小麦籽粒游离多胺对土壤干旱的响应及其与籽粒灌浆的关系
张伟杨 徐云姬 钱希 旸 李银银 王志琴 杨建昌扬州大学江苏省作物遗传生理重点实验室 / 粮食作物现代产业技术协同创新中心,江苏扬州 225009
小麦籽粒游离多胺对土壤干旱的响应及其与籽粒灌浆的关系
张伟杨 徐云姬 钱希旸 李银银 王志琴 杨建昌*
扬州大学江苏省作物遗传生理重点实验室 / 粮食作物现代产业技术协同创新中心,江苏扬州 225009
摘 要:为探明干旱胁迫下小麦内源游离多胺在籽粒灌浆过程中的作用,2013—2014和2014—2015年度选用高产品种扬麦16和宁麦13进行不同水分条件的盆栽试验。自分蘖末期至成熟期设置正常供水(WW)、土壤轻度干旱(MD)和土壤重度干旱(SD) 3种处理,观察不同土壤水分对籽粒中游离多胺和籽粒灌浆的影响。2个品种的结果一致表明,与WW相比,MD处理对叶片水势及光合作用没有显著影响,显著增加弱势粒灌浆速率(12.5%)和粒重(11.8%),对强势粒灌浆无显著影响;SD处理则严重抑制叶片光合作用,显著降低叶片水势,强势粒的灌浆速率和粒重分别下降10.1%和9.5%,弱势粒的灌浆速率和粒重分别下降14.5%和11.7%。MD处理显著提高了灌浆期弱势粒中游离亚精胺(Spd)和精胺(Spm)含量及其与腐胺(Put)的比值,而SD处理的结果则相反。籽粒灌浆速率、粒重与籽粒中Spd和Spm含量及Spd/Put和Spm/Put值呈极显著正相关,与Put含量呈极显著负相关。喷施Spd和Spm,显著增加3个处理弱势粒及SD处理强势粒的灌浆速率(11.2%~25.9%)和粒重(9.9%~17.7%),但对WW和MD处理的强势粒无显著影响;喷施Spd和Spm合成抑制剂[甲基乙二醛-双脒基腙(MGBG)]后,3个处理强、弱势粒的灌浆速率和粒重均显著降低,分别下降 20.5%~28.8%和 16.9%~28.5%。表明小麦籽粒中多胺对土壤水分的响应因土壤干旱程度而异,通过轻度土壤干旱处理增加籽粒中Spd和Spm含量以及Spd/Put和Spm/Put值,可以促进籽粒灌浆,增加粒重。
关键词:小麦;土壤干旱;多胺;籽粒灌浆;粒重
本研究由国家自然科学基金项目(31271641,31471438),中央级科研院所基本科研业务费(农业)专项(201103003,201203079),国家科技支撑计划项目(2011BAD16B14,2012BAD04B08,2013BAD07B09,2014AA10AS605),江苏省农业三新工程项目(SXG2014313)和江苏高校优势学科建设工程专项资助。
This study was supported by the National Natural Science Foundation of China (31271641,31471438),China National Public Welfare Industry (Agriculture) Plan (201103003,201203079),the National Key Technologies R&D Program of China (2011BAD16B14,2012BAD04B08,2013BAD07B09,2014AA10AS605),Jiangsu “Three-innovation” Agricultural Project (SXG2014313),and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
第一作者联系方式∶ E-mail∶ wyzhangyzu1990@163.com
URL∶ http∶//www.cnki.net/kcms/detail/11.1809.S.20160328.1116.006.html
小麦等禾谷类作物的生长发育极易遭受极端温度、干旱以及营养缺乏等不良环境因素的影响,其中土壤干旱是限制作物产量的最主要逆境因子之一[1-2]。小麦生长发育过程中土壤水分不足会引起小麦植株体内生理代谢的紊乱、光合性能降低,抑制小麦的正常发育,使小麦产量低而不稳,从而限制其产量潜力的发挥[3-5]。因此,研究不同灌水量及灌溉方式对小麦生理特性及产量的影响,对制定科学合理的灌溉制度,提高小麦水分生产效率具有重要意义。关于土壤干旱对小麦产量影响的报道较多,有学者指出,灌浆期适度的土壤落干或轻度土壤干旱处理能显著加快光合产物向籽粒的转移,提高灌浆速率和最终粒重[6-9]。然而,对于这种适度土壤干旱处理促进籽粒灌浆的生理基础,目前仍不清楚。
植物内源激素调控许多生理过程,如细胞分裂、形态发生、胚胎发生、生长发育、种子形成、衰老以及对环境压力的响应等[10-12]。小麦籽粒的形态建成、灌浆充实及最终产量都受到内源激素的调控。多胺作为一种新型植物激素,是一种具有强烈生物活性的物质,广泛存在于植物体内,在植物的生长发育、形态建成以及对环境胁迫的响应过程中发挥重要的调控作用,其中最常见的类型是腐胺(Put)、亚精胺(Spd)和精胺(Spm)[13-16]。多胺能够调节细胞膜的稳定性,在干旱胁迫条件下,使细胞受到的伤害最小化[17-18]。研究表明,高含量的 Spd和Spm 与玉米籽粒的形成和发育呈极显著正相关[19],能够极显著地促进水稻籽粒的灌浆[20];小麦体内的多胺含量与其抗旱性密切相关,较高的 Spd和Spm含量能够拮抗干旱对小麦生长的抑制作用[21-22];喷施Spm或Spd显著促进冬小麦籽粒灌浆,而喷施Put对冬小麦籽粒灌浆无显著影响[23]。然而,在不同土壤干旱处理下,小麦籽粒中游离多胺的变化特点及其与籽粒灌浆的关系少有研究。本试验比较了 3种土壤水分条件下,小麦分蘖末期至成熟籽粒中游离多胺含量的变化及其与籽粒灌浆的关系,进一步明确小麦产量形成对土壤水分的响应规律,为小麦节水高产栽培提供理论依据。
1 材料与方法
1.1 试验概况
2013—2014和2014—2015小麦生长季,选用当地大面积应用的高产小麦品种扬麦16和宁麦13,在扬州大学江苏省作物遗传生理重点实验室试验农牧场进行盆栽试验。盆钵容积14.72 L (高30 cm,直径25 cm),填装过筛沙壤土13 kg。填装土取自大田表层,含有机质2.02%、有效氮为105.0 mg kg-1、速效磷34.2 mg kg-1、速效钾68.0 mg kg-1。按照高产栽培进行肥料运筹,全生育期施用尿素折合纯氮 180 kg hm-2(折合纯氮0.84 g pot-1),基肥(播种前1 d)、壮蘖肥(5叶时)、拔节肥(叶龄余数2.5)、孕穗肥(叶龄余数1.2)的比例为5∶1∶2∶2。播种前一次性施过磷酸钙(含P2O513.5%),折合纯磷90 kg hm-2(折合纯磷0.42 g pot-1)和氯化钾(含K2O 52%),折合纯钾90 kg hm-2(折合纯钾0.42 g pot-1)。全生育期严格控制病虫草害。
两年度播种期均为10月29日,每品种270盆,每盆播25粒,三叶期定苗,每盆留8苗。扬麦16和宁麦13的开花日期分别为2014年4月9日和4月11日以及2015年4月10日和4月13日。
1.2 水分胁迫处理
自分蘖末期至成熟,设置供水充足(WW,土壤水势-20 ~ -30 kPa)、轻度土壤干旱(MD,土壤水势为-40 ~ -50 kPa)和重度土壤干旱(SD,土壤水势为-60 ~ -70 kPa),每处理90盆;3个处理的含水量分别相当于 0~20 cm 土层田间最大持水量的 80%~85%、60%~65%和45%~50%。下雨时用可移动式塑料大棚挡雨。每天6∶00—7∶00、12∶00—13∶00、17∶00—18∶00时记录负压计读数,当读数达到设计阈值时,WW、MD和SD处理每盆分别浇水0.4、0.3和0.2 L。
根据在预备试验中土壤干旱对小麦产量有无显著影响作为划分轻度和重度干旱的标准。与供水充足相比,如果土壤干旱对产量无显著影响甚至还有所提高,这种干旱定义为轻度干旱或适度干旱;如果土壤干旱显著降低了产量,这种干旱定义为重度干旱。在盆钵内安装真空表式负压计(中国科学院南京土壤研究所生产)监测土壤水势,负压计陶土头埋设离土表15~20 cm。用土壤水势作为指标可以克服土壤类型的差异。
1.3 籽粒灌浆动态测定
于开花期选择同一日开花、长势一致的穗子挂牌,标记开花日期,每处理标记400穗。根据小穗在花序轴上着生次序不同,把同一穗上的籽粒分为强势粒和弱势粒。从穗基部向上数第4至第12小穗(中部小穗),取其第 1、第 2位籽粒为强势粒。上部和下部小穗上若第 3、第 4位籽粒能正常形成则取其作为弱势粒,若无3、4位籽粒则取第1、第2位籽粒作为弱势粒。开花后的第6、第12、第18、第24、第30、第36、第42天分7次取样,每次取挂牌单穗 20~30个,按强、弱势粒分样品。同类籽粒样品一部分用于测定多胺,一部分烘干后称重。参照朱庆森等[24]描述的方法分析强、弱势粒的灌浆动态,用Richards方程[25]拟合。

式中,W为籽粒重量(mg),A为最大粒重,t为开花后的时间(d),B、k和N为回归方程所确定的参数。对方程(1)求导,得灌浆速率F。
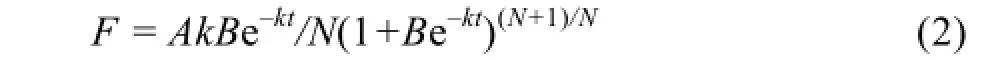
定义活跃灌浆期D (d)为籽粒粒重(W)由最终粒重A的5% (t1)增加到95% (t2)所经历的时间(t2- t1),这段时间内麦粒增加的重量除以灌浆活跃期(t2- t1)为平均籽粒灌浆速率Fmean。
1.4 旗叶水势及光合速率的测定
花后第 21天和第 23天(灌浆中期),于6∶00—18∶00采用压力室法(Model 3000,Soil Moisture Equipment Corp,Santa Barbara,CA,USA)每隔2 h测定一次旗叶水势,取2次测定的平均值作为一个观测值。
开花后第 6、12、18、24、30、36、42天,于晴天 9∶00,采用 LI-6400光合测定仪(Li-Cor,USA)测定旗叶的光合速率,每处理重复10次。
1.5 多胺含量的测定
预备试验发现小麦灌浆期籽粒中结合型多胺含量很低,且在干旱与供水处理间无显著差异,其中结合型Put含量为180~230 nmol g-1DW,结合型Spd和结合型Spm含量仅为61~66 nmol g-1DW和35~42 nmol g-1DW。因此,本研究参照Flores和Galston[26]的方法提取游离多胺,苯甲酰化[27]后用高效液相色谱仪测定游离多胺含量。用10 μL甲醇(60%,v/v)溶解样品,色谱柱为C18反相柱(4.6 mm × 250.0 mm,5 μm,流速0.6 mL min-1),进样体积为20 μL,柱温为25℃,检测器为Perkin-Elmer LC-95,吸收峰波长为254 nm。以1,6-已二胺为内标,重复测定4次,取平均值,多胺含量单位为nmol g-1DW。
1.6 化学调控处理
从开花结束后的第 2天开始连续 4 d,每天16∶00—17∶00时,对麦穗分别喷施2 mmol L-1Put、1 mmol L-1Spd、1 mmol L-1Spm和5 mmol L-1MGBG,每盆喷施20 mL。MGBG即甲基已二醛-双(脒基腙),是Spd和Spm合成抑制剂。喷施液中含0.1%乙醇和0.01% (v/v) Tween-20作为展开剂,对照为喷施等量清水(含有相同浓度的展开剂)。3个处理每品种每种激素喷施5盆,重复3次。在喷施调控剂之前,用塑料膜遮盖叶片,以防止激素喷在叶片上。于花后第18、21、24天(灌浆中期)取样,测定籽粒中游离多胺含量,取 3次重复平均值。籽粒内源多胺含量和籽粒灌浆速率测定同上述。
1.7 数据处理
采用Microsoft Excel 2003、SPSS 16.0和SAS (Version 6.12;SAS Institute,Cary,NC,USA)软件处理和分析数据,用SigmaPlot 10.0绘图。
2 结果与分析
2.1 不同土壤水分处理的叶片水势和光合速率
不论何种处理的叶片水势在中午 12∶00时之前都不断下降,12∶00—14∶00期间处在最低状态,此后又逐渐回升,2个品种的结果趋势表现一致(图1)。与WW相比,MD和SD处理都显著加快了中午旗叶水势的下降,MD处理的叶片水势最低值大于-1.5 MPa,SD处理下的叶片水势显著小于-1.5 MPa。中午叶片水势在-1.5 MPa被认为是植物在灌浆期遭受干旱胁迫的临界值[28]。早晨(6∶00时),SD处理下的叶片水势显著低于对照WW,MD处理则与WW无显著差异。表明 MD处理未对植株造成伤害,其水分状况在夜间可以恢复到正常水平,而 SD处理的叶片水势则不能恢复。
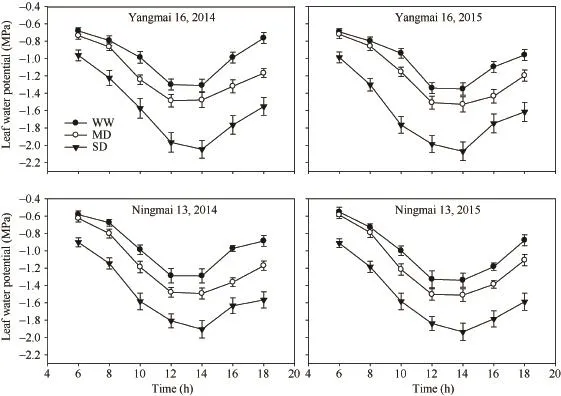
图1 不同干旱处理下小麦旗叶水势变化Fig. 1 Changes in water potential of wheat flag leaf under different drought treatments
随生育进程,叶片光合速率不断降低。MD处理的光合速率与WW处理无显著差异,而SD处理极显著地降低光合速率(图2)。表明MD处理叶片光合作用未受到明显抑制,SD处理则显著抑制了叶片光合作用。
2.2 不同土壤水分处理的粒重及籽粒灌浆速率
籽粒灌浆速率随灌浆进程呈先增大后降低的单峰变化趋势,强势粒在花后18 d左右达到峰值,弱势粒在花后24 d左右达到峰值,此后急剧下降。强势粒粒重及灌浆速率MD与WW间没有差异,SD处理显著降低了强、弱势粒粒重、最大灌浆速率和平均灌浆速率。2个小麦品种结果趋势一致(表1和图3)。
2.3 不同土壤水分处理的籽粒游离多胺含量
与籽粒灌浆速率变化类似,游离 Put、Spd和Spm含量以及 Spd/Put和Spm/Put比值在灌浆前期不断增加,强势粒在花后第18天前后达到峰值,弱势粒在花后第24天前后达到峰值,此后急剧下降。在灌浆前期,强势粒游离多胺含量大于弱势粒,在灌浆后期则相反,弱势粒的 Put峰值显著高于强势粒,而强势粒的Spd、Spm含量及Spd/Put和Spm/Put比值的峰值明显高于弱势粒(图4和图5)。与WW处理相比,MD处理显著增加了弱势粒中Spd和Spm含量以及Spd/Put和Spm/Put比值,而对强势粒中这4项指标没有显著影响;但SD处理却造成强、弱势粒中Spd、Spm含量及Spd/Put和Spm/Put比值的显著降低(图4和图5)。
在籽粒灌浆过程中,无论MD或SD处理,均显著增加弱势粒中游离Put的积累,且SD处理比MD的增幅大。SD处理下亦显著增加了强势粒的游离Put含量,MD处理对强势粒的Put含量无显著影响(图4-A-D)。
2.4 籽粒多胺含量与籽粒灌浆的关系
相关分析表明,2个供试小麦品种在活跃灌浆期内籽粒中游离多胺含量与其平均灌浆速率、最大灌浆速率以及最终粒重密切相关。籽粒中游离Spd、Spm含量以及Spd/Put和Spm/Put值与平均灌浆速率、最大灌浆速率以及最终粒重呈极显著正相关(r = 0.769~0.878,P < 0.01);游离Put含量与平均灌浆速率、最大灌浆速率以及最终粒重呈极显著负相关(r = -0.673~-0.714,P < 0.01)。可见,弱势粒中较低的游离Spd、Spm含量以及Spd/Put和Spm/Put值是导致其灌浆差、粒重低的重要生理原因(表2)。
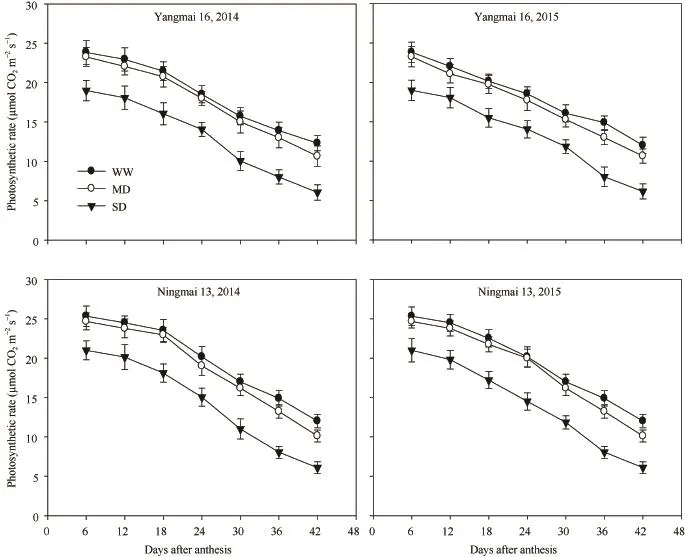
图2 不同干旱处理下小麦品种旗叶光合速率变化Fig. 2 Changes in photosynthetic rate of wheat flag leaf under different drought treatments
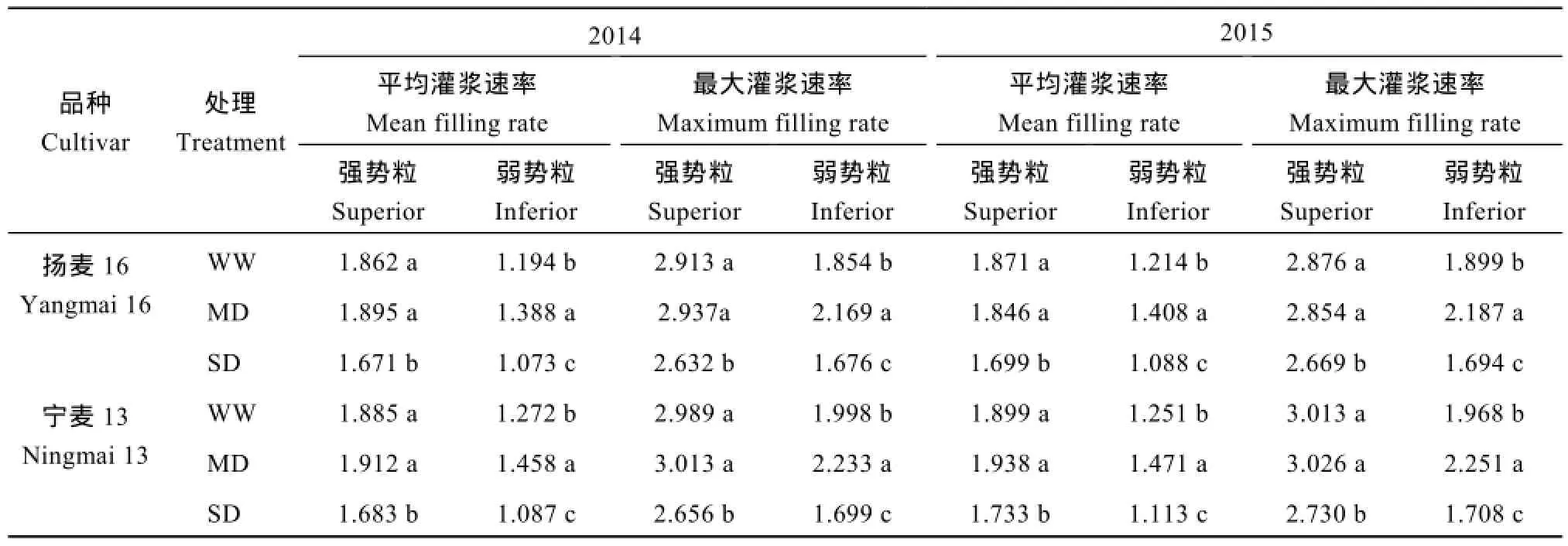
表1 土壤水分对小麦强、弱势粒平均灌浆速率和最大灌浆速率的影响Table 1 Effects of soil moisture on mean and maximum grain-filling rates of superior and inferior grains of wheat (mg grain-1d-1)
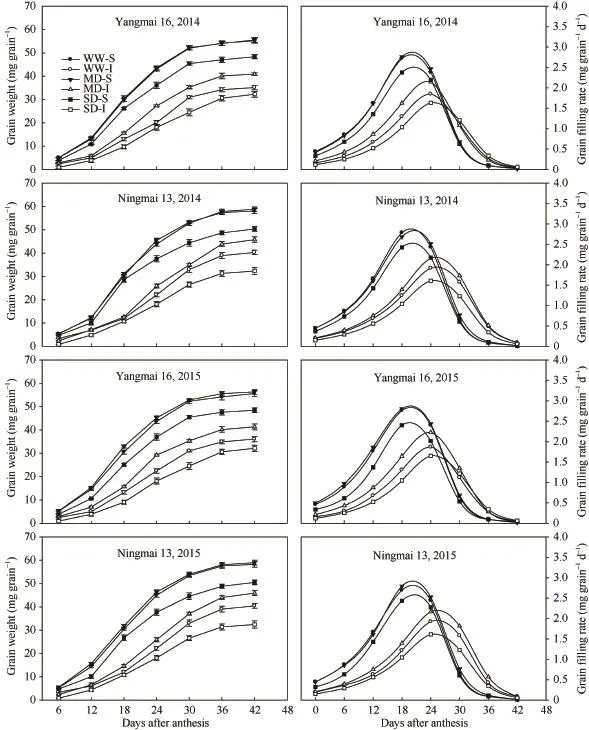
图3 不同干旱处理小麦籽粒增重与灌浆速率的变化Fig. 3 Changes in grain weight and grain-filling rate of wheat under different drought treatments
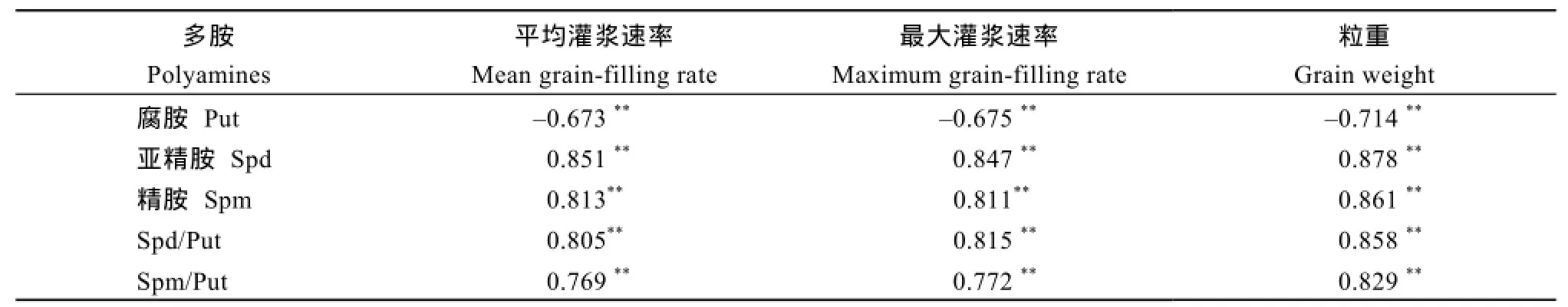
表2 活跃灌浆期籽粒多胺含量与灌浆速率和粒重的相关性Table 2 Correlations between polyamines concentrations in grains and the filling rate and final weight of grains during active filling period
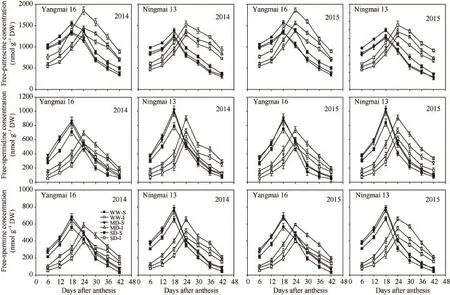
图4 不同干旱处理下小麦强、弱势粒的游离多胺含量的变化Fig. 4 Changes in free-polyamine concentrations in superior and inferior grains of wheat under different drought treatments
2.5 化学调控对内源多胺和籽粒灌浆速率的影响
2个小麦品种的测定结果一致显示,喷施Put、Spd和Spm显著增加了3个处理弱势粒中相对应的内源多胺含量;WW和MD处理相比,强势粒中的游离多胺含量没有显著影响;而SD较WW处理显著提高了强势粒中相对应的游离多胺含量(表 3和表4)。喷施外源Put显著降低3个处理弱势粒以及SD处理强势粒中的 Spd/Put和 Spm/Put值;喷施外源Spd或Spm则显著提高3个理弱势粒以及SD处理强势粒中的Spd/Put和Spm/Put值。外源多胺对WW 和MD处理强势粒中的游离多胺比例影响不显著。
喷施Spd和Spm抑制剂MGBG后,3个处理2种类型籽粒中的Put含量均显著升高,而Spd和Spm含量及Spd/Put和Spm/Put值显著降低,两品种结果趋势一致(表3和表4)。
喷施Put显著降低了3个处理弱势粒及SD处理强势粒的平均灌浆速率、最大灌浆速率和粒重,喷施Spd和Spm的结果则相反。喷施MGBG后,3个处理强、弱势粒的平均灌浆速率、最大灌浆速率和粒重均比对照显著降低。喷施 Put、Spd、Spm对 WW和MD处理下强势粒平均灌浆速率、最大灌浆速率和粒重影响很小,与未喷施多胺的对照差异不显著(表5)。2个小麦品种结果趋势一致。
3 讨论
对内源多胺调节植物生长发育、形态建成和对环境逆境响应已有不少报道[29-31],但小麦内源游离多胺含量对土壤水分的响应及其与强、弱势粒籽粒灌浆及粒重关系却很少研究。本试验设计 3种水分条件,发现与WW处理相比,MD处理显著增加籽粒灌浆速率和粒重,SD处理则相反。在不同土壤水分条件下籽粒灌浆速率和粒重的增加或降低与籽粒中Spd及Spm含量的增加或降低密切相关;在灌浆初期喷施Spd或Spm可以显著增加3个处理下弱势粒中Spd或Spm含量,弱势粒灌浆速率和粒重则显著增加,而对WW和MD处理的强势粒没有显著影响;对SD处理喷施Spd或Spm后,其强势粒和弱势粒中Spd或Spm含量、灌浆速率和粒重均显著增加。喷施Spd和Spm合成抑制物质MGBG,显著降低了3个处理籽粒中Spd或Spm含量,强、弱势粒灌浆速率和粒重则均显著降低。说明游离多胺,特别是Spd和Spm,对小麦籽粒灌浆有重要调控作用。MD处理或SD处理通过调控籽粒中 Spd 和Spm合成,实现对籽粒灌浆和粒重的调控,同时也验证了小麦体内的多胺含量水平与其抗旱性密切相关,较高的Spd和Spm含量能够拮抗干旱对小麦生长的抑制作用[22]。
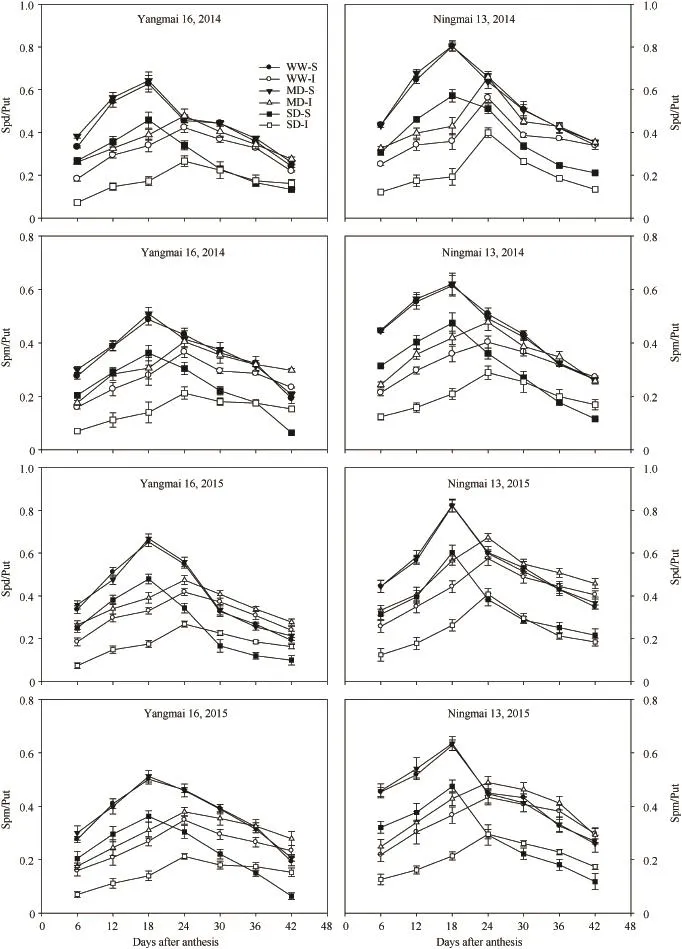
图5 不同干旱处理下小麦强、弱势粒的Spd/Put和Spm/Put比值的变化Fig. 5 Changes in ratios of Spd/Put and Spm/Put in superior and inferior grains of wheat under different drought treatments
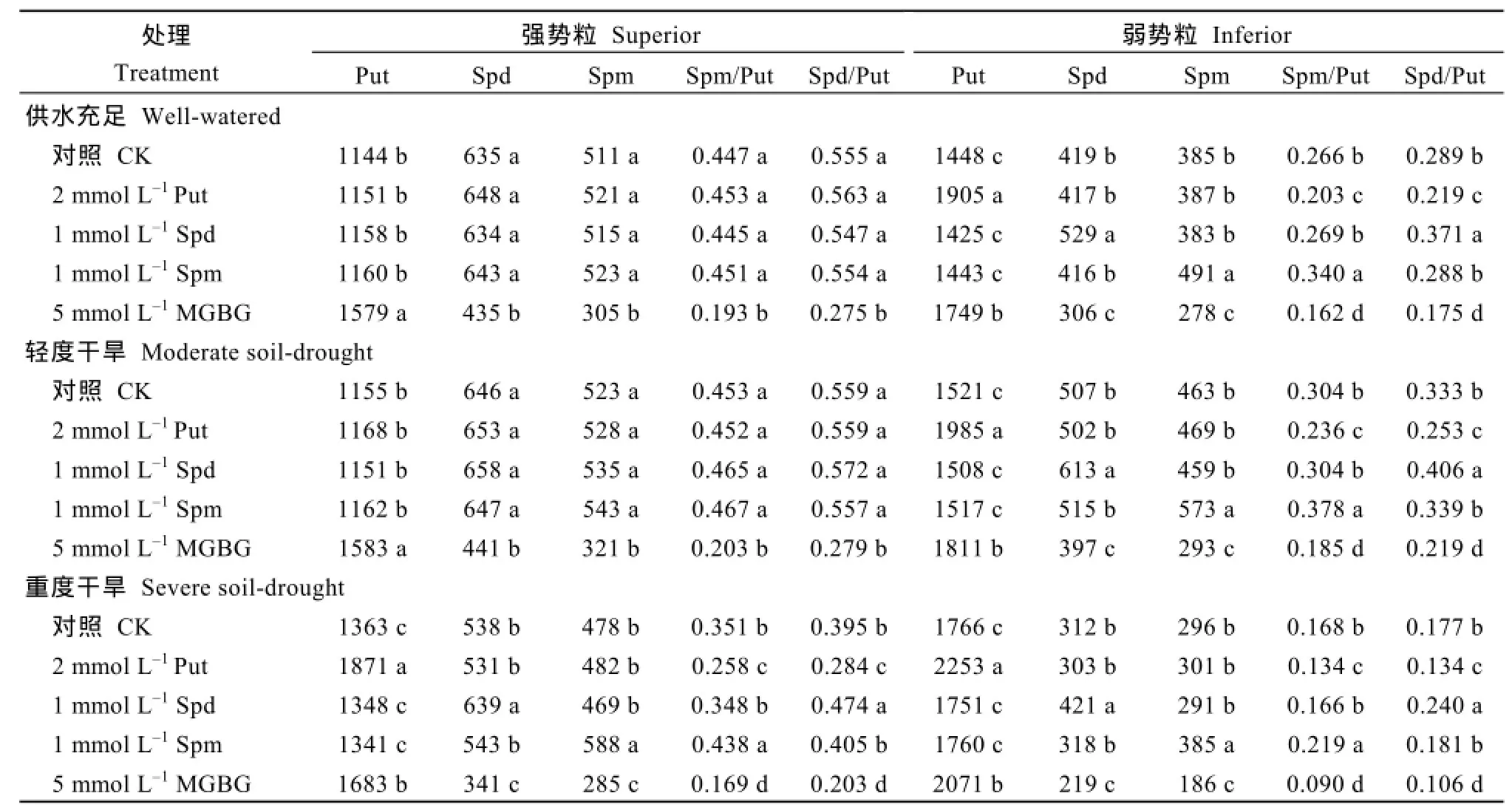
表3 外源多胺及其抑制剂对扬麦16强、弱势粒中Put、Spd和Spm含量的影响Table 3 Effects of exogenous PAs and MGBG on Put,Spd,and Spm concentrations in superior and inferior grains of Yangmai 16(nmol g-1DW)
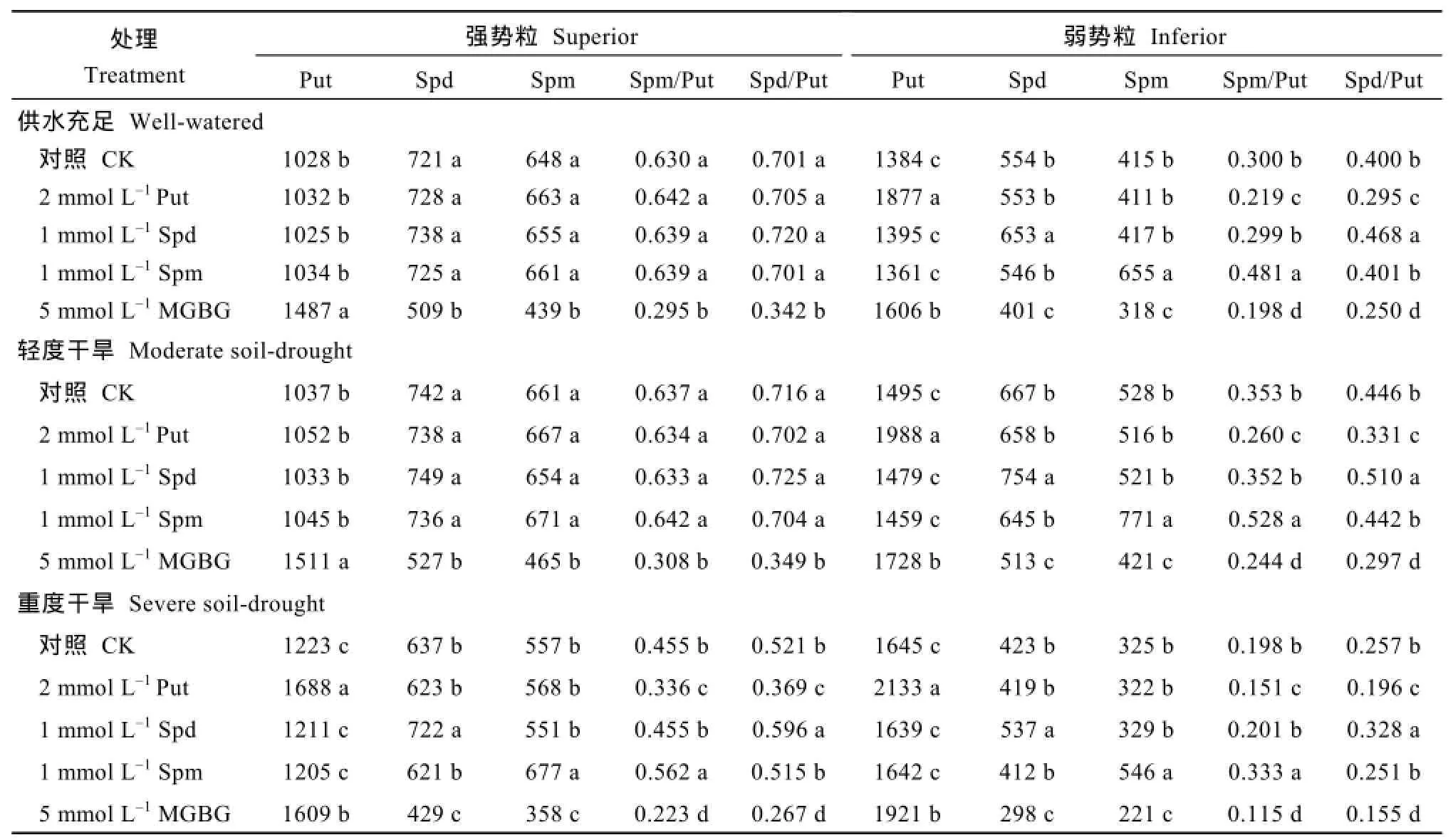
表4 外源多胺及其抑制剂对宁麦13强、弱势粒中Put、Spd和Spm含量的影响Table 4 Effect of exogenous PAs and MGBG on Put,Spd,and Spm concentrations in superior and inferior grains of Ningmai 13
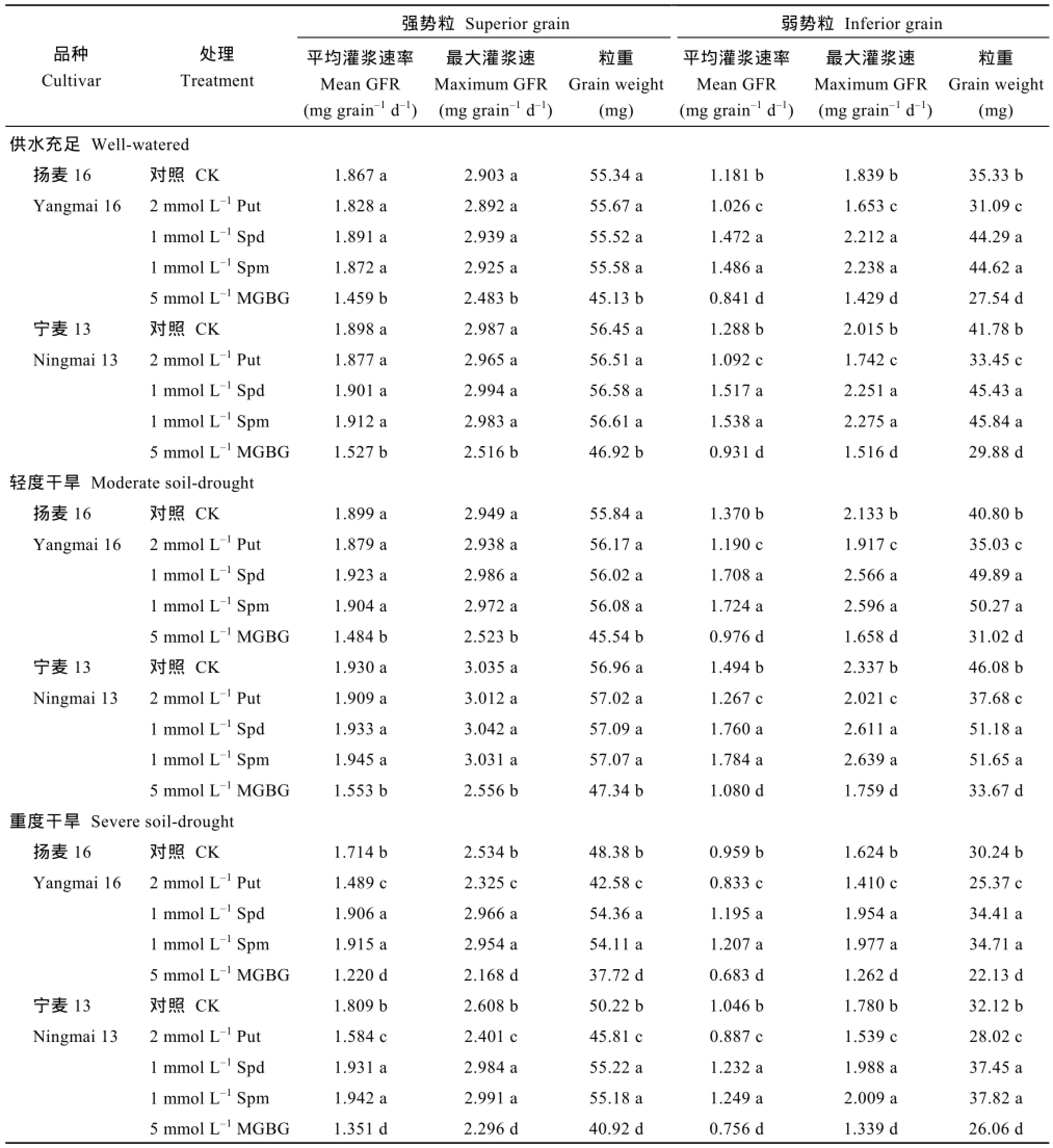
表5 外源多胺及多胺抑制剂对小麦强、弱势粒灌浆速率和粒重的影响Table 5 Effects of exogenous Put,Spd,Spm,and MGBG on grain-filling rate and grain weight in superior and inferior grains of wheat
目前尚不清楚小麦灌浆过程中籽粒中游离多胺的作用机制。本课题组研究发现,在灌浆初期外源Spd 或Spm显著增强了水稻弱势粒中蔗糖-淀粉代谢途径的关键酶活性,而外源 Put的作用则相反[32-33]。另外,多胺能直接参与植株活性氧清除,植株体内较高的Spd和Spm含量能够显著提高抗氧化酶(SOD、POD 和CAT)活性,降低MDA含量[23,34-36]。由此推测,不同土壤水分通过改变内源多胺的含量,进而调节籽粒蔗糖-淀粉代谢途径关键酶及抗氧化酶活性,实现对籽粒灌浆的调节。
从多胺的生物合成途径分析,Spd和 Spm的合成分别通过亚精胺合成酶和精胺合成酶在腐胺上按一定顺序加上由 S-腺苷-L-甲硫氨酸脱羧酶(SAMDC)催化S-腺苷-L-甲硫氨酸(SAM)生成的氨丙基单位[37]。S-腺苷-L-甲硫氨酸(SAM)同时也是乙烯合成的前体,SAM在ACC合成酶和ACC氧化酶催化作用下合成1-氨基环丙烷-1-羧酸(ACC)进而合成乙烯。研究表明,乙烯可以增强细胞分裂素的分解,而细胞分裂素在保持胚乳的细胞分裂方面起着重要作用[38-39]。籽粒中较高的乙烯水平能抑制胚乳细胞分裂,导致较低的籽粒灌浆速率和粒重,水稻灌浆期土壤轻度落干处理能促进籽粒中Spd和Spm的合成并能显著抑制乙烯的生物合成[40]。这些结果表明,MD处理通过增强Spd和Spm的合成,抑制乙烯的产生,进而促进籽粒灌浆,增加粒重。有关多胺调控小麦籽粒灌浆的机制有待深入研究。
国内外关于游离 Put在植物体内的生理作用的报道有很多,结论尚不统一[15,41-42]。在正常生长条件下,Put作为Spd和Spm合成的供体,籽粒中较高的Put有利于 Spd和Spm的合成,进而促进籽粒生长发育,但过多的游离 Put在器官中的累积会产生毒害作用[40,43]。本试验也观察到,SD处理显著提高了籽粒中游离 Put的含量,籽粒灌浆速率和粒重显著降低;活跃灌浆期内源游离 Put含量与灌浆速率及粒重呈极显著的负相关;通过喷施Put或MGBG增大籽粒Put含量,灌浆速率和粒重显著降低。再次表明游离 Put在籽粒中的过度积累,会对籽粒产生毒害作用,从而不利于籽粒灌浆。
Davies[44]指出,激素往往不是单一地发挥作用,而是通过与其他激素相互作用、平衡最终共同决定植物的生长发育,即所谓的协同或拮抗作用。本试验观察到,小麦的籽粒灌浆速率和粒重不仅与灌浆过程中籽粒中游离多胺的含量密切相关,同时还与Spd/Put及Spm/Put值呈极显著正相关;在MD处理下弱势粒中Spd/Put和Spm/Put值增大,籽粒灌浆速率和粒重也增加。相反,SD处理显著降低了Spd/Put 和Spm/Put值,籽粒灌浆速率和粒重也随之降低。这一结果说明,小麦籽粒内源游离Put、Spd和Spm间的平衡对籽粒灌浆有重要调控作用,较高的 Spd或Spm与Put的比值,有利于小麦籽粒灌浆。
4 结论
与WW相比,MD处理能显著提高小麦弱势粒灌浆速率和粒重,SD处理则显著降低籽粒灌浆速率和粒重,这与籽粒中游离Spd和Spm含量有密切关系。小麦弱籽粒中较低的游离Spd和Spm含量以及较低的Spd/Put和Spm/Put比值是导致籽粒灌浆差、粒重低的重要生理原因。MD处理通过增加籽粒中Spd和Spm含量以及Spd/Put和Spm/Put值,进而促进籽粒灌浆,增加粒重。
References
[1] Yang W B,Yin Y P,Li,Cai T,Ni Y L,Peng D L,Wang Z L. Interactions between polyamines and ethylene during grain filling in wheat grown under water deficit conditions. Plant Growth Regul,2014,72∶ 189-201
[2] Shao H B,Chu L Y,Jaleel C A,Manivannan P,Panneerselvam R,Shao M A. Understanding water deficit stress-induced changes in the basic metabolism of higher plants-biotechnologically and sustainably improving agriculture and the eco-environment in arid regions of the globe. Crit Rev Biotechnol,2009,29∶ 131-151
[3] Kobata T,Palta J A,Turner T C. Rate of development of post anthesis water deficits and grain filling of spring wheat. Crop Sci,1992,32∶ 1238-1242
[4] 杨桂霞,赵广才,许轲,常旭虹,杨玉双,马少康. 灌水及化控对不同粒色小麦籽粒灌浆及叶绿素含量的影响. 华北农学报,2010,25(4)∶ 152-157 Yang G X,Zhao G C,Xu K,Chang X H,Yang Y S,Ma S K. Effect of irrigation and chemical control on grain filling and chlorophyll content in wheat with different grain colors. Acta Agric Boreali-Sin,2010,25(4)∶ 152-157 (in Chinese with English abstract)
[5] Yang J C,Zhang J H,Ye Y X,Wang Z Q,Zhu Q S,Liu L J. Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant Cell Environ,2004,27∶ 1055-1064
[6] Yang J C,Zhang J H Wang Z Q,Zhu Q S,Liu L J. Water deficit-induced senescence and its relationship to remobilization of prestored carbon in wheat during grain filling. Agron J,2001,93∶ 196-206
[7] Yang J C,Zhang J C. Grain filling of cereals under soil drying. New Phytol,2006,169∶ 223-236
[8] 王维,张建华,杨建昌,朱庆森. 适度土壤干旱对贪青小麦茎鞘贮藏性糖运转及籽粒充实的影响. 作物学报,2004,30∶1019-1025 Wang W,Zhang J H,Yang J C,Zhu Q S. Effects of controlled soildrought on remobilization of stem-stored carbohydrate and grain filling of wheat with unfavorably-delayed senescence. Acta Agron Sin,2004,30∶ 1019-1025 (in Chinese with English abstract)
[9] 吕丽华,胡玉昆,李雁鸣,王璞. 灌水方式对不同小麦品种水分利用效率和产量的影响. 麦类作物学报. 2007,27∶88-92 Lü L H,Hu Y K,Li Y M,Wang P. Effect of irrigating treatments on water use efficiency and yield of different wheat cultivars. J Triticeae Crops,2007,27∶ 88-92 (in Chinese with English abstract)
[10] Duan H G,Yuan S,Liu W J,Xi D H,Qing D H,Liang H G,Lin H H. Effects of exogenous spermidine on photosystem II of wheat seedlings under water stress. J Integr Plant Biol,2006,48∶ 920-927
[11] Yang J C,Zhang J H,Liu K,Wang Z Q,Liu L J. Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol,2006,171∶ 293-303
[12] Liu Y E,Liu P. Hormonal changes caused by the Xenia effect during grain filling of normal corn and high-oil corn crosses. Crop Sci,2010,50∶ 215-221
[13] Tomosugi M,Ichihara K,Saito K. Polyamines are essential for the synthesis of 2-ricinoleoyl phosphatidic acid in developing seeds of castor. Planta,2006,223∶ 349-358
[14] Kasukabe Y,He L,Nada K,Misawa S,Ihara I,Tachibana S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stress and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol,2004,45∶ 712-722
[15] Paschalidis K A,Roubelakis-Angelakis K A. Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age,cell division/expansion,and differentiation. Plant Physiol,2005,138∶ 142-152
[16] Alcazar R,Marco F,Cuevas J C,Patron M,Ferrando A,Carrasco P,Tiburcio A F,Altabella T. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett,2006,28∶ 1867-1876
[17] Liu J H,Kitashiba H,Wang J,Ban Y,Moriguchi T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol,2007,24∶ 117-126
[18] Goyal M,Asthir B. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul,2010,60∶ 13-25
[19] Feng H Y,Wang Z M,Kong F N,Zhang M J,Zhou S L. Roles of carbohydrate supply and ethylene,polyamines in maize kernel set. J Integr Plant Biol,2011,53∶ 388-398
[20] Yang J C,Cao Y Y,Zhang H,Liu L J,Zhang J H. Involvement of polyamines in the post-anthesis development of inferior and superior spikelets in rice. Planta,2008,228∶ 137-149
[21] 牛明功,胡炳义,张胜,朱自学,刘怀攀. 小麦种子脱水过程中多胺水平的变化. 种子,2006,25(11)∶ 61-63 Niu M G,Hu B Y,Zhang S,Zhu Z X,Liu H P. Changes of polyamine during dewatering of wheat seed. Seed,2006,25(11)∶ 61-63 (in Chinese with English abstract)
[22] Liu H P,Zhu Z X,Liu T X,Li C H. Effect of osmotic stress on the kinds,forms and levels of polyamines in wheat coleoptiles. J Plant Physiol Mol Biol,2006,32∶ 293-299
[23] 刘杨,温晓霞,顾丹丹,郭强,曾爱,李长江,廖允成. 多胺对冬小麦籽粒灌浆的影响及其生理机制. 作物学报,2013,39∶ 712-719 Liu Y,Wen X X,Gu D D,Gu Q,Zeng A,Li C J,Liao Y C. Effect of polyamine on grain filling of winter wheat and its physiological mechanism. Acta Agron Sin,2013,39∶ 712-719 (in Chinese with English abstract)
[24] 朱庆森,曹显祖,骆亦奇. 水稻籽粒灌浆的生长分析. 作物学报,1988,14∶ 182-193 Zhu Q S,Cao X Z,Luo Y Q. Growth analysis on the progress of grain filling in rice. Acta Agron Sin,1988,14∶ 182-193 (in Chinese with English abstract)
[25] Richards F J. A flexible growth functions for empirical use. J Exp Bot,1959,10∶ 290-300
[26] Flores H E,Galston A W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol,1982,69∶ 701-706
[27] DiTomaso J M,Shaff J E,Kochian L V. Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of interaction seeding. Plant Physiol,1989,90∶ 988-995
[28] Yang J C,Liu K,Wang Z Q,Du Y,Zhang J H. Water-saving and high-yielding irrigation for lowland rice by controlling limiting values of soil water potential. J Integr Plant Biol,2007,49∶ 1445-1454
[29] Harsh Pal Bias,G A Ravishankar. Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell,Tiss Organ Cult,2002,69∶ 1-34
[30] Bueno M,Lendinez M L,Aparicio C,Cordovilla M P. Effect of salinity on polyamines and ethylene in Atriplex prostrate and Plantago coronopus. Biol Plant,2015,59∶ 596-600
[31] Rossetto M R M,Vianello F,Saeki M J,Lima G P P. Polyamines in conventional and organic vegetables exposed to exogenous exposed to ethylene. Food Chem,2015,188∶ 218-224
[32] 谈桂露,张耗,付景,王志琴,刘立军,杨建昌. 超级稻花后强、弱势粒多胺含量变化及其与籽粒灌浆的关系. 作物学报,2009,35∶ 2225-2233 Tan G L,Zhang H,Fu J,Wang Z Q,Liu L J,Yang J C. Post-anthesis changes in concentrations of ployamines in superior and inferior splikelets and their relation with grain filling of super rice. Acta Agron Sin,2009,35∶ 2225-2233 (in Chinese with English abstract)
[33] Yang J,Cao Y,Zhang H,Liu L,Zhang J. Involvement of polyamines in the post-anthesis development of inferior and superior spikelets in rice. Planta,2008,228∶ 137-149
[34] 张木清,陈如凯,余松烈. 多胺对渗透胁迫下甘蔗愈伤组织诱导和分化的作用. 植物生理学通讯,1996,32∶ 175-178 Zhang M Q,Chen R K,Yu S L. Effect of polyamines on induction and differentiation of calli from leaves of sugarcane under osmotic stress. Plant Physiol Commun,1996,32∶175-178 (in Chinese)
[35] 徐仰仓,王静,刘华,王根轩. 外源精胺对小麦幼苗抗氧化酶活性的促进作用. 植物生理学报,2001,27∶ 349-352 Xu Y C,Wang J,Liu H,Wang G X. Promoting effect of ex-ogenous spermine on anti-oxidative enzyme activity in wheat seedlings. Acta Phytophysiol Sin,2001,27∶ 349-352 (in Chinese with English abstract)
[36] 璟李,胡晓辉,郭世荣,王素平,王鸣华. 外源亚精胺对根际低氧胁迫下黄瓜幼苗根系多胺含量和抗氧化酶活性的影响. 植物生态学报,2006,30∶ 118-123 Li J,Hu X H,Guo S R,Wang S P,Wang M H. Effect of exogenous spermidine on polyamine content and antioxidant enzyme activities in roots of cucumber seedlings under root zone hypoxia stress. Chin J Plant Ecol,2006,30∶ 118-123 (in Chinese with English abstract)
[37] Maiale S,Sánchez D H,Guirado A,Vidal A,Ruiz O A. Spermine accumulation under salt stress. J Plant Physiol,2004,161∶ 35-42
[38] Bollmark M,Eliasson L. Ethylene accelerates the breakdown of cytokinin and thereby stimulates rooting in Norway spruce hypocotyl cuttings. Physiol Plant,1990,80∶ 534-540
[39] Yang J C,Zhang Z J,Wang Z Q,Zhu Q S,Liu L J. Correlation of cytokinin levels in the endosperms and roots with cell number and cell division activity during endosperm development in rice. Ann Bot (London),2002,90∶ 369-377
[40] Chen T T,Xu Y J,Wang J C,Wang Z Q,Yang J C,Zhang J H. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J Exp Bot,2013,64∶ 2523-2538
[41] Bouchereau A,Aziz A,Larher F,Tanguy J M. Polyamines and environmental challenges∶ recent development. Plant Sci,1999,140∶ 103-125
[42] Capell T,Bassie L,Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA,2004,101∶ 9909-9914
[43] Hummel I,Amrani A E,Gouesbet G,Hennion F,Couée I. Involvement of polyamines in the interacting effects of low temperature and mineral supply on Pringlea antiscorbutica (Kerguelen cabbage) seedlings. J Exp Bot,2004,55∶ 1125-1134
[44] Davies P J. The plant hormones∶ their nature,occurrence and function. In∶ Davies P J ed. Plant Hormones,Biosynthesis,Signal Transduction,Action! Dordrecht∶ Kluwer Academic Publishers,2004. pp 1-15
Free Polyamines in Grains in Response to Soil Drought and Their Relationship with Grain Filling of Wheat
ZHANG Wei-Yang,XU Yun-Ji,QIAN Xi-Yang,LI Yin-Yin,WANG Zhi-Qin,and YANG Jian-Chang*
Jiangsu Provincial Key Laboratory of Crop Genetics and Physiology / Co-innovation Center of Modern Production Technology for Grain Crops,Yangzhou University,Yangzhou 225009,China
Abstract:For understanding the role of endogenous free polyamines on grain filling of wheat under drought stress,we conducted a two-year pot experiment from September 2013 to June 2015 using high-yield wheat cultivars Yangmai 16 and Ningmai 13 grown in different soil moisture conditions. Three treatments,namely�well-watered (WW),moderate soil-drought (MD),and severe soil-drought (SD),were imposed from late-tillering to maturity stage. Grain filling rate and free polyamines levels in both superior and inferior grains were determined. The results showed the consistency between the two cultivars. Compared with WW,MD treatment had significantly increased grain-filling rate and grain weight in inferior grains by 12.5% and 11.8%,respectively;whereas no effect on grain filling in superior grains. In contrast,SD treatment showed negative influences on leaf water potential,photosynthetic rate,and grain filling. Under SD treatment,grain-filling rate and grain weight of superior grains reduced by 10.1% and 9.5% and those of inferior grains reduced by 14.5% and 11.7%,respectively. During grain filling,concentrations of freespermidine (Spd) and spermine (Spm) as well as their ratios to putrescine (Put) in inferior grains increased significantly under MD treatment and decreased significantly under SD treatment. Grain-filling rate and grain weight were positively correlated with concentrations of Spd and Spm,and the ratios of Spd/Put and Spm/Put (P < 0.01),whereas negatively correlated with Put concentration (P < 0.01). Exogenous Spd or Spm resulted in significant increases of grain-filling rate (11.2-25.9%) and grain weight (9.9-17.7%) in inferior grains under the three soil moistures and in superior grains under SD treatment,and had no significant difference in superior grains between WW and MD treatments. The positive effects of exogenous Spd and Spm were eliminated when their synthesis inhibitor,methylglyoxal-bis guanylhydrazone (MGBG),was applied together with Spd and Spm. Both superior and inferior grains showed great decreases of grain-filling rate (20.5-28.8%) and grain weight (16.9-28.5%) after spraying MGBG under the three soil moistures. These results indicate that the responses of polyamines in grain to soil moisture vary with drought strength,and moderate drought stress has a positive effect on grain filling through increasing concentrations of Spd and Spm and the ratios of Spd/Put and Spm/Put in grains.
Keywords:Wheat;Soil drought;Polyamines;Grain filling;Grain weight
DOI:10.3724/SP.J.1006.2016.00860
*通讯作者(
Corresponding author)∶ 杨建昌,E-mail∶ jcyang@yzu.edu.cn
收稿日期Received()∶ 2015-09-21;Accepted(接受日期)∶ 2016-03-14;Published online(网络出版日期)∶ 2016-03-28.

