticles, TEOS was hydrolyzed to form the corresponding hydroxylated products and alcohol;meanwhile, the condensation reaction between silicic acid itself and TEOS occurred. The reaction equationsshow as below:

During the modification step of the procedure, OTS reacted with the silanols from the surface of the silicon spheres, resulting in the loss of Cl-and H+; eventually Si-R and Si-OH groups coexisting at the surface of the silica particles. If the concentration of the OTS was high enough, the hydroxyl groups on the surface of the nanoparticles were almost all replaced, causing lipophilic nano-SiO2composite particles with Si-R groups on their surfaces. By adjusting the weight ratios of OTS and TEOS, the ratio of Si-R and Si-OH groups on the silica nanoparticle could be changed, therefore,different levels of modifi ed amphiphilic silica particles were offered.
3.2 Structural Characterization
3.2.1 FEGFEG-SEMSEM Imagesmages
The FEG-SEM images of the hydrophilic silica material before modification showed that the particles were highly soluble in water, but not in hexane (Fig.1). This implied that hydrophilic silica particles would not dissolve in the amino acid component of latent fi ngerprint residue. This was the main reason why hydrophilic silica particles were not appropriate for detecting latent fi ngerprints. On the other hand, silicon spheres, modified by OTS, showed excellent solubility in hexane. The solubility appeared to increase with the weight ratio (WR) of SiO2to OTS (Fig.2). According to the results, the bigger the weight ratio of SiO2to OTS, the better the nanoparticles adhere to each other. The weight ratio of SiO2to OTS became the key factor to affect the ability of amphiphilic silica nanoparticles to detect latent fi ngerprints.
3.2.2 FTIRFTIR Spectraectra forfor Amphiphilichilic Silicailica Nanoparticleticle
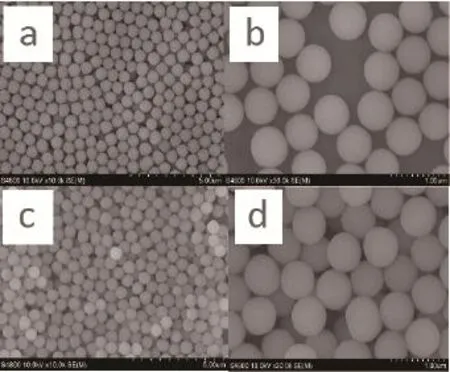
Fig.1 SEM images of the hydrophilic silica particles in water bath (a, b) and in hexane bath (c, d)
Fourier transform infrared spectra provided important corresponding data for the characterization of the structure of the nanoparticles in the amphiphilic silica material by offering information on the microstructure of the material. The spectra of silica sphere before modification could be seen in Fig.3a. In Fig.3a, three peaks at 797, 1100 and 1400 cm-1were observed. The peak at 1100 cm-1was the antisymmetric stretching vibration,belonging to Si-O-Si, and the peak at 1400 cm-1was the stretching vibration of C-O. These two peaks showed that some Si-C groups were present on the surface of the silica sphere before modification. The peaks at 935 and 3414 cm-1refl ected the bending vibration of Si-OH and the stretching vibration of -O-H. On Fig.3b~3d, the peaks near 2921 and 2852 cm-1reflected the antisymmetric stretching vibration arising from -CH2- alkyl chain, which showed that alkyl groups had successfully replaced some of the hydroxyl groups on the surface of silica spheres. The peak at 1469 cm-1reflecting the asymmetric bending vibration of -C-H-, also explained above view. The peak near 1632 cm-1might be caused by the absorbed water during the particles' preservation process. Fig.3a-3d showed that the intensity of the characteristic absorption peaks gradually increased with the rise of the weight ratios. It also meant the function of adherence could be strengthened by the increase of the weight ratios.
3.2.3 2.3 X-rayray Photoelectronctron Spectroscopyscopy
XPS analysis was conducted in order to obtain information about the surface and the feature of the chemical bonds. Fig.4 showed the XPS binding energy (eV)values of C1s, O1s and Si2p were acquired for the silica nanoparticles. The binding energy (B.E.) value of C1s at 284.7 eV was assigned to carbon bonded atoms in polyaromatic structures in graphite (B.E.¼284.6 eV), or carbon presented in alcohol[32-35]. As a consequence, the binding energy value of O1s at 532.8 eV was assigned to carbonyl oxygen atoms in esters, and anhydrides and oxygen atoms in hydroxyl groups. This implied that the modified silica material was ideal for further efficient chemical modifi cations.
3.2.4 2.4 X-rayray Powderowder Diffractionction

Fig.2 SEM images of the amphiphilic silica particles in hexanebath with the weight ratio of SiO2: OTS at 2:1 (a), 10:1 (b),50:1 (c) and 100:1 (d), respectively
To show that the silica material had a disordered structure, the XRD patterns of the unmodified silica sphereand amphiphilic silica nanoparticle were obtained and compared (Fig.5). It showed that both the unmodified material and amphiphilic silica presented an amorphous structure, which could be inferred from the absence of peak refl ection in the XRD diffractogram. Based on these results, it was safe to say that neither of the particle types had periodicity, but both were disordered.
3.3 Dispersion Capacity of Modifi ed Silica Particle
The dispersion performance of amphiphilic nanoparticle in water and organic solvent was studied (Fig.6). The modifi ed silica material was found well-dispersed in hexane environment, indicating that amphiphilic silica particles were highly lipophilic. To facilitate the observation,a small amount of methyl orange was added to color the water. Silica nanoparticles of 1% mass fraction was added to the water and hexane mixed system (water and hexane
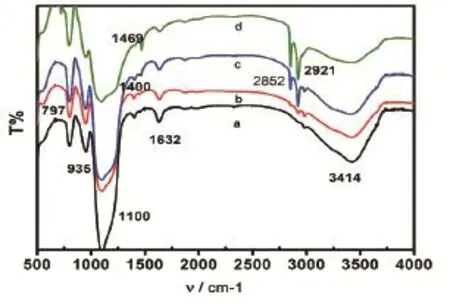
Fig.3 FTIR spectra of unmodifi ed silica sphere (a), amphiphilic silica particle modifi ed by the weight ratio of SiO2: OTS at 100:1 (b), 50:1 (c) and 10:1 (d)
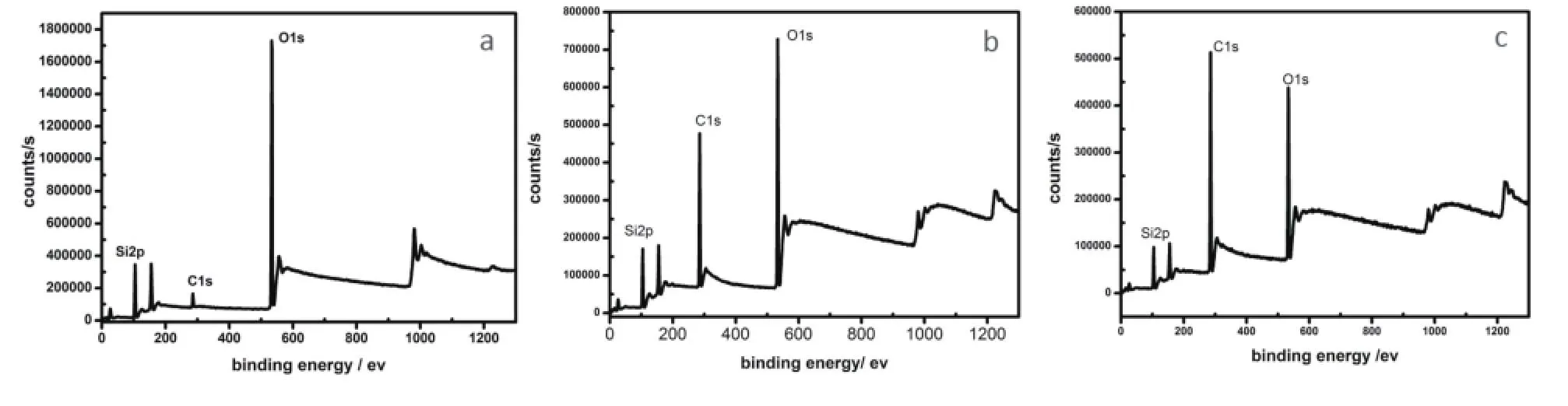
Fig.4 XPS images of unmodifi ed silica sphere (a), amphiphilic silica particle modifi ed by the weight ratio of SiO2: OTS at 50:1 (b), 10:1 (c)
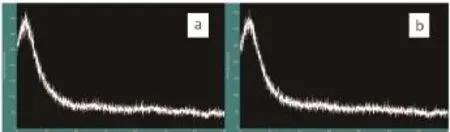
Fig.5 XRD images of unmodifi ed silica sphere (a), amphiphilic silica particle modifi ed by the weight ratio of SiO2: OTS at 10:1 (b)

Fig.6 Modifi ed silica particle in hexane environment (a), reference solutions of 1:1 water and hexane (b), and modifi ed silica particle in mixed system of water and hexane (c)
volume ratio is 1:1). It showed that the modified silica nanoparticles were well dispersed in the hexane phase.
3.4 Optimization for Fingerprint Development Effectiveness
3.4.1 Effectffect ofof Weighteight RatioRatio ofof Unmodifi edfi ed Silicailica Spherephere to to OTSOTS
The images acquired using the SEM (Fig.2) showed that as the weight ratio of SiO2: OTS increased, adhesive ability between particles improved. In order to find the most effective amphiphilic silica particle for detecting latent fi ngerprint, the effectiveness of 4 different weight ratios (100:1,50:1,10:1,2:1) was compared. All types of modifi ed particles were used to detect fresh fi ngerprints, which were left on glass surfaces (Fig.7). Even though all of modifi ed nano-silica particles' powder appeared to be able to detect latent fingerprints, the best weight ratio of SiO2: OTS was 10:1.
3.4.2 Effectffect ofof Particleticle Diametermeter
The diameter of the amphiphilic silica particles depended on the hydrophilic silica material used. SiO2nano-particles prepared by the Stober method usually had uniform spherical surface with a large number of bonded hydroxyl groups at different states (e.g., isolated hydroxyl, hydrogen bonded hydroxyl and twins hydroxyl). Many factors might infl uence the preparation of the monodisperse silica spherical particles, such as the type of catalyst, the concentration of silicon source, the type of solvent, hydrolysis temperature, and reaction time. In the case of using ammonia as a catalyst, its concentration was the main factor infl uencing the particle size, followed by the silicon source density.
Different sizes of amphiphilic silica particles (250, 550 and 700 nm) were synthesized. Results showed that both the size of the nanoparticles and the weight ratio of SiO2:OTS could affect the fi ngerprint detection. The best diameter for the amphiphilic silica particles (DASP) was 700

Table 1 Summary of the results obtained when different sets of latent fi ngerprints on glass slices developed with different weight ratio of SiO2: OTS and different diameter of modifi ed silica particles
nm and best weight ratio of SiO2: OTS was 10:1 (Table 1).
3.4.3 Resultssults ofof Fingerprintsrints Developmentpment
The modifi ed amphiphilic silica particles with a diameter of 700 nm and a weight ratio of SiO2: OTS at 10:1 were used to develop latent fingerprints on nonporous objects such as glass, ceramic and plastic. With a subtle size, the high lipophilic particle could absorb onto the fi ngerprints' oil residual much well. Compared with the traditional powders as golden, silver and magnetic powder, the amphiphilic silica nanoparticle could generate much more recognizable fi ngerprint image with continuous ridges and visible pores (Fig.8).
4 Conclusions
A new type of modifi ed amphiphilic silica nanoparticles was synthetized using OTS, and was successfully applied to detect fresh and aged latent fi ngerprints on slide glass. Such particles are very sensitive and selective to amino acids due to their good lipophilic and hydrophilic properties. Different particle sizes and SiO2:OTS weight ratios were investigated to optimize the formulation of the nanoparticles. Silica sphere with a diameter of 700 nm and modifi ed at a weight ratio of 10:1 showed the best performance when used to detect and develop latent fi ngerprints. These findings enable to propose a method for latent fingerprints detection on smooth nonporous objects. The wider range of applications of the new powder is currently under investigation, as well as the use of other types of silica nanoparticles for the detection of latent fi ngerprints on various other surfaces.
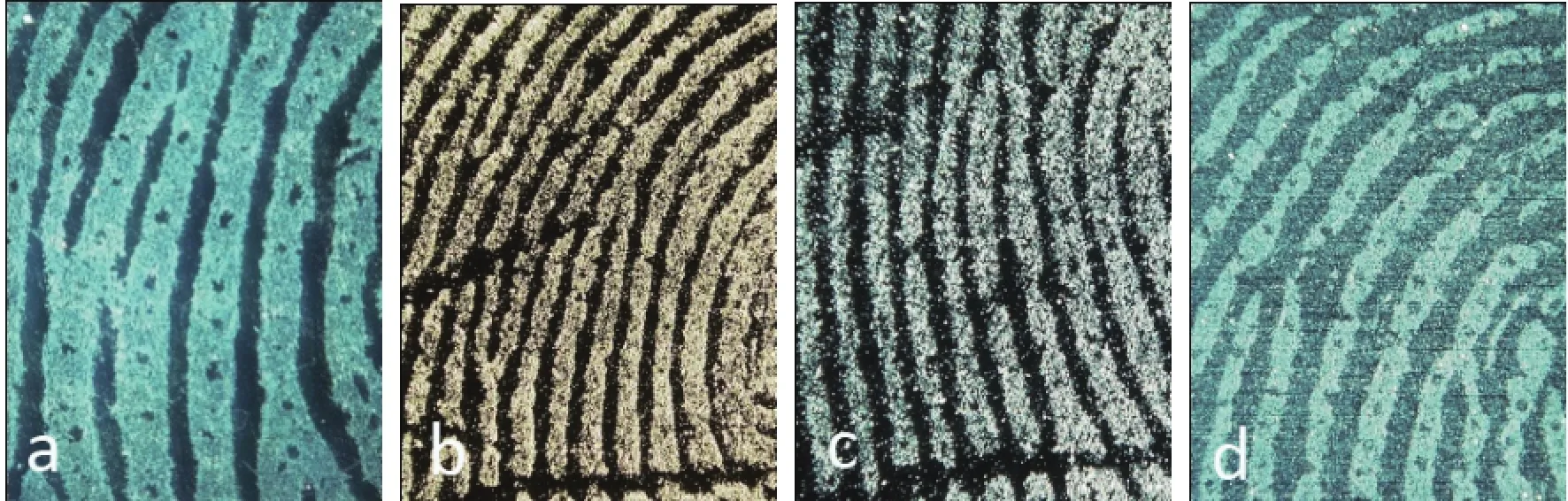
Fig.8 Images of latent fi ngerprints on glass developed by nanoparticle (a), golden powder (b), silver powder (c) and magnetic powder (d)
Reference
[1] Croxton RS, Baron MG, Butler D, et al. Variation in amino acid and lipid composition of latent fingerprints. Forensic Sci Int,2010,199(3):93-102.
[2] Schwarz L. An amino acid model for latent fingerprints on porous surfaces. J Forensic Sci, 2009, 54:1323-1326.
[3] Salama J, Donovan SA, Lennard C, et al. Evaluation of the fingermark reagent oil red O as a possible replacement for physical developer. J Forensic Ident, 2008,58:203-207.
[4] Tahtouh M, Despland P, Shimmon R. The application of infrared chemical imaging to the detection and enhancement of latent fingerprints: method optimization and further findings. J Forensic Sci, 2007,52:1089-1096.
[5] Thomas GL. The physics of fingerprints and their detection. Phys E Sci Instrum J, 1978,11:722-731.
[6] Kerr FM, Barron IW, Haque F. Organic based powders for latent fingerprint detection on smooth surfaces. J Forensic Sci,1983, 16: 39-44.
[7] Morimoto SI, Kamingo A, Hirano T. A new method to enhance visualization of latent fingermark by sublimating dyes and its practical use with a combination of cyanoacrylate fuming. Forensic Sci Int, 1998,97:101-108.
[8] Baileya JA, Crane JS. Use of nitrogen cryogun for separating duct tape and recovery of latent fingerprints with a powder suspension method. Forensic Sci Int, 2011,210:170-173.
[9] Muthyala R. Chemistry and application of Leuco dyes. New York: Kluwer Academic Publishers, 2002.
[10] Brennan J, Bramble S, Crabtree S, et al. Fuming of latent fingerprints using dimethylaminocinnamaldehyde. J Forensic Id ent, 1995,45:373-380.
[11] Marriott C, Lee R, Wilkes Z. Evaluation of fingermark detection sequences on paper substrates. Forensic Sci Int,2014,236:30-37.
[12] Menzel ER, Ulvick SM. Photoluminescent semiconductor nanocrystals for fingerprint detection. J Forensic Sci,2000,45:545-551.
[13] Shellya DC, Quarlesb JM, Warner IM. Preliminary evaluation of mixed dyes for fingerprinting non-fluorescent bacteria. Analytical Letters, 1981,14:1111-1124.
[14] Thonglon T, Chaikum N. Magnetic fingerprint powder from a mineral indigenous to Thailand. J Forensic Sci, 2010,55:1343-1346.
[15] Choi MJ, McDonagh AM, Maynard PJ. Preparation and evaluation of metal nanopowders for the detection of fingermarks on nonporous surfaces. J Forensic Ident, 2006,56:756-768.
[16] Haruta M, Yamada N, Kobayashi T. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J Catal, 1989,115:301-309.
[17] Xu W, Kong JS, Yeh Y. Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat Mater, 2008,7:992-996.
[18] Becue A, Moret S, Champod C. Use of quantum dots in aqueous solution to detect blood fingermarks on non-porous surfaces. Forensic S ci Int, 2009,191:36-41.
[19] Cheng KH, Ajimo J, Chen W. Exploration of functionalized CdTe nanoparticles for latent fingerprint detection. J Nanosci Nanotechnol, 2008,8:1170-1173.
[20] Moret S, Andy B, Champod C. Cadmium-free quantum dots in aqueous solution: Potential for fingermark detection, synthesis and an application to the detection of fingermarks in blood on non-porous surfaces. Forensic Sci Int, 2013,224:101-110.
[21] Gao F, Lv CF, Han JX. CdTe-Montmorillonite nanocomposites: control synthesis, UV radiation-dependent photoluminescence, and enhanced latent fingerprint detection. J Phys Chem C, 2011,115:21574-21583.
[22] Alaoui IM, Menzel ER. Substituent effects on luminescence enhancement in europium and terbium Ruhemann’s purple complexes. Forensic Sci Int, 1996,77:3-11.
[23] Brusaferri L, Sanguinetti S, Grilli E. Thermally ac tivated carrier transfer and luminescence line shape in self-organized InAs quantum dots. Applied Physics Letters, 1996,69:3354-3356.
[24] Walcarius A, Alexander K. Ordered porous thin films in electrochemical analysis. Trends Anal Chem, 2008,27:593-603.
[25] Walcarius A, Mandler D, Cox JA, et al. Exciting new directions in the intersection of functionalized sol-gel materials with electrochemistry. J Materials Chem, 2005,15:3663-3689.
[26] Collinson MM. Recent trends in analytical applications of organically modified silicate materials. Trends Anal Chem,2002,21:31-39.
[27] Jal PK, Patel S, Mishra BK. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta, 2004, 62:1005-1028.
[28] Lin JH, Wei ZJ, Zhang HH. Sensitive immunosensor for the label-free determination of tumor marker based on carbon nanotubes/mesoporous silica and graphene modified electrode. Biosensors and Bioelectronics, 2013,41:342-347.
[29] Knowles AM. Aspects of physicochemical methods for the detection of latent fingerprints. J Phys E, 2001,11:713.
[30] Theaker T. Doped hydrophobic silica nano- and micro-particles as novel agents for developing latent fingerprints. Forensic Sci Int, 2008,174:26-34.
[31] Liu T. Exploration of the use of novel SiO2nanocomposites doped with fluorescent eu3+/sensitizer complex for latent fingerprint detection, Forensic Sci Int, 2008,176:163-172.
[32] Martínez MT, Callejas MA, Benito AM. Sensitivity of single wall carbon nanotubes to oxidative processing: Structural modification, intercalation and functionalization. Carbon,2003,41:2247-2256.
[33] Kundu S, Wang Y, Xia W. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: a quantitative high-resolution XPS and TPD/TPR study. J Phys Chem, 2008,112:16869-16878.
[34] Zhou JH, Sui ZJ, Zhu J. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon, 2007,45:785-796.
[35] Kim JH, Song MJ, Lee CJ. A comparative study of electrochemical and biointerfacial properties of acid- and plasmatreated single-walled carbon-nanotube-film electrode systems for use in biosensors. Carbon, 2013,52:398-407.
Author: HUANG Wei, Ph.D., associate research fellow in photograph and video examination. E-mail: huangwei@cifs.gov.cn
This work was supported by grants from the Basic Research Program Exclusive for central Government-class Institutes of Public welfare (2014JB008).
CLC number:DF793.2
Document Code:A Article ID:1008-3650(2016)01-0025-07
Received date:2015-06-23
Corresponding Author:LI Xiaojun, M.S., assistant research fellow in fingerprint examination. E-mail: lixiaojun@cifs.gov.cn

