两个2,4-二羟基苯甲醛缩甘氨酸配合物的合成、结构及其对醇的选择性氧化催化
李成娟 燕彩鑫 杨欣欣 任永和 薛泽春 崔传生 黄现强(山东省化学储能与新型电池技术重点实验室,聊城大学化学化工学院,聊城 252059)
两个2,4-二羟基苯甲醛缩甘氨酸配合物的合成、结构及其对醇的选择性氧化催化
李成娟燕彩鑫杨欣欣任永和薛泽春崔传生黄现强*
(山东省化学储能与新型电池技术重点实验室,聊城大学化学化工学院,聊城252059)
摘要:合成和表征了两个2,4-二羟基苯甲醛缩甘氨酸(H3L)席夫碱配合物[Cu(Py)2(HL)](1)和[Zn(Py)3(HL)]·2Py(Py=吡啶)(2),并通过X射线单晶衍射分析确定了其结构。配合物1通过分子间的O-H…O氢键形成了一维链状结构,配合物2通过分子间的O H…O和C-H…O氢键形成了二维网状结构。重要的是,配合物1在醇的选择性氧化反应中显示出了良好的催化效率(转化率高达94.8%,选择性高达98.3%)。
关键词:席夫碱配合物;晶体结构;醇氧化
0 Introduction
In the past decades,design and construction of transition metal coordination complexes and supramolecular architectures,are of mainly interest in view of their potential applications in fluorescence,chemical absorption,magnetism,electricalconductivityand molecular recognition[1-2].Now,lots of chemists from various disciplines have redirected their efforts to the field of catalytic reactions due to their vital role in ou modern society[3].Schiff base as a class of readily available,versatileimportantorganicintermediate
国家自然科学基金(No.21401094)、国家大学生科技创新基金(No.142081408,142081502)和聊城市科技发展计划(No.12014GJH01)资助项目。
*通信联系人。E-mail:hxqqxh2008@163.comwith excellent coordination capability and advantageous performance has received intensively attention in organic and inorganic chemistry[4-6].Their flexibility and efficiency are just two of the many reasons as to why these compounds are of great interest to the chemical community[7-9].Beyond exceptional coordination properties,Schiff bases also offer numerous opportunitiesforfine-tuninginacontrolledwayby choosing the constituent ligand and counter cations in a myriad of distinct structures,to enhance the solubility and stability of either homogeneous or heterogeneous catalysts[10-12],and many excellent results have been achieved[13-14].However,to our best knowledge,Schiff base complexes derived from 4-hydroxysalicylaldehyde and amino acid are very little[15],and the studies of catalytic properties of the Schiff base complexes are rather rare.
Herein,two novel 2,4-dihydroxybenzaldehydeglycine Schiff base copper(Ⅱ) and zinc(Ⅱ) complexes 1 and2havebeensynthesizedandstructurally characterized.In addition,the two complexes were further investigated as heterogeneous catalysts for the oxidative dehydrogenation of alcohols with hydrogen peroxide as an oxidant.
1 Experimental
1.1General methods and materials
All reagents and solvents for synthesis were purchased from commercial sources and used without further purification.The FT-IR spectra were recorded from KBr pellets in the range of 4 000~400 cm-1on Nicolet 170 SXFT/IR spectrometer.The GC analyses were performed on Shimadzu GC-2014C with a FID detector equipped with an Rtx-1701 Sil capillary column.The C,H,and N elemental analyses were conducted on Perkin-Elmer 240C elemental analyzer.
1.2Synthesis of complexes
Complex 1:2,4-Dihydroxybenzaldehyde(0.138 g,1 mmol),glycine(0.075 g,1 mmol)and NaOH(0.04g,1 mmol)were dissolved in MeOH(30 mL)and refluxed for 3 h.This solution was allowed to cool to room temperature.Copper chloride(0.17 g,1 mmol)were added.After stirring for another 2 h,a green solid was separated out and was filtered off and washed with methanol(5 mL),diethyl ether(5 mL),and then dried in air.The solid was dissolved with pyridine,and stirred for 30 min,filtered and left at room temperature.The blue crystals formed after a few days and were collected by filtration.Yield:64% (based on Cu).Anal.Calcd.(Found)for C19H17CuN3O4(%):C,55.00(55.17);H,4.13(4.25);N,10.13(10.09). IR spectrum(cm-1):3 070(m),2 618(m),1 631(s),1 594 (m),1 533(m),1 448(m),1 228(s),1 178(m),1 074(s),851(s),754(s),708(m).
Complex 2:The synthetic procedure was the same as for the complex 1,except that ZnCl2was used instead of CuCl2.Yield:72%(based on Zn).Anal. Calcd.(Found)for C34H31N6O4Zn(%):C,62.53(62.67);H,4.79(4.65);N,12.87(12.69).IR spectrum(cm-1): 3 069(m),2 887(m),2 696(m),1 622(s),1 569(m),1 528 (m),1 447(m),1 218(s),1 176(m),1 066(m),853(s),754(s),703(s).
1.3Procedure for the catalytic oxidation of aromatic alcohols
Aromatic alcohols(2 mmol),6.0%(molar fraction,the same below)catalystwere added to a 20 mL Schlenk Tube.And then 2 mL of 30%H2O2was added to the mixture.The reaction mixture was subsequently heated at 333 K in a Wattecs Parallel Reactor for 3 h with stirring.After the reaction was completed,the resulting mixture was analyzed by GC and the catalyst was retrieved by filtration,washed with CH2Cl2,and air-dried.
1.4X-ray crystallography
Single-crystal X-ray diffraction data for complexes 1 and 2 were conducted on a Bruker-AXS CCD diffractometer equipped with a graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at 296 K.All absorption corrections were applied using multi-scan technique.The structures were solved by the direct method and refined through full-matrix least-squares techniques method on F2using the SHELXTL 97 crystallographic software package[16-17].The hydrogen atoms of the organic ligands were refined as rigid groups.Crystallographic data for two complexes and selected bond distances,bond angles for the structures are listed in Table 1 and Table 2,respectively.
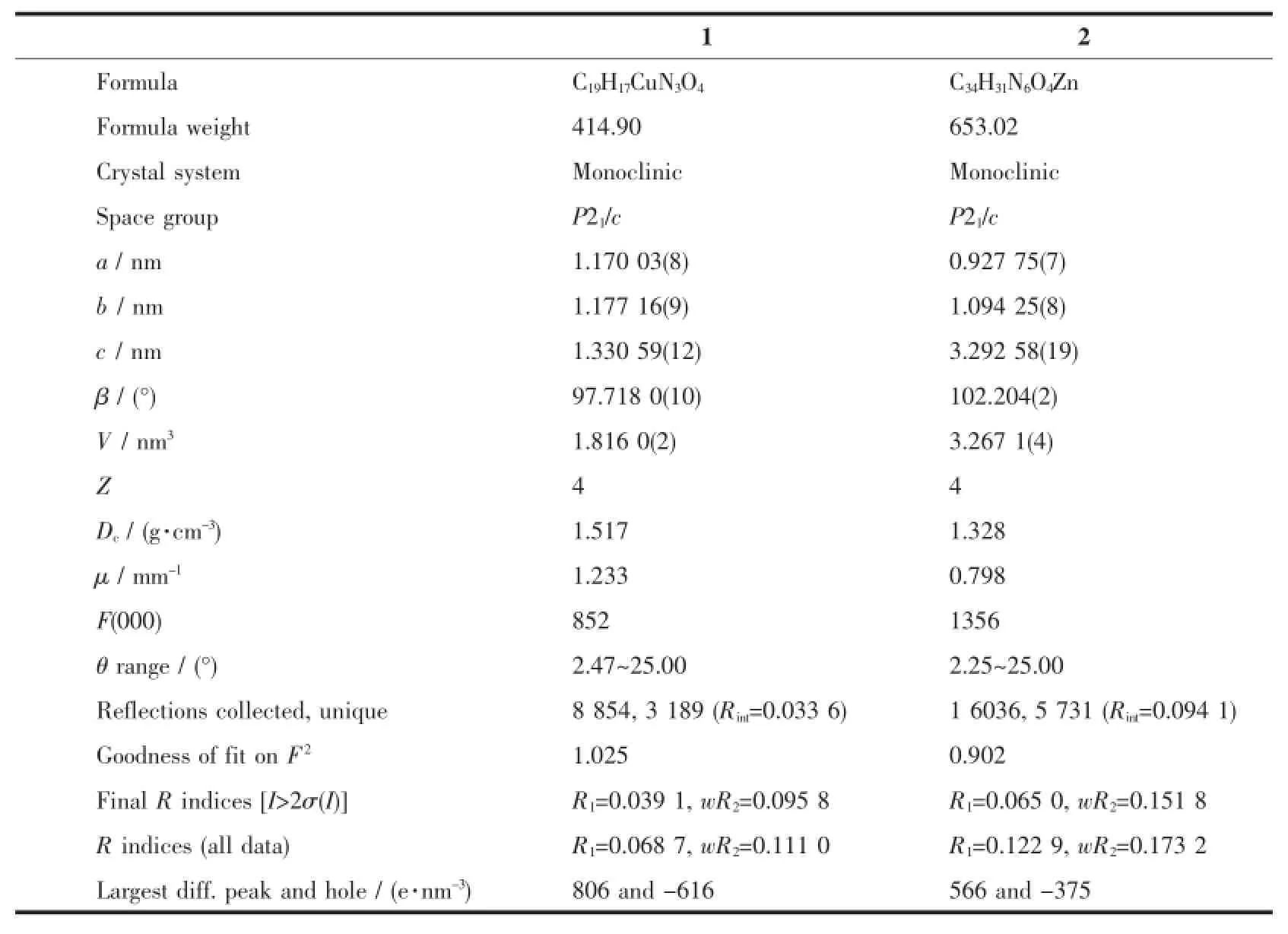
Table 1 Crystallographic data for complexes 1 and 2
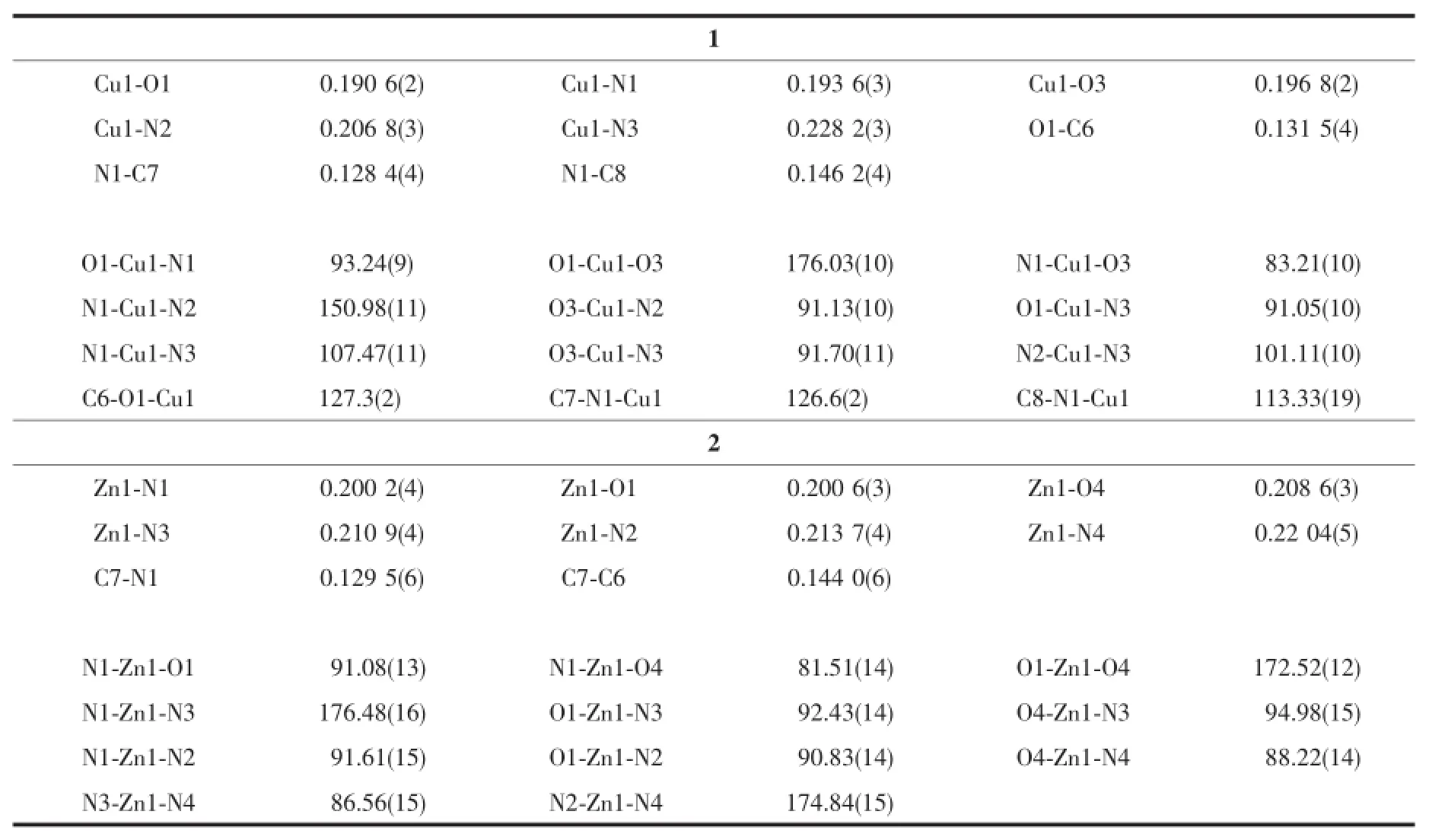
Table 2 Selected bond distances(nm)and bond angles(°)for the complexes 1 and 2
CCDC:1062408,1;1407540,2.
2 Results and discussion
2.1Structure description
The complex 1 crystallizes in the monoclinic system,space group P21/c.As depicted in Scheme 1 and Fig.1,the crystal structure of complex 1 consist of one discrete centrosymmetric copper(Ⅱ) atom with formula[Cu(Py)2(HL)].According to the previous work we define the geometric parameter τ=(β-α)/60 whichis applicable to five-coordinate structures such as that represented(Fig.2),as an index of the degree of trigonality,within the structural continuum between trigonal bipyramidal and rectangular pyramidal[18]. Thus,the copper atom in complex 1 is five-coordinated in the form of a slightly distorted square pyramidal geometry with τ=0.41.The basal plane of the square pyramid is occupied by the phenolic oxygen atom (O1),the carboxyl oxygen(O3),the nitrogen atom(N1)of the imine group and one terminal N atom(N2)of pyridine ligand.The corresponding bond lengths of Cu1-O1,Cu1-O3,Cu1-N1 and Cu1-N2 are 0.190 6(2),0.196 8(2),0.193 6(3)and 0.206 8(3)nm,respectively,which are in agreement with those of the previously reported copper complex with formula Cu(C9H7NO4)(Py)]2·2CH3OH(The bond distances of Cu-O,Cu-N range from 0.192 9(2)to 0.197 8(2)nm,0.193 3(2)to 0.201 5(2)nm,respectively)[19].It is noted that the phenolic oxygen atom and carboxyl oxygen atom are nearly linear with the copper(Ⅱ)moiety,and show bent coordination with the metal atom(the bond angles of O1 -Cu1-O3,N1-Cu1-O3 and O1-Cu1-N1 are 176.03(10)°,83.21(10)°and 93.24(9)°,respectively),which suggests that the organic substrate may attack the catalytic center from the side or the other of copper atom in the catalytic reaction.
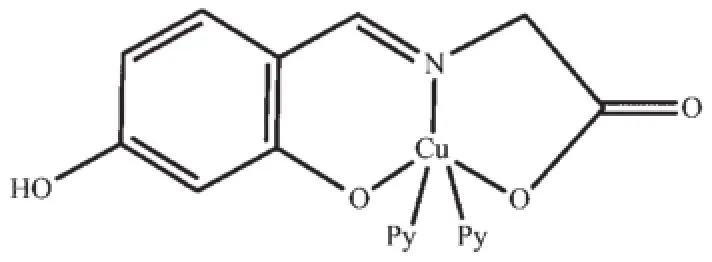
Scheme 1 Molecular structure of the complex 1
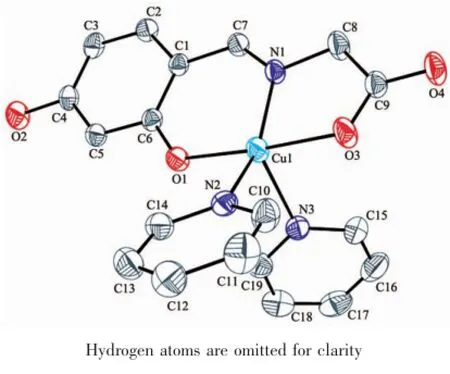
Fig.1 Crystal structure of the complex 1 with thermal ellipsoids at 30%probability

Fig.2 Pyramidal geometry of five-coordinate system
The supramolecular assembly of the complex 1 is determined by a intermolecular interaction of an O-H …O hydrogen bond(Table 3),and the discrete copper (Ⅱ) units of the complex 1 are further linked into a one-dimensional chain(Fig.3)by the O2-H2…O4ihydrogen-bonding interactions of the phenolic OH groups with the uncoordinated carboxyl O atoms of the adjacent molecules.

Fig.3 One-dimensional chain of complex 1

Table 3 Hydrogen-bonding geometry for the complexes 1 and 2
The crystal structure of complex 2 is depicted in Fig.4 and Scheme 2.Complex 2 consists of a discrete zinc(Ⅱ) centre,three coordinated pyridine molecules and two free pyridine molecules with formula[Zn(Py)3(HL)]·(Py)2.In contrast to 1,The Zn(Ⅱ) ion is in anoctahedralenvironmentcoordinatedtoone2,4-dihydroxybenzaldehydeglycineSchiffbaseanions acting as tridentate ligand through their adjacent carbonyl,imine and phenol oxygen atoms(the bond distances of Zn-O1,Zn-O4 and Zn-N1 are 0.200 6(3),0.208 6(3)and 0.200 1(4)nm,respectively).The other positions around the metal are occupied by three pyridine molecules(Zn-N bond distances are in the range of 0.210 9(4)~0.220 5(4)nm].O-Zn-O and NZn-N trans angles in the ZnN4O2 core are in the range of 172.51(12)°~176.49(16)°and cis O-Zn-O angles are within the 81.50(14)°~95.00(15)°interval. Thetwo-dimensionsupramolecularstructureof complex 2(Fig.5)is determined by an intermolecular interaction of O2-H2…O3iiand C16-H16…O3ihydrogen bonds(Table 3).
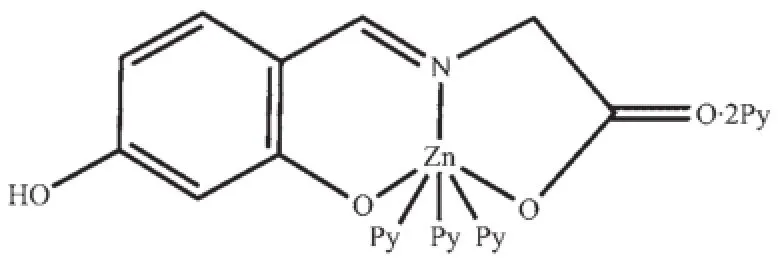
Scheme 2 Molecular structure of the complex 2
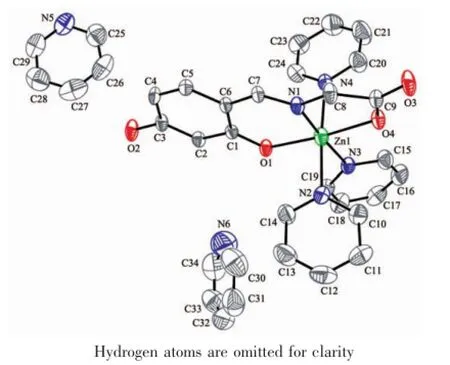
Fig.4 Crystal structure of the complex 2 with thermal ellipsoids at 30%probability
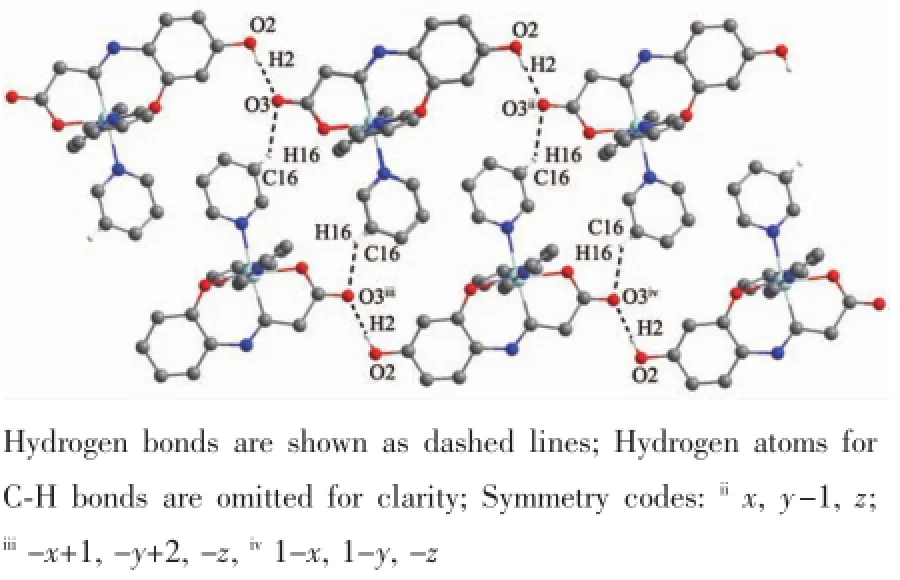
Fig.5 Two-dimensional supramolecular structure of 2
2.2Selective oxidation of alcohols catalyzed by complexes 1 and 2
The selective oxidation of alcohols to aldehyde is a practically important reaction for the production of chlorine-free aldehydes required in the perfumer andpharmaceuticalindustries,andconsequentl catalysts involved have received extensive attention[20-22]Usinghydrogenperoxideasanoxidantoffer significant environmental and economic benefits ove traditional stoichiometric oxidants[23-25].Our oxidation studies focused on the case of benzyl alcohol(Scheme 3),with the aim to understand the catalytic property o Schiff base complexes for developing a new oxidation system in organic chemistry.To optimize the produc conversion and selectivity,the oxidation of benzy alcoholtobenzaldehyde was selected as a model reaction to evaluate the catalytic activities of the differen catalysts in the presence of hydrogen peroxide.The influences of different reaction conditions(i.e.catalyst reaction temperature,reaction time,the amount o hydrogen peroxide and catalyst)about the oxidation o benzyl alcohol have been investigated.The conversion and selectivity of each reaction are summarized and illustrated in Fig.6.After the preliminary optimization the best conversion of benzaldehyde(90.5%)wa obtained during the oxida tion of benzyl alcohol(2 mmol),with catalyst 1 (6%)and 2 mL 30%hydrogen peroxide at 333 K for 3 h.

Scheme 3 Conversion of benzyl alcohol to benzaldehyde
The higher catalytic activity of the complex 1 i probably attributed to the coordination mode of coppe ionsandthecoordinationpyridinemoleculeso copper ions,which may leave enough space for the organic substrates and functions as potential active sites for oxidation through the departure of pyridine molecules.These preliminary results exhibit that the complex 1 can facilitate the oxidation of benzy alcohol and serve as highly efficient and selective catalyst.Andindeed,catalyticactivitiesofthecomplex 1 outperforms many effective catalysts reported to date,i.e.ZSM-5 zeolite catalysts[26],some oxovanadium(Ⅱ) complexes[27].
Furthermore,control catalytic experiments of the catalytic oxidation of benzyl alcohol were performed under the same reaction conditions.The conversion was 8.9%in the absence of the catalyst.When the Cu(OAc)2were used as catalysts,the conversions of benzyl alcohol were 35.8%.However,using the novel complex 1,the reaction can be finished in 3 h and the conversion reaches 90.5%,which overmatch the Cu(OAc)2and complex 2.Based on the abovementioned facts,we conclude that the introduce of Schiff base ligand into the framework bring new coordination environment for the copper ion center,which can enhance its oxidative capacity for it leaves potential coordination sites for the reactant.
As green catalyst,complex 1 was further used to explore the influence of catalyst recycles on the catalytic properties of oxidation of alcohols in a heterogeneous system.In the recycle experiments,the catalyst can easily be separated by filtration or centrifugation and washed using CH2Cl2.The IR spectra of the recovered complex 1 were identical to those of the freshly prepared complex 1(Fig.7).The experiment results displayed that no obvious loss of activity was observed after three runsas shown in Fig.8.

Fig.6 Conversion and selectivity of benzyl alcohol to benzaldehyde with different reaction conditions
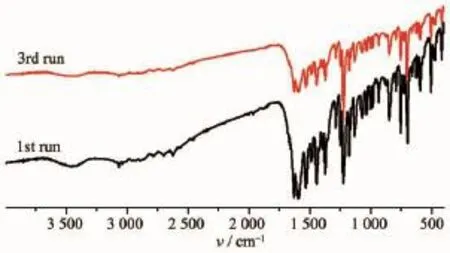
Fig.7 FT-IR spectra of comlex 1 after three runs of catalytic cycles
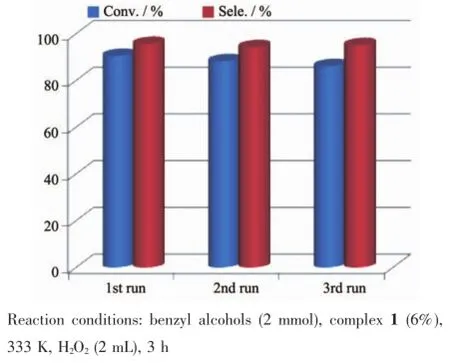
Fig.8 Recycle experiments of complex 1 on oxidation of benzyl alcohol
Following the success of the oxidation of benzyl alcohol and evaluating the scope and limitations of the current procedure,oxidation reactions with an array of aromatic alcohols were examined.A compilation of results for the oxidation of various additional primary and secondary alcohols,along with the corresponding aldehydes and ketones is presented in Table 4.It is noteworthy that,without any further optimization,the coppercomplex1exhibitsremarkablecatalytic activities for the oxidation of various aromatic alcohols to corresponding aldehydes or ketones with high conversion(90.5%~94.8%)and excellent selectivity (93.5%~98.3%).These preliminary results exhibit that the complex 1 can facilitate the oxidation of aromatic alcohols and serve as a highly efficient and selective catalyst.
Basedonthecomparablestudiesreported previously[28-31],a plausible catalytic mechanism of theoxidation of benzyl alcohol can be proposed and is shown in Fig.9.Specifically,the catalytic cycle begins by the formation of an anticipated perhydroxy-Cu complex(B)in the presence of benzyl alcohol and H2O2[32].B further reacts with benzyl alcohol and affords the copper-alcoholate intermediate C[33],and the alcoholate species undergoes typical β-elimination to afford the corresponding carbonyl compound,and then reacts with one pyridine molecule to form A again[34].

Table 4 Results of oxidation of aromatic alcohols catalyzed by the complex 1a
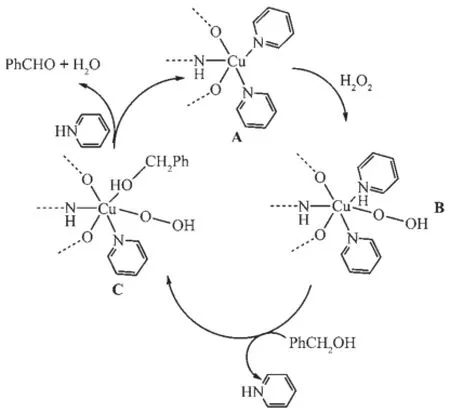
Fig.9 Plausible catalytic mechanism of oxidation of benzyl alcohol
3 Conclusions
In summary,two Schiff base complexes have been successfully synthesized and it is found that the Schiff base copper complex can used as highl efficient and selective oxidation catalysts of aromatic alcohol with high conversion(90.5%~94.8%)and excellent selectivity(93.5%~98.3%).The use of Schif base complexes in other organic reactions is in process,which will be reported timely.
References:
[1]Guo P,Dutta D,Wong-Foy A G,et al.J.Am.Chem.Soc. 2015,137:2651-2657
[2]Manna P,Das S K.Cryst.Growth Des.,2015,15:1407-1421
[3]Kakati N,Maiti J,Lee S H,et al.Chem.Rev.,2014,114 12397-12429
[4]Chang M C,Lu W Y,Chang H Y,et al.Inorg.Chem.,2015 54:11292-11298
[5]Wang W M,Zhang H X,Wang S Y,et al.Inorg.Chem. 2015,54:10610-10622
[6]Tabthong S,Nanok T,Sumrit P,et al.Macromolecules,2015 48:6846-6861
[7]Bassolino G,Sovdat T,Duarte A S,et al.J.Am.Chem.Soc.2015,137:12434-12437
[8]Huang N,Zhang S,Yang L,et al.ACS Appl.Mater.Interfaces,2015,7:17935-17946
[9]Gao F,Yang F L,Zhu G Z,et al.Dalton Trans.,2015,44: 20232-20241
[10]Kong W L,Wang Z X.Dalton Trans.,2014,43:9126-9135
[11]Liu Q,Yang X,Huang Y,et al.Energy Environ.Sci.,2015,8:3204-3207
[12]Hazra S,Martins L M D R S,Fátima M,et al.RSC Adv.,2015,5:90079-90088
[13]Leenders S H A M,Gramage-Doria R,Bruin B,et al.Chem. Soc.Rev.,2015,44:433-448
[14]Buchwald J R,Kal S,Dinolfo P H.J.Phys.Chem.C,2014,118:25869-25877
[15]Lu Z,Zhang D,Gao S,et al.Inorg.Chem.Commun.,2005,8:746-750
[16]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,Göttingen University,Germany,1997.
[17]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,Göttingen University,Germany,1997.
[18]Addison A W,Rao T N,Reedijk J,et al.J.Chem.Soc. Dalton Trans.,1984,7:1349-1356
[19]Gou Y,Yu M,Li Y.Inorg.Chim.Acta,2013,404:224-229
[20]Lenoir D.Angew.Chem.,Int.Ed.,2006,45:3206-3210
[21]Guo Z,Liu B,Zhang Q,et al.Chem.Soc.Rev.,2014,43: 3480-3524
[22]Ryland B L,Stahl S S.Angew.Chem.Int.Ed.,2014,53: 8824-8838
[23]Richardson R D,Wirth T.Angew.Chem.Int.Ed.,2006,45: 4402-4404
[24]Uyanik M,Ishihara K.Chem.Commun.,2009,16:2086-2099
[25]Uyanik M,Akakura M,Ishihara K.J.Am.Chem.Soc.,2009,131:251-262
[26]Jia A,Lou L L,Zhang C,et al.J.Mol.Catal.A:Chem.,2009,306:123-129
[27]Parihar S,Jadeja R N,Gupta V K.RSC Adv.,2014,4:10295 -10302
[28]Bansal V K,Thankachan P P,Prasad R.Appl.Catal.A,2010,381:8-17
[29]Kato C N,Hasegawa M,Sato T,et al.J.Catal.,2005,230: 226-236
[30]Yamaguchi K,Mizuno N.Angew.Chem.Int.Ed.,2002,41: 4538-4542
[31]Klinman J P.Chem.Rev.,1996,96:2541-2561
[32]Ahmad J U,Räisänen M T,Leskel M,et al.Appl.Catal.A: Gen.,2012,411-412:180-187
[33]Corma A,Esteve P,Martinez A.Appl.Catal.A,1996,143: 87-100
[34]Chen B,Huang X,Wang B,et al.Chem.Eur.J.,2013,19: 4408-4413
中图分类号:O614.121
文献标识码:A
文章编号:1001-4861(2016)05-0891-08
DOI:10.11862/CJIC.2016.101
收稿日期:2016-01-06。收修改稿日期:2016-03-06。
Two 2,4-Dihydroxybenzaldehydeglycine Schiff Base Complexes:Syntheses,Structures and Selective Oxidation Catalytic Properties for Alcohols
LI Cheng-JuanYAN Cai-XinYANG Xin-XinREN Yong-He XUE Ze-ChunCUI Chuan-ShengHUANG Xian-Qiang*
(Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology,School of Chemistry&Chemical Engineering,Liaocheng University,Liaocheng,Shandong 252059,China)
Abstract:Two 2,4-dihydroxybenzaldehydeglycine(H3L)Schiff base complexes:[Cu(Py)2(HL)](1)and[Zn(Py)3(HL)·2Py(2)(Py=pyridine)have been synthesized and structurally characterized.The single crystal X-ray diffraction analysis reveals that the copper center in complex 1 is five-coordinated in a(O,N)fashion of a distorted square pyramidal geometry,while the zinc center in complex 2 possesses a normal octahedral geometry.Importantly complex 1 exhibit excellent heterogeneous catalytic performance(Conversion:up to 94.8%;Selectivity:up to 98.3% in the oxidation of aromatic alcohols using hydrogen peroxide as the oxidant.CCDC:1062408,1;1407540,2.
Keywords:Schiff base complexes;crystal structure;oxidation of alcohols

