混合二甲苯系统热力学平衡组成的计算
赵 岩,任冬梅,夏云生,李新华,包德才
(渤海大学化学化工学院,辽宁 锦州 121013)
有机化工与催化
混合二甲苯系统热力学平衡组成的计算
赵岩*,任冬梅,夏云生,李新华,包德才
(渤海大学化学化工学院,辽宁 锦州 121013)
摘要:研究混合二甲苯模型中3种组分在不同温度下的热力学平衡组成,依据状态函数特点,分别计算各平衡的标准摩尔反应熵变、标准摩尔反应焓变及标准摩尔吉布斯自由能变,进而计算平衡组成。结果表明,在该混合二甲苯理想气体模型中,反应温度由298.15 K升至1 023.15 K,混合二甲苯中3个平衡组成随着反应温度升高呈如下变化规律:间二甲苯含量y(MX)由0.599 0逐渐降至0.496 3,降幅达17.15%;邻二甲苯含量y(OX)由0.162 6逐渐升至0.275 9,升幅达69.68%;对二甲苯含量y(PX)由0.238 4缓慢升至0.242 6,而后逐渐降至0.227 7,最大值出现在423.15 K,最大降幅仅为6.14%,表明升高温度不利于提高对二甲苯的平衡组成。
关键词:化学热力学;混合二甲苯;平衡组成;对二甲苯;异构化
CLC number:TQ241.1+3;O643.12Document code: AArticle ID: 1008-1143(2016)05-0075-06
混合二甲苯是间二甲苯(MX)、邻二甲苯(OX)和对二甲苯(PX)构成的混合体系,如指定了某一温度和压力,则该系统的状态函数不再变化,即此时系统处于热力学动态平衡,其组分含量是定值。当混合二甲苯看作理想气体或理想液体混合物时,可以对系统热力学性质进行演绎推理,为深入开发混合二甲苯转化工艺提供重要的热力学基础数据。工业合成对二甲苯的工艺(如甲苯歧化、烷基转移和汽油裂解等)主要产物为混合二甲苯[1-3],因此要设计高效分离设备,对生产操作(如冷凝、气化、闪蒸、精馏、吸收、萃取、结晶和吸附)进行优化。本文对混合二甲苯系统热力学平衡组成的计算进行研究。
1模型建立
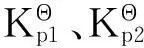

图1 理想二甲苯混合系统模型Figure 1 Ideal model of xylene mixed system
2计算过程
计算平衡组成热力学方程式及计算路线:

3结果与讨论


表1 二甲苯的标准摩尔恒压热容与温度的关系[4-5]

表2 不同反应温度下二甲苯平衡体系的标准摩尔反应熵变(T)


表3 不同反应温度下二甲苯平衡体系的标准摩尔反应焓变(T)
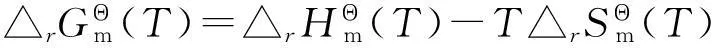
表4 不同反应温度下二甲苯平衡体系的标准摩尔反应吉布斯自由能变(T)
从表4可以看出,反应温度低于673.15 K,仅有邻二甲苯向对二甲苯的异构化反应可以自发进行,且随着反应温度升高,自发进行的趋势越来越弱;反应温度高于673.15 K,上述3个异构化反应均不能自发进行,且随着反应温度升高,自发程度越来越难。
3.5平衡常数及平衡组成
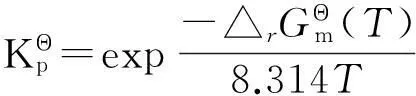
幅为17.15%;邻二甲苯含量随着反应温度升高逐渐提高,增幅为69.68%;对二甲苯含量随着反应温度升高先增后降,降幅为6.14%。从热力学角度看,反应温度变化对二甲苯含量影响较小,而升高温度更有利于间二甲苯向邻二甲苯异构。因此,若要提高对二甲苯选择性,需要从反应动力学着手[9-11]。选择合适的择形催化剂并适当改性[12-23]以及设计新的分离工艺[24]或合成路线[25]均至关重要。

表5 不同反应温度下二甲苯平衡体系的气相组成
4结论
(1) 通过对混合二甲苯热力学平衡组成的计算可知,间二甲苯含量随着反应温度升高逐渐降低,邻二甲苯含量随着反应温度升高逐渐提高,对二甲苯含量随着反应温度升高先增加后降低,但降幅较小。升高温度有利于促进间二甲苯向邻二甲苯异构,而对二甲苯含量变化不大。若要提高对二甲苯选择性,需从选择催化剂、分离工艺和新合成路线入手。
(2) 混合二甲苯系统热力学平衡组成的计算结果将为产品质量控制提供至关重要的热力学参数和多元相平衡数据,经过对某些参数的适当修正,该方法适用于其他理想混合体系的热力学平衡计算。
参考文献:
[1]Chen N Y,Kaeding W W,Dwyer F G.Para-directed aromatic reactions over shape-selective molecular sieve zeolite catalysts[J].Journal of the American Chemical Society,1979,101(22):6783-6784.
[2]Paul B Weisz.Molecular shape selective catalysis[J].Pure and Applied Chemistry,1980,52:2091-2103.
[3]Kaeding W W,Chu C,Young B,et al.Shape-selective reactions with zeolite catalysts Ⅱ.Selective disproportionation of toluene to produce benzene and p-xylene[J].Journal of Catalysis,1981,69:392-398.
[4]John A Dean.Lange’s Handbook of Chemistry[M].United States of America:McGraw-Hill Book Company,1967.
[5]Robert C Weast.Handbook of Chemistry and Physics[M].70th ed.London:Wolfe Medical Publications Ltd.,1989.
[6]Rozanska X,van Santen R A,Hutschka F,et al.A periodic DFT study of intramolecular isomerization reactions of toluene and xylenes catalyzed by acidic mordenite[J].Journal of the American Chemical Society,2001,123(31):7655-7667.
[7]康承琳,龙军,周震寰,等.二甲苯异构化的反应化学[J].石油学报(石油加工),2012,28(4):533-537.
Kang Chenglin,Long Jun,Zhou Zhenhuan,et al.Reaction chemistry of xylene isomerization[J].Acta Petrolei Sinica(Petroleum Processing Section),2012,28(4):533-537.
[8]李玲玲,聂小娃,宋春山,等.H-ZSM-5分子筛催化二甲苯异构化的反应机理[J].物理化学学报,2013,29(4):754-762.
Li Lingling,Nie Xiaowa,Song Chunshan,et al.Isomerization mechanism of xylene catalyzed by H-ZSM-5 molecular sieve[J].Acta Physico-Chimica Sinica,2013,29(4):754-762.
[9]李玉光,胡军.二甲苯在改性ZSM-5沸石上异构化动力学[J].化学物理学报,1996,9(4):339-344.
Li Yuguang,Hu Jun.Kinetics of xylene isomerization on modified ZSM-5 zeolites[J].Chinese Journal of Chemical Physics,1996,9(4):339-344.
[10]李增和,刘秀英,马丽景,等.HZSM-5催化剂上间二甲苯异构化反应动力学研究[J].北京化工大学学报,1997,24(4):67-70.
Li Zenghe,Liu Xiuying,Ma Lijing,et al.Reaction kinetics of m-xylene isomerization over zeolite ZSM-5[J].Journal of Beijing University of Chemical Technology,1997,24(4):67-70.
[11]陈金仙,张春,娄玥芸,等.HZSM-5分子筛上间二甲苯异构化反应动力学研究[J].南京工业大学学报(自然科学版),2012,34(3):66-69.
Chen Jinxian,Zhang Chun,Lou Yueyun,et al.Reaction kinetics simulation of m-xylene isomerization over HZSM-5 zeolite catalyst[J].Journal of Nanjing Universsity of Technology(Natural Science Edition),2012,34(3):66-69.
[12]Zhao Y,Wu H Y,Tan W,et al.Effect of metal modification of HZSM-5 on catalyst stability in the shape-selective methylation of toluene[J].Catalysis Today,2010,156(1/2):69-73.
[13]Zhao Y,Tan W,Wu H Y,et al.Effect of Pt on stability of nano-scale ZSM-5 catalyst for toluene alkylation with methanol into p-xylene[J].Catalysis Today,2011,160(1):179-183.
[14]Ahn J H,Kolvenbach R,Al-Khattaf S S,et al.Methanol usage in toluene methylation with medium and large pore zeolites[J].ACS Catalysis,2013,3:817-825.
[15]Lu P,Fei Z Y,Li L,et al.Effects of controlled SiO2deposition and phosphorus and nickel doping on surface acidity and diffusivity of medium and small sized HZSM-5 for para-selective alkylation of toluene by methanol[J].Applied Catalysis A:General,2013,453:302-309.
[16]Tan W,Liu M,Zhao Y,et al.Para-selective Methylation of toluene with methanol over nano-sized ZSM-5 catalysts:synergistic effects of surface modifications with SiO2,P2O5and MgO[J].Microporous and Mesoporous Materials,2014,196:18-30.
[17]Magdolna R M,Márton K,Szilvia K,et al.Transformation of ethylbenzene-m-xylene feed over MCM-22 zeoliteswith different acidities[J].Applied Catalysis A:General,2014,476:19-25.
[18]Zepeda T A,Pawelec B,Infantes-Molina A,et al.Ortho-xylene hydroisomerization under pressure on HMS-Ti mesoporous silica decorated with Ga2O3nanoparticles[J].Fuel,2015,158:405-415.
[19]Zepeda T A,Infantes-Molina A,Díaz de Leon J N,et al.Synthesis and characterization of Ga-modified Ti-HMS oxidematerials with varying Ga content[J].Journal of Molecular Catalysis A:Chemical,2015,397:26-35.
[20]Wu Y L,Li J C,Chai Y M,et al.Synergetic effect of H-ZSM-5/silicalite-1@Pt/Al2O3core-shell catalyst to enhance the selective hydrogenation of p-xylene[J].Journal of Membrane Science,2015,496:70-77.
[21]Ding W J,Li Hui,Pfeifer P,et al.Crystallite-pore network model of transport and reaction of multicomponent gas mixtures in polycrystalline microporous media[J].Chemical Engineering Journal,2014,254:545-558.
[22]Wu Q M,Wang X,Meng X J,et al.Organotemplate-free,seed-directed,and rapid synthesis of Al-rich zeolite MTT with improved catalytic performance in isomerization of m-xylene[J].Microporous and Mesoporous Materials,2014,186:106-112.
[23]Ahn J H,Kolvenbach R,Gutiérrez O Y,et al.Tailoring p-xylene selectivity in toluene methylation on medium pore-size zeolites[J].Microporous and Mesoporous Materials,2015,210:52-59.
[24]Gonalves J C,Rodrigues A E.Simulated moving bed reactor for p-xylene production:adsorbent and catalyst homogeneous mixture[J].Chemical Engineering Journal,2014,258:194-202.
[25]Zhang Y Y,Li Y F,Chen L,et al.A newcatalytic process for the synthesis of para-xylene through benzene methylation with CH3Br[J].Catalysis Communications,2014,54:6-10.
Calculation of thermodynamic equilibrium compositions of mixed xylenes
Zhao Yan*, Ren Dongmei, Xia Yunsheng, Li Xinhua, Bao Decai
(College of Chemistry and Chemical Engineering, Bohai University, Jinzhou 121013, Liaoning, China)
Abstract:The thermodynamic equilibriums of three components of mixed xylenes model under different temperatures were researched.Based on the characteristics of the state function,three thermodynamic functions (T) and (T) of three equilibriums components under different temperatures were calculated,respectively.And then the equilibrium compositions were calculated.The results showed that in the ideal gas model of mixed xylene,the change rules of the three equilibrium compositions with the increase of reaction temperatures from 298.15 K to 1 023.15 K were as follows:the y(MX)of m-xylene contents reduced gradually from 0.599 0 to 0.496 3 with the drop of 17.15%;the y(OX)of o-xylene contents enhanced gradually from 0.162 6 to 0.275 9 with the increase of 69.68%;the y(PX)of p-xylene contents increased slowly from 0.238 4 to 0.242 6,and then decreased gradually to 0.227 7.Its maximum of 0.242 6 at 423.15 K with the biggest drop of 6.14% was obtained,which indicated that increasing temperature was unfavorable to improving balance composition of p-xylene.
Key words:chemical thermodynamics; mixed xylenes; equilibrium composition; p-xylene; isomerization
收稿日期:2016-03-16
基金项目:渤海大学博士启动基金(BSQD201416,BSQD201417)资助项目;国家自然科学基金项目(21076026);辽宁省教育厅项目(L2013430)
作者简介:赵岩,1976年生,男,辽宁省锦州市人,博士,讲师,研究方向为多相催化。
doi:10.3969/j.issn.1008-1143.2016.05.015 10.3969/j.issn.1008-1143.2016.05.015
中图分类号:TQ241.1+3;O643.12
文献标识码:A
文章编号:1008-1143(2016)05-0075-06
通讯联系人:赵岩。

