Sensitivity Enhancement in Uranium Determination by UV-Visible Spectroscopy Using Ion Imprinted Polymer
Tulin M, Mehmet YAMAN
Firat University, Sciences Faculty, Department of Chemistry, Elazig, Turkey
Sensitivity Enhancement in Uranium Determination by UV-Visible Spectroscopy Using Ion Imprinted Polymer
Firat University, Sciences Faculty, Department of Chemistry, Elazig, Turkey
There is need to determination of uranium concentration at ppb level in environmental matrices. Due to low sensitivity of FAAS, UV-Visible Spectroscopy is generally used as measurement technique. In this study, ion-imprinted polymers (IIP) were prepared for uranyl ion (imprint ion) by formation of ternary (salicylaldoxime and 4-vinylpyridine) complex in 2-methoxy ethanol (porogen) following copolymerization with methacrylic acid (MAA) as a functional monomer and ethylene glycol dimethacrylate (EGDMA) as crosslinking monomer using 2,2-azobisisobutyronitrile as initiator. The synthesized polymers were characterized by FT-IR and TGA analysis. Arsenazo Ⅲ in 3 M HClO4was used as complexing agent in the measurement step. The optimal pH for preconcentration was found to be between 3.5~6.5 values. The developed method was applied to uranium (Ⅵ) determination in natural water samples.
Uranium; Ion imprinted polymer; Arsenazo Ⅲ; UV-vis Spectroscopy
Introduction
Uranium and its compounds are highly toxic which cause progressive or irreversible renal injury and in acute cases may lead to kidney failure and death. The inhalation of uranium compounds results in deposition of uranium in the lungs and reaches kidneys through blood stream. The tolerable daily intake of uranium is established by World Health Organization (WHO) as 0.6 μg·kg-1of body weight per day[1]. The WHO, United States and European United-Member States related with Health drinking water guidelines fixed the maximum uranium concentration in drinking waters to be less than 10~30 μg·L-1[2-4]. The inhalation of uranium compounds results in deposition of uranium in lungs and reaches kidneys through blood stream. The uranium concentration of seawaters is about 3.0 ng·mL-1, below 2.0 ng·mL-1in freshwaters. Thus, highly sensitive methods are required for preconcentration and determination of uranium in water samples. Traditionally used neutron activation analysis (NAA) and more popular ICP-mass spectrometry (ICP-MS) are the techniques which are widely sought after for the determination of not only uranium, thorium and their radionuclides but also other actinides. However, the direct analysis of geological and environmental samples by NAA or ICP-MS is still difficult because of the very low concentrations of uranium and thorium and also the presence of complex matrix. Unlike other trace metals such as copper, lead cadmium and nickel[5-14], sensitivity of uranium by flame AAS is too poor due to the formation of refractory oxides in the flame region and has been used only scarcely. Even with the use of high temperatures and a nitrous oxide-acetylene flame, a poor sensitivity is achieved for uranium, approximately 50 g·mL-1for 1% absorbance, while the nitrous oxide-acetylene flame gives an absorbance reading of about 0.09 for 1 000 g·mL-1uranium if sufficient alkali has been added to suppress ionization[15]. GFAAS is useful for the trace determination of uranium (Ⅵ) and is sensitive unlike FAAS[16]. On the other hand, the interference due to matrix (in case of real sample analysis) is more pronounced and is not that popular. Energy dispersive, wavelength dispersive and total reflectance X-ray fluorescence spectrometric techniques are multi-element techniques but requires elaborate sample preparation, in addition to being not sensitive for liquid samples. Inductively coupled plasma atomic emission spectrometry (ICP-AES) is also multi-elemental technique but cannot be used for differentiating various radionuclides of uranium and thorium. Although the measurement of the fluorescence of the uranyl ion-ligand is one of the used methods for determining trace amounts of uranium, this method is of low accuracy as well as repeatability. So, researchers have focused to UV-vis. Spectrometry for U determination after preconcentration procedures[17-20]. For the spectrophotometric determination of uranium, several organic and inorganic reagents have been used among which are Arsenazo Ⅲ (2,7-bis(2-arsenophenylazo)-1,8-dihydroxynaphthalene-3,6-disulfonic acid disodium salt), morin, calmagite, pyrocathecol viole and dibenzoylmethane[17-20]. One way of achieving high sorption capacity is by the use of ligands of small size which can extensively functionalize an appropriate crosslinked polymer. In addition to the nature of the reagents, the role of the medium of determination is also very important. Various procedures have been reported for the determination of uranium in organic and mineral acid media. Arsenazo Ⅲ was found to be more stable in perchloric acid than in acids, such as sulfuric, hydrochloric and nitric acids[17-20]. Nitric acid among them, being an oxidizing agent, can easily decompose azo-dyes at room temperature[17-20]. On the other hand, because of the low concentration of uranium in water, the formation of complexes with these reagents by other trace elements, and the similarity of the absorption curve of the uranium complex with the curves of the complexes formed by other trace elements, most spectrophotometric determinations of uranyl ion are applied by a separation and preconcentration step. Among preconcentration methods, the imprinted polymer has greater selectivity for the target ion than does either the monomer ionophore or the unimprinted polymer. The polymer also provides remarkable concentration efficiency and good selectivity for the uranyl ion. The unique shape of the uranyl ion may be expected to lead to much greater selectivity and high adsorption capacity for the uranyl ion by molecular imprinting than for other metal ions. Briefly, ion imprinted molecular polymeric adsorbents are popular in recent years[21-27].
In this study, ion-imprinted polymers (IIPs) were prepared for uranyl ion by formation of ternary (salicylaldoxime and 4-vinylpyridine) complex in 2-methoxy ethanol following copolymerization with methacrylic acid (MAA) as a functional monomer and ethylene glycol dimethacrylate (EGDMA) as crosslinking monomer using 2,2-azobisisobutyronitrile as initiator. Then, the supported U was removed from resin and determined by UV-Vis. Spectrometry.
1 Experimental
1.1 Reagents

1.2 Instrumentation
A Digital pH meter 100 (Cyberscan) was used for pH measurements. FT-IR spectra were recorded in the frequency range 4 000~400 cm-1by KBr pellet method using Perkin Elmer FT-IR. Temperature controlled rotary shaking machine was used for shaking. UV-Vis spectrometry (PG instruments-T80+) was used for determination of U. ICP-MS was also used to determine U in some samples for check the accuracy.
1.3 Synthesis of ion-imprinted and control polymers
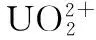
As a result, ion-imprinted polymers (IIPs) were prepared for uranyl ion by formation of ternary (salicylaldoxime and 4-vinylpyridine) complex in 2-methoxy ethanol following copolymerization with methacrylic acid (MAA) as a functional monomer and ethylene glycol dimethacrylate (EGDMA) as crosslinking monomer using 2,2-azobisisobutyronitrile as initiator. Control polymer (CP) was also prepared under identical experimental conditions without using imprint ion. The above synthesized polymers were characterized by FT-IR analysis techniques.
1.4 Removal of imprint ion from the polymer
The imprint ion was removed from 2 g of the synthesized polymer by stirring with 100 mL of 4.0 mol·L-1HCl for 1 h, two times, and 100 mL of 2 mol·L-1HCL. The activated polymer obtained after filtration was dried in an oven at 80 ℃ to get leached ion-imprinted polymer for possible selective extraction of uranium (Ⅵ) from dilute aqueous solution. The effect of various leachants: HCl, HNO3or H2SO4(2.0 and 4.0 mol·L-1) was studied.

Fig.1 Scheme of imprinted uranium removal and preconcentration steps
1.5 Preconcentration procedure
A 0.1 g of activated (U removed) IIP was added to the 100 mL solutions of 10~500 ppb U and the pH of these solutions were adjusted to 4.5 by addition of 10 mL of CH3COOH/CH3COONa buffer. The solutions were stirred for 30 min. After that, the uranyl ions preconcentrated on polymers were eluted by heating and evaporating added 5 mL of concentrated nitric acid to the polymer. Then, 10 mL of 3 mol·L-1HClO4was added, stirred and filtrated. The clear solution was measured for U. The detailed scheme steps were given in Figure 1.
1.6 Determination of uranium by UV-Vis. spectrophotometry
The uranyl ion content in eluent was determined spectrophotometrically using the modified Arsenazo Ⅲ method. Two mL of Arsenazo Ⅲ in 3 mol·L-1HClO4were added to 2.0 mL of eluent. After stirring and waiting for 30 min to equilibrate the complex, the absorbance of uranium (Ⅵ)-Arsenazo Ⅲ complex was measured at 651.0 nm. The calibration solutions were also measured in this way. Using calibration curve, U concentrations in the samples studied were found. Spectra of different U solutions were displayed in Fig.2.

Fig.2 UV-Vis spectra of different U solutions
Related with statistically consideration, One-Way Analyses of Variance (ANOVA) were conducted to test the equality of mean values for each sample of interest. One of the pairwise comparisoo tests, Tukey HSD, was carried out to find which of the sample means is different from each other. SPSS (version 15) statistical program was used for all statistical computations. Statistical significance was considered when P was equal to=or higher than 0.05.
2 Results and Discussion
2.1 FT-IR spectra and TGA for characterization of IIP


Fig.3 FTIR spectrum of IIP
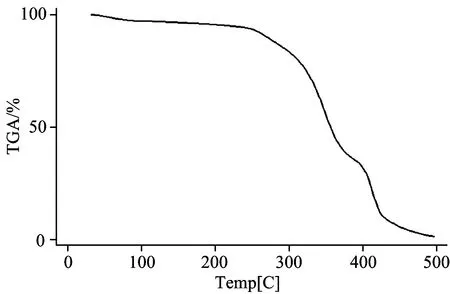
Fig.4 TGA curve of the synthesized IIP
2.2 Optimization of reagent species and concentrations in measurement step
As it is described above, the nature of the reagents and the role of the medium of determination are very important in UV-Vis. Spectrophotometric measurement. Morine, pyrocathecol and Arsenazo Ⅲ were examined to determine maximum absorbance using the same concentrations of uranyl ions. Arsenazo Ⅲ was found the best reagent for this purpose. Then, the same concentrations of perchloric, sulfuric, hydrochloric and nitric acids were used in dilution procedure at the measurement step to determine the best solvent. Maximum absorbance signals were obtained by using perchloric acid. Thus, different concentrations of perchloric acid were examined to determine maximum sensitivity. The obtained results were given in Table 1.
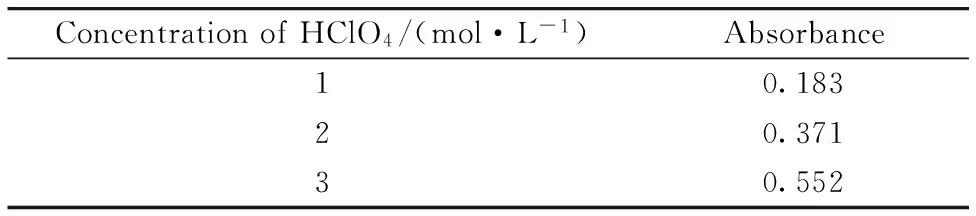
Table 1 Effect of HClO4 concentration on absorbance signal of 10 mg·L-1 U
2.3 Effect of pH

2.4 Optimization of other experimental variables in preconcentration procedure
It was found that 30 min of stirring time for the preconcentration at pH 4.5 is enough for maximum recovery. The results obtained depending on nature of eluent, eluent concentration and eluent volume are summarized in Table 2. It was found that hydrochloric acid alone offers quantitative elution of imprinted uranium (Ⅵ). The required minimum concentration of HCl was found to be 4.0 mol·L-1to elute the imprinted uranium (Ⅵ). Further, the increase of the initial volume of solution up to 500 mL did not affect the quantitative recovery of uranium (Ⅵ). So, calibration curve and real samples were also carried out using 500 mL of solutions.

Fig.5 Effect of pH on preconcentration
Table 2 Effect of acid species and concentration on leaching of uranium (Ⅵ)

ParameterRecovery/%1.0mol·L-1HCl602.0mol·L-1HCl703.0mol·L-1HCl804.0mol·L-1HCl921.0mol·L-1HNO3632.0mol·L-1HNO3683.0mol·L-1HNO3824.0mol·L-1HNO3931.0mol·L-1H2SO4502.0mol·L-1H2SO4604.0mol·L-1H2SO4753-times30min.4mol·L-1HCl873-times60min.4mol·L-1HCl923-times120min.4mol·L-1HCl92
2.5 Calibration graph and accuracy studies
Under the optimum conditions described above, the calibration curve was linear over the concentration range 2.0~50 μg·L-1of uranium (Ⅵ) using 500 mL of initial solutions according Fig.1. The linear equation with regression is as follows.
Y=22.692x+0.269 7 R2=0.999 9
where correlation coefficient is 0.999 9,Yis the absorbance andxis the concentration in μg·L-1. The LOD and LOQ were found to be 0.7 and 2.0 μg·L-1. All the statistical calculations are based on the average of triplicate readings for each standard solution in the given range. Some sample solutions were determined by ICP-MS to check the accuracy of results. It was found that there are no significant differences (the recoveries higher than 90% were obtained for the results from UV-Vis and ICP-MS) between the data obtained by the UV-Vis and ICP-MS methods using t test at a confidence level of 90%. Furthermore, A recovery test was performed by determining the spiked concentration in the samples in which 20 μg·L-1of uranium (Ⅵ) was added. The recoveries of uranium (Ⅵ) from real water were 96%±7% which could make the procedure a reliable method. Related the selectivity studies, Fe, Ca, Mg, Ni, Al, Mn, Zn, Cr and Co metal ions were added to U solutions. The results from Table 3 show that those ions do not affect quantitative determination of uranyl ions from solutions by using the optimized method.

Table 3 Effect of metal ions on recovery of U
2.6 Applications
The applicability of the method was tested to the natural water samples. The ground, river and Lake waters were subjected to uranium analysis by employing the developed preconcentration method in conjunction with the Arsenazo Ⅲ spectrophotometric method. The obtained results for the studied water samples were given in Table 4. It was found that uranium in river and ground water is under sensitivity (2.0 μg·L-1) of the developed method. The mean U concentration in Lake Van Water was found to be 60 μg·L-1.


Table 4 Results obtained for real samples.
3 Conclusions

[1] Zhivin S, Laurier D, Canu I G. International Journal of Radiation Biology, 2014, 90(11): 1104.
[2] World Health Organization. WHO, Geneva, 4th ed. 2011. 430.
[3] EU 98/83/ECD, European Commission Directive, Related with Drinking Water Quality Intended for Human Consumption, European Commission, Brussels, Belgium, 1998.
[4] United States Environmental Protection Agency, National Primary Drinking Water Regulations, EPA 816-F-09-0004, http://water.epa.gov/drink/contaminants/index.cfm#Inorganic. 2009.
[5] Ozen O A, Songur A, Sarsilmaz M, et al. Journal of Trace Elements in Medicine and Biology, 2003, 17(3): 207.
[6] Yaman M, Kaya G, Simsek M. International Journal of Gynecological Cancer, 2007, 17: 220.
[7] Yaman M, Cokol N. Atomic Spectroscopy, 2004, 25(4): 185.
[8] Yaman M, Kaya G, Yekeler H. World Journal of Gastroenterology, 2007, 13(4): 612.
[9] Yaman M, Gucer S. Analyst, 1995, 120: 101.
[10] Yaman M. Journal of Analytical Atomic Spectrommetry, 1999, 14: 275.
[11] Yaman M, Atici D, Bakirdere S, et al. Journal of Medicinal Chem., 2005, 48(2): 630.
[12] Kaya G, Akdeniz I, Yaman M. Atomic Spectroscopy, 2008, 29(3): 99.
[13] Yaman M. Analytical Biochemistry, 2005, 339: 1.
[14] Kaya G, Yaman M. Talanta, 2008, 75: 1127.
[15] Santos J S, Teixeira L S G, Dos Santos W N L, et al. Anal. Chim. Acta, 2010, 674: 143.
[16] Shamsipur M, Ghiasvand A R, Yamini Y. Anal. Chem., 1999, 71: 4892.
[17] Park C, Huang H, Cha K. Bull. Corean Chem., 2001, 22: 84.
[18] Jauberty L, Drogat N, Decossas J L, et al. Talanta, 2013, 115: 751.
[19] Ozdemir S, Kilinc E. Microchim. Acta, 2012, 178: 389.
[20] Golmohammadi H, Radhidi A, Safdari S J. Chemistry and Chemical Technology, 2012, 6(3): 245.
[21] Khan M H, Warwick P, Evans N. Chemosphere, 2006, 63: 1165.
[22] Singh D K, Mishra S. Anal. Chim. Acta, 2009, 644: 42.
[23] Metilda P, Gladis J M,et al. Anal. Chim. Acta, 2007, 587: 263.
[24] Gladis J M,Rao T P. Microchim. Acta, 2004, 146: 251.
[25] Metilda P, Gladis J M, Rao T P. Anal. Chim. Acta, 2004, 512: 63.
[26] Monier M, Alatawi R A S, Abdel-Latif D A. Journal of Molecular Recognition, 2015, 28: 306.
[27] Qian J, Zhang S, et al. Rsc. Advances, 2015, 5: 4153.
[28] Say R, Ersoz A, Denizli A. Separation Science And Technology, 2003, 38(14): 3431.
[29] Yaman M, Ince M, Erel E, et al. Clean-Soil Air Water, 2011, 39: 530.
[30] Kolpakova M. Procedia Earth Planetary Sci., 2014, 10: 164.
O657.3
A
Foundation item: the Scientific Investigate Projects of Firat University, Turkey (FF.14.10)
10.3964/j.issn.1000-0593(2016)06-1992-06
Received: 2015-10-14; accepted: 2016-02-02
Biography: Professor Yaman received his Ph.D. in 1990 from the University of Inonu, Malatya-Turkey e-mail: myaman@firat.edu.tr; ijpacmy@gmail.com
- 光谱学与光谱分析的其它文章
- 基于光声光谱联合主成分回归法的血糖浓度无损检测研究
- HP-β-CD降低油田地层水中SDBS胶束对其检测光谱的干扰
- 近红外高光谱成像技术用于转基因大豆快速无损鉴别研究
- 采用小波分析方法降低可调谐半导体激光吸收光谱技术测量下限的实验研究
- 钠钾替代条件下不同基因型棉花叶片的FTIR光谱研究
- Structural, Morphological and Optical Properties of Well-Ordered CdO Nanostructures Synthesized by Easy-Economical Chemical Bath Deposition Technique

