巯基聚苯乙烯树脂对FGD系统中Hg2+的脱除性能
付康丽,姚明宇,钦传光,程广文,聂剑平(西安热工研究院有限公司国家能源清洁高效火力发电技术研发中心,陕西 西安 70054;西北工业大学理学院,陕西 西安 7007)
巯基聚苯乙烯树脂对FGD系统中Hg2+的脱除性能
付康丽1,姚明宇1,钦传光2,程广文1,聂剑平1
(1西安热工研究院有限公司国家能源清洁高效火力发电技术研发中心,陕西 西安 710054;2西北工业大学理学院,陕西 西安 710072)
摘要:以氯甲基化聚苯乙烯微球为原料,经过两步反应制得巯基聚苯乙烯树脂,用红外光谱测试、比表面分析、元素分析及热重分析表征了该巯基聚苯乙烯树脂,测试了此巯基聚苯乙烯树脂对含Hg2+烟气、脱硫废水及脱硫浆液的脱汞性能。分析测试表明:巯基聚苯乙烯树脂热稳定性好,能有效脱除烟气、脱硫废水及脱硫浆液中的Hg2+。巯基聚苯乙烯树脂对烟气中Hg2+的脱除效率大于90%,对脱硫废水及脱硫浆液的脱汞率接近100%。将此树脂置于脱硫系统内,能捕捉脱硫系统内的Hg2+,避免Hg2+进入脱硫石膏而造成脱硫石膏中汞的再释放。用6 mol·L−1盐酸洗脱捕捉了Hg2+的巯基聚苯乙烯树脂,再生3次后,巯基聚苯乙烯树脂的再生率仍高达90.2%。
关键词:烟道气;煤燃烧;废水;汞;巯基树脂
2015-11-06收到初稿,2015-11-23收到修改稿。
联系人:付康丽,钦传光。第一作者:付康丽(1986—),女,博士后,高级工程师。
Received date: 2015-11-06.
Foundation item: supported by the China Postdoctoral Science Foundation (2015M570849) and the National Key Technology Research and Development Program of China (2014BAA07B04).
引 言
汞污染问题已成为社会的焦点问题之一。煤燃烧是人为汞排放的最主要来源[1-2]。烟气中的汞分为3种形态,即元素汞(Hg0)、氧化汞(Hg2+)和颗粒汞(Hgp)。在这3种形态中,Hgp能被布袋除尘或静电除尘有效除去;Hg2+水溶性好[3],湿法脱硫系统能脱除高达99%的Hg2+,此法被认为是经济可行的燃煤电厂多污染物控制方法。然而,Hg0易挥发且不溶于水,不利于Hg0的脱除。许多研究报道[4-6]集中于将Hg0氧化成Hg2+,再借助湿法脱硫系统将Hg2+脱除。然而,捕集在湿法脱硫系统中的Hg2+与此系统中的还原性粒子(如亚硫酸根离子)反应生成Hg0,造成汞的再释放[7]。对此,一些研究集中于降低汞的再释放的影响因素[1-2,8-12]。
目前,几乎所有关于避免烟气中汞的再释放的研究都集中于尽可能地将Hg2+保留于脱硫系统中[13-17]。脱硫系统捕集的汞最终将流向脱硫废水和脱硫石膏[18]。脱硫废水被净化处理后作为脱硫工艺水使用。脱硫石膏是脱硫系统中产生的唯一产物,广泛用作墙板的制作原料。此外,脱硫石膏还用作水泥和混凝土的添加剂及填埋料[19-21]。脱硫石膏中汞的浓度高达656~1919 ng·g−1[22],在石膏墙板的制作过程中将会有12.1%~55%的汞发生再释放[23-24],造成环境汞污染。在长期的化学和物理作用下,脱硫石膏填埋料中的汞会渗透土壤进入地下水,而且目前还未见到关于脱除石膏中汞的报道。此外,将添加剂加入石膏中能抑制汞的再释放,但也会降低石膏品质,不利于石膏的资源化利用。
因此,基于湿法脱硫系统的Hg2+脱除技术只能暂时脱除烟气中的汞。若要从根本上解决汞污染排放问题,就必须研发新的脱汞剂,而且要求该脱汞剂能将Hg2+从湿法脱硫系统中分离出来。有机树脂材料由于其优异的吸附性而越来越受关注,已有研究将改性酚醛树脂材料用于燃煤烟气脱硫脱硝处理,并获得了优异的脱硫性能及较好的脱硝性能[25-28]。大孔聚苯乙烯树脂常用于重金属废水处理[29-31]。然而还未见到关于用树脂脱除烟气中Hg2+的报道。
本工作的研究目的在于脱除烟气中的Hg2+,防止Hg2+进入脱硫石膏和脱硫废水,以从根本上脱除烟气中的Hg2+,并解决汞的再释放问题。制备了一种新型可再生巯基聚苯乙烯树脂,并用红外、比表面分析、元素分析及热重分析对其进行表征;研究了巯基聚苯乙烯树脂对烟气中和脱硫废水中Hg2+的脱除性能;最后探讨了巯基聚苯乙烯树脂的再生性能。
1 实验材料和方法
1.1材料
氯甲基化聚苯乙烯树脂(交联度8%)由南开化工厂提供,硫脲(分析纯)、NaOH(分析纯)、无水乙醇(分析纯)、37%浓盐酸皆由国药集团化学试剂有限公司提供。
1.2巯基聚苯乙烯树脂的制备及表征
将硫脲和氯甲基化聚苯乙烯树脂置于无水乙醇中回流反应5 h得硫脲基聚苯乙烯树脂,在氮气保护下用50% NaOH溶液于80℃水解12 h得巯基聚苯乙烯树脂粗产物,经水洗—稀盐酸洗—水洗后,再将巯基聚苯乙烯树脂置于无水乙醇中回流萃取8 h,真空干燥得巯基聚苯乙烯树脂[32]。具体制备过程如图1所示。制备巯基聚苯乙烯树脂后,用红外光谱、比表面积分析、元素分析、热重分析对巯基聚苯乙烯树脂进行化学表征。
1.3汞浓度分析
使用Ohio Lumex RA-915+型塞曼汞分析仪,根据安大略法(ASTM D678—2002)测试汞浓度。
1.4Hg0的氧化
V2O5基催化剂对Hg0具有良好的氧化性[33-35]。O2和HCl的加入能促进SCR催化剂对Hg0的氧化效率[36-37]。配制含300 mg·m−3HCl和6% O2及一定浓度Hg0的模拟烟气(N2为平衡气体),烟气流速为0.5 L·min−1,此模拟烟气流经装有粉末状SCR催化剂的U形管(置于管式炉中,350℃)。
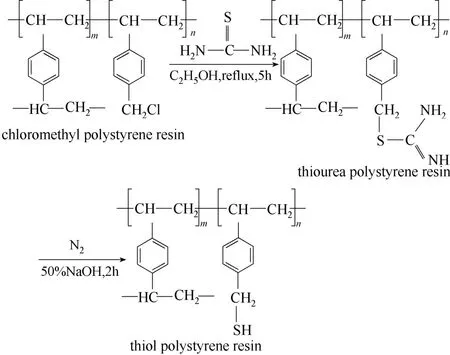
图1 巯基聚苯乙烯树脂的制备反应Fig.1 Synthetic route of thiol polystyrene resin
1.5 巯基聚苯乙烯树脂对烟气中Hg2+的脱除
此组实验的目的在于测定巯基聚苯乙烯树脂对烟气中Hg2+的脱除效率。图2是Hg0的氧化吸收反应系统。具体实验过程如下:将1.0 g巯基聚苯乙烯树脂和30 g石英砂混合均匀,将此混合物置于装有滤气板的U形管中,使含Hg2+烟气通过巯基聚苯乙烯树脂。烟气流速为0.5~1.5 L·min−1,测试温度为25~80℃,入口烟气中Hg2+浓度为32.5 μg·m−3。
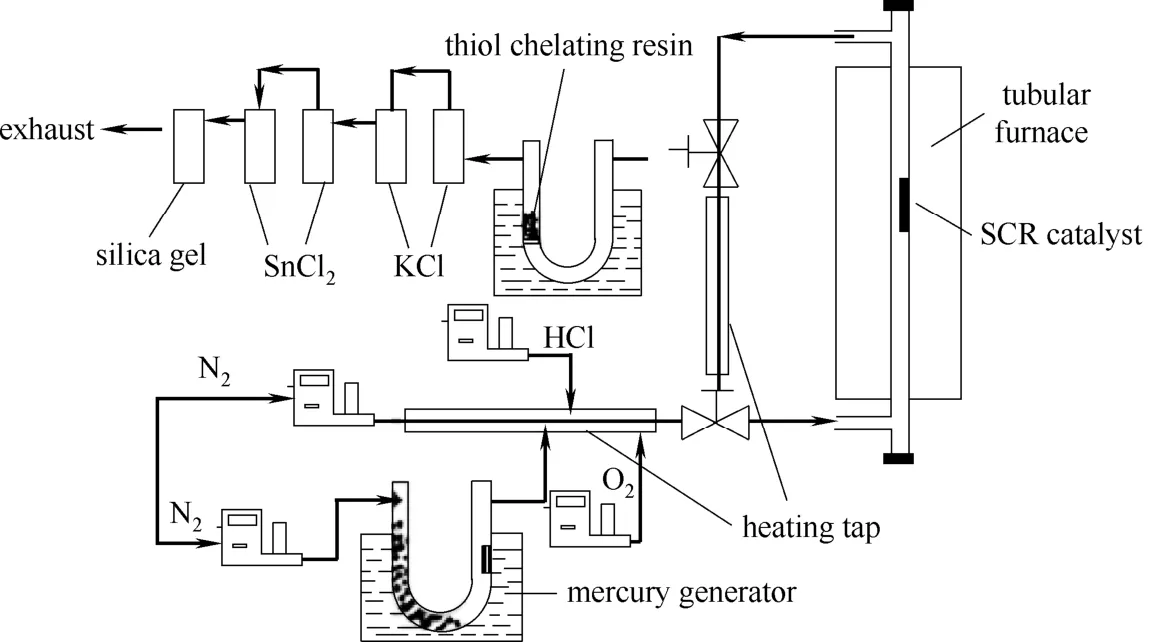
图2 Hg0的氧化及Hg2+的吸收反应系统Fig.2 Schematic diagram of mercury adsorption
Hg2+脱除效率(ηHg2+)计算公式如式(1)所示。

1.6 巯基聚苯乙烯树脂对脱硫废水和脱硫浆液中Hg2+的脱除
脱硫废水和脱硫浆液均由华能铜川照金电厂和杨柳青电厂提供。分别取150 ml脱硫废水、脱硫浆液,向其中加入3 g巯基聚苯乙烯树脂,将此体系置于30℃下,以150 r·min−1的搅拌速率搅拌30 min,随后静置1 h,过滤出巯基聚苯乙烯树脂,测试初始脱硫废水及脱硫浆液和处理后脱硫废水及脱硫浆液中的汞浓度,计算脱汞效率。
1.7巯基聚苯乙烯树脂的再生
用6 mol·L−1盐酸和去离子水连续洗涤捕集了Hg2+的巯基聚苯乙烯树脂,测试洗脱液中的汞含量。
2 实验结果与讨论
2.1巯基聚苯乙烯树脂的性质
表1给出了各树脂的元素分析结果。如表中所示,相比氯甲基化聚苯乙烯树脂,巯基聚苯乙烯树脂中的硫含量具有显著的提升。氯甲基化聚苯乙烯树脂的氯含量为18.8%(此数据由南开化工厂提供),其他组分含量主要是氯含量。巯基聚苯乙烯树脂中的其他组分含量显著降低,这意味着巯基成功取代了氯基。表中的氮含量主要是在巯基聚苯乙烯树脂制备过程中残余的少量硫脲基所致。
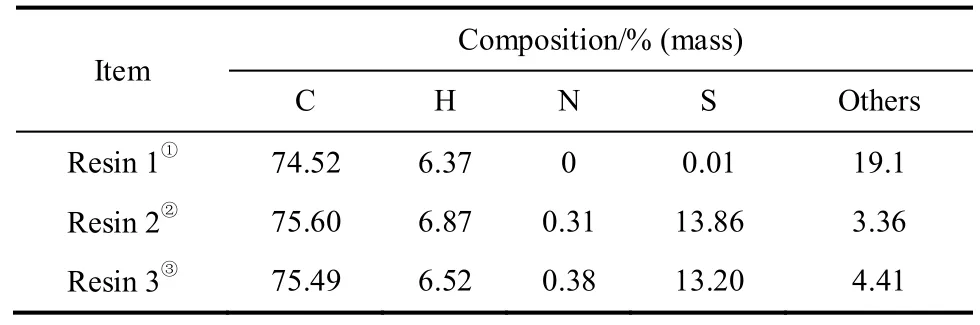
表1 树脂的元素分析Table 1 Elemental analysis for resins
用红外分析进一步确定巯基是否成功嫁接在树脂上。如图3所示,2562 cm−1和2507 cm−1处的吸收峰分别归属于S H和结合Hg2+的S H的伸缩振动[38],672、1250和1264 cm−1处的吸收峰归属于C Cl的伸缩振动[39]。对比氯甲基化聚苯乙烯树脂和巯基聚苯乙烯树脂的红外光谱图,巯基聚苯乙烯树脂中C Cl的吸收峰强度减弱,而且在2562 cm−1处出现了新的吸收峰,这说明S H被成功嫁接在树脂上。测试样品中含有水,故在图中3200 cm−1以上波数处出现的宽吸收峰为样品中的水的吸收峰[40]。
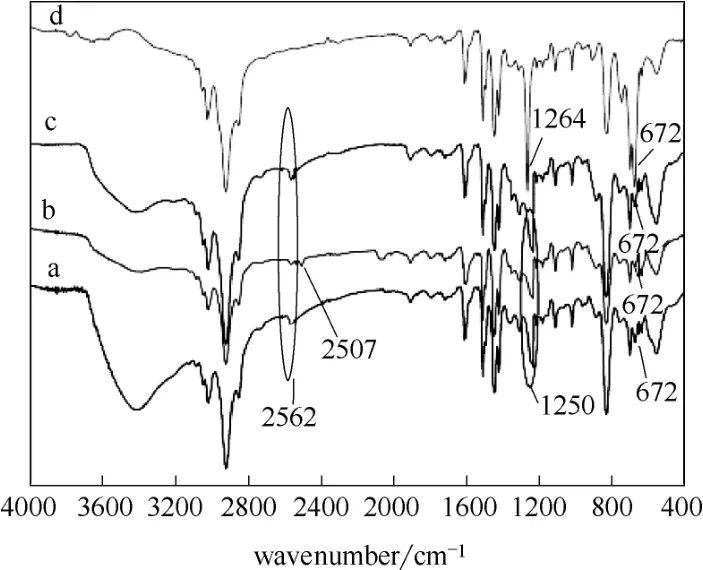
图3 树脂的红外光谱图Fig.3 FTIR spectra for resinsa—thiol polystyrene resin; b—thiol polystyrene resin captured Hg2+; c—regenerated thiol polystyrene resin; d—chloromethyl polystyrene resin, respectively
由图4可知,巯基聚苯乙烯树脂在300℃以下耐热稳定性好。在升温到800℃的过程中主要发生了3次明显的质量损失;第1降解阶段为100℃左右,这是由于样品中含的微量水所引起;第2降解阶段始于327℃,此阶段降解是树脂中的巯基的降解;第3降解阶段(427℃)为聚苯乙烯树脂的碳链降解[41]。
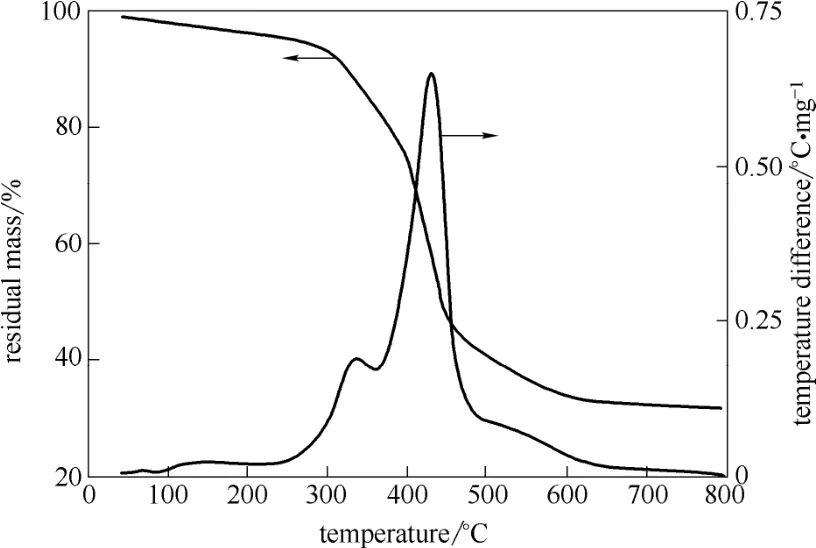
图4 巯基聚苯乙烯树脂热失重曲线Fig.4 TGA curve of thiol polystyrene resin
此外,为了测定巯基聚苯乙烯树脂的吸附性能,测试了其比表面性。从理论上说,树脂的比表面积越高,树脂的吸附容量越大[42]。从表2可知,SH的引入在一定程度上降低了树脂的比表面积、孔径和孔容。表中的树脂都具有比表面积较大、孔容较宽、孔径较长的特点,这为树脂对烟气中Hg2+的吸附做好了准备。

表2 树脂的孔径表面积分析Table 2 Pore and surface characteristics of resins
2.2各因素对烟气脱汞的影响规律
将制备的巯基聚苯乙烯树脂用于烟气脱汞处理,研究了影响脱汞性能的因素。考察处理时间对脱汞性能的影响时,以巯基聚苯乙烯树脂作为脱汞吸收剂,其烟气流速为1.0 L·min−1,处理温度为40℃。实验结果如图5所示,处理1 h后脱汞效率为95.3%,而且脱汞效率随处理时间延长而降低。分析其原因为巯基聚苯乙烯树脂对烟气中Hg2+的捕获机理为软硬酸碱理论。根据软硬酸碱理论,巯基为软碱,Hg2+为软酸,软酸和软碱极易结合成络合物[43-44],具体吸附机理如下[45]
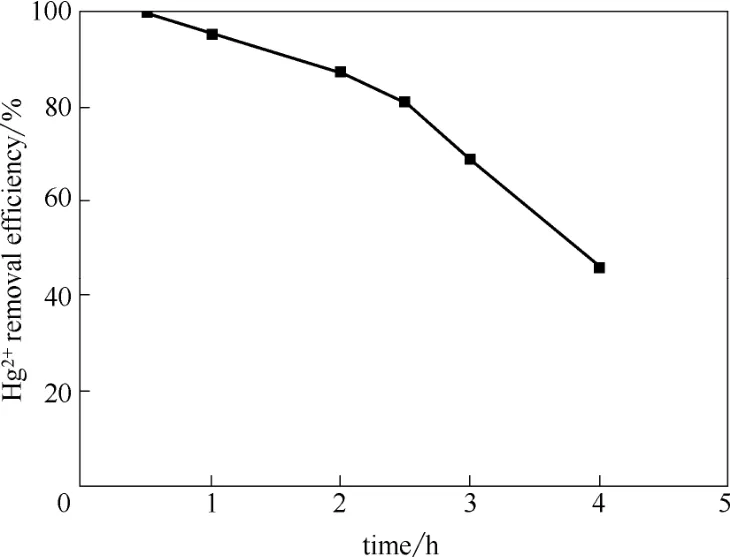
图5 处理时间对Hg2+脱除效率的影响Fig.5 Influence of treatment time on Hg2+removal efficiency
巯基聚苯乙烯树脂对Hg2+的捕集消耗了其中的巯基,故使得脱汞效率随处理时间延长而降低。此外,由图3可知巯基和Hg2+的结合不仅削弱了巯基的吸收峰(2562 cm−1),而且产生了巯基和Hg2+结合物的吸收峰(2506 cm−1),这证实Hg2+与巯基发生了化学吸附。当处理时间大于2 h时脱汞效率低于87.5%,故处理时间不宜长于2 h。
为了测定处理温度对烟气脱汞效率的影响,以巯基聚苯乙烯树脂为脱汞吸收剂,烟气流速为0.5 L·min−1,处理时间为1 h。由图6可知脱汞效率随处理温度升高而降低。这意味着巯基聚苯乙烯树脂与Hg2+的结合属于放热反应,升高温度不利于其结合[46-48]。当处理温度不高于60℃时脱汞效率均能达80%以上,而湿法脱硫系统的浆液温度约为50℃[49],这为该树脂应用于湿法脱硫系统做好了准备。巯基聚苯乙烯树脂与脱硫浆液混合,当烟气通过脱硫塔时巯基树脂捕获烟气中的Hg2+,形成稳定配合物,避免Hg2+进入脱硫废水和脱硫石膏,从根本上解决汞的再释放问题,从而克服了湿法脱硫系统脱汞的弊端。
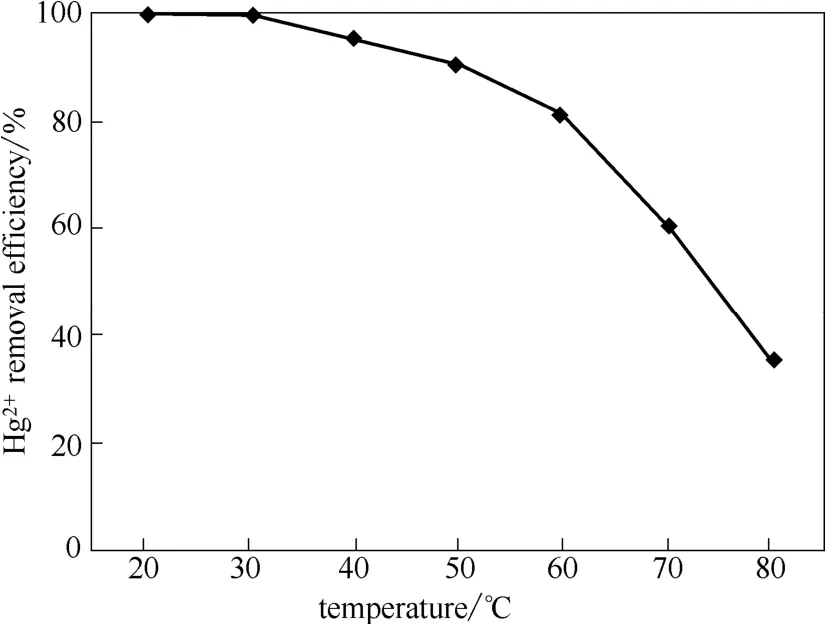
图6 处理温度对烟气脱汞效率的影响Fig.6 Influence of treatment temperature on Hg2+removal efficiency
此外,还探讨了烟气流速对巯基聚苯乙烯树脂脱汞效率的影响,实验结果如图7所示。脱汞效率随烟气流速增大而降低,当烟气流速不大于1.2 L·min−1时脱汞效率在90%以上。其原因为烟气流速的增大降低了烟气和巯基聚苯乙烯树脂的接触时间。为了获得高效脱汞,烟气流速应不高于1.2 L·min−1。

图7 烟气流速对脱汞效率的影响Fig.7 Influence of flue gas flow rate on Hg2+removal efficiency
2.3脱硫废水及脱硫浆液的脱汞处理
脱硫废水及脱硫浆液中均含一定浓度的Hg2+,汞会对环境和人体健康带来危害,因此有必要探讨巯基聚苯乙烯树脂对脱硫废水及脱硫浆液的脱汞效果。本实验所使用的脱硫废水和脱硫浆液均取自华能铜川照金电厂和杨柳青电厂,巯基聚苯乙烯树脂对脱硫废水的脱汞效率均为100%,对脱硫浆液的脱汞率分别为100%和99.5%。因此,将巯基聚苯乙烯树脂置于脱硫系统内,不仅能捕集烟气中的Hg2+,也能捕集从烟气中进入脱硫废水和脱硫浆液中的Hg2+,进而避免Hg2+进入脱硫石膏而造成汞的还原再释放。
2.4巯基聚苯乙烯树脂的再生
对巯基聚苯乙烯树脂的再生处理不仅能降低脱汞成本,而且有助于汞资源的集中处理。从2.2节中讨论的吸附机理反应方程式可知,当H+浓度较高时化学反应平衡向左移动,吸附于树脂上的Hg2+将释放至溶液中。以下探讨了盐酸洗脱液pH与树脂洗脱效果间的关系。此树脂对Hg2+的饱和吸附量为3.1 mmol·g−1。

图8 pH对巯基聚苯乙烯树脂吸附Hg2+的影响Fig.8 Hg2+adsorption over thiol polystyrene resins at various initial pH
从图8可以看出,当pH>4时此树脂对Hg2+具有强吸附能力,当pH<1时此树脂几乎不能吸附Hg2+。基于此,常用浓盐酸再生巯基聚苯乙烯树脂[46,50-51]。用6 mol·L−1盐酸和去离子水交替洗涤巯基聚苯乙烯树脂,再生处理3次后,其再生率仍高达90.2%。用红外分析、比表面分析及元素分析表征再生后的巯基聚苯乙烯树脂,从表1、表2和图3可以看出再生后的巯基聚苯乙烯树脂和原始聚苯乙烯树脂基本性质几乎一样,这也说明了巯基树脂被成功再生。用再生的巯基聚苯乙烯树脂于40℃下处理烟气1 h,其脱汞效率为95.0%。此外,从巯基聚苯乙烯树脂上洗脱下来的Hg2+进入再生处理液中,这便于实现Hg2+的集中处理,为实现汞的资源化利用打下基础。
本研究制备的巯基聚苯乙烯树脂为球状乳白色颗粒,其平均粒径为1.15 mm。此树脂稳定性好,不溶于脱硫浆液、水以及6 mol·L−1盐酸,通过简单过滤处理即可将树脂从脱硫浆液中分离出来。此外,经再生处理后该树脂捕获的Hg2+进入盐酸洗脱液,树脂沉于盐酸洗脱液底部,过滤即可实现树脂与洗脱液的分离。
3 结 论
(1)以氯甲基化聚苯乙烯树脂为原料制得了巯基聚苯乙烯树脂,该树脂具有良好的吸附性能。
(2)巯基聚苯乙烯树脂能有效吸附烟气中和脱硫废水以及脱硫浆液中的Hg2+。巯基与Hg2+的结合属于放热反应,升高温度不利于二者的结合。当处理温度低于60℃时,烟气脱汞率大于80%,而且对脱硫废水的脱汞率高达100%。
(3)此树脂与Hg2+间以化学吸附为主,当pH<1时此树脂对Hg2+几乎不具吸附能力,当pH>4时此树脂对Hg2+具有强吸附能力。
(4)用6 mol·L−1盐酸再生处理捕集了Hg2+的巯基聚苯乙烯树脂,并分别用红外光谱、比表面分析、元素分析测试巯基聚苯乙烯树脂。测试结果均显示捕集了Hg2+的巯基聚苯乙烯树脂被成功再生。再生处理3次后其再生率仍高达90.2%,这使得巯基聚苯乙烯树脂得以重复利用。
(5)采用巯基聚苯乙烯树脂脱汞具有经济性高和低污染的优点,巯基聚苯乙烯树脂有望用于实际燃煤电厂的脱汞处理中。
References
[1]WO J J, ZHANG M, CHENG X Y, et al. Hg2+reduction and re-emission from simulated wet flue gas desulfurization liquors [J]. J. Hazard. Mater., 2009, 172 (2/3): 1106-1110.
[2]LIU Y, WANG Y J, WU Z B, et al. Amechanism study of chloride and sulfate effects on Hg2+reduction insulfite solution [J]. Fuel, 2011, 90 (7): 2501-2507.
[3]STERGARŠEK A, HORVAT M, KOTNIK J, et al. The role of flue gas desulphurisation in mercury speciation and distribution in a lignite burning power plant [J]. Fuel, 2008, 87 (17/18): 3504-3512.
[4]GOODISE M E, PLANE J M C, SKOV H. Correction to a theoretical study of the oxidation of Hg0to HgBr2in the troposphere [J]. Environ. Sci. Technol., 2012, 46 (9): 5262-5262.
[5]FINLEY B D, JAFFE D A, CALL K, et al. Development, testing, and deployment of an air sampling manifold for spiking elemental and oxidized mercury during the reno atmospheric mercury inter comparison experiment (RAMIX) [J]. Environ. Sci. Technol., 2013, 47 (13): 7277-7284.
[6]LIM D H, WILCOX J. Heterogeneous mercury oxidation on Au(Ⅲ) from first principles [J]. Environ. Sci. Technol., 2013, 47 (15): 8515-8522.
[7]LIU Y, WANG Q, MEI R, et al. Mercury re-emission in flue gas multipollutants simultaneous absorption system [J]. Environ. Sci. Technol., 2014, 48 (23): 14025-14030.
[8]OCHOA-GONZÁLEZ R, DÍAZ-SOMOANO M, MARTÍNEZ-TARAZONA M R. Effect of anion concentrations on Hg2+reduction from simulated desulphurization aqueous solutions [J]. Chem. Eng. J., 2013, 214: 165-171.
[9]OMINE N, ROMERO C E, KIKKAWA H, WU S, et al. Study of elemental mercury re-emission in a simulated wet scrubber [J]. Fuel, 2012, 91 (1): 93-101.
[10]WU C L, CAO Y, HE C C, et al. Study of elemental mercury re-emission through a lab-scale simulated scrubber [J]. Fuel, 2010, 89 (8): 2072-2080.
[11]DIAZ-SOMOANO M, UNTERBERGER S, HEIN K R G. Mercury emission control in coal-fired plants: the role of wet scrubbers [J]. Fuel Process. Technol., 2007, 88 (3): 259-263.
[12]DEBERRY D W, BLYTHE G M. Bench-scale kinetics study of mercury reactions in FGD liquors[R]. Baltimore: Air and Waste Management Association, 2006.
[13]WANG Y J, LIU Y, MO J S, et al. Effects of Mg2+on the bivalent mercury reduction behaviors in simulated wet FGD absorbents [J]. J. Hazard. Mater., 2012, 237-238: 256-261.
[14]HEIDEL B, HILBER M, SCHEFFKNECHT G. Impact of additives for enhanced sulfur dioxide removal on re-emissions of mercury in wet flue gas desulfurization [J]. Appl. Energy., 2014, 114: 485-491.
[15]LU R, HOU J, XU J, et al. Effect of additives on Hg2+reduction and precipitation inhibited by sodium dithiocarbamate in simulated flue gas desulfurization solutions [J]. J. Hazard. Mater., 2011, 196: 160-165.
[16]TANG T, XU J, LU R, et al. Enhanced Hg2+removal and Hg0re-emission control from wet fuel gas desulfurization liquors with additives [J]. Fuel, 2010, 89: 3613-3617.
[17]SUN M, HOU J, TANG T, et al. Stabilization of mercury in flue gas desulfurization gypsum from coal-fired electric power plants with additives [J]. Fuel Process. Technol., 2012, 104: 160-166.
[18]DUNHAM G E, DE WALL R A, SENIOR C L. Fixed-bed studies of the interactions between mercury and coal combustion fly ash [J]. Fuel Process. Technol., 2003, 82: 197-213.
[19]TRUMAN C C, NUTI R C, TRUMAN L R, et al. Feasibility of using FGD gypsum to conserve water and reduce erosion from an agricultural soil in Georgia [J]. Catena, 2010, 81: 234-239.
[20]ÁLVARE-AYUSO E, QUROL X, TOMÁS A. Implications of moisture content determination in the environmental characterisation of FGD gypsum for its disposal in landfills [J]. J. Hazard. Mate., 2008, 153: 544-550.
[21]KAIRIES C L, SCHROEDER K T, CARDONE C R. Mercury in gypsum produced from flue gas desulfurization [J]. Fuel, 2006, 85: 2530-2536.
[22]PUDASAINEE D, KIM J H, YOON Y S, et al. Oxidation, reemission and mass distribution of mercury in bituminous coal-fired power plants with SCR, CS-ESP and wet FGD [J]. Fuel, 2012, 93: 312-318.
[23]LIU X, WANG S, ZHANG L, et al. Speciation of mercury in FGD gypsum and mercury emission during the wallboard production inChina [J]. Fuel, 2013, 111: 621-627.
[24]MARSHALL J, BLYTHE G M, RICHARDSON M. Fate of mercury in synthetic gypsum used for wallboard production [R]. Topical Reports, Prepared for the U.S. Department of Energy National Energy Technology Laboratory, Cooperative Agreement No. DE-FC26-04NT42080, 2008.
[25]程辛, 许绿丝. 钴、猛改性方法对酚醛炭泡沫除SO2/NO的影响 [J].华侨大学学报(自然科学版), 2014, 35 (5): 552-557. CHENG X, XU L S. Effect of the modification method with Co and Mn on simultaneous removal of SO2and NO of phenolic carbon foam [J]. J. Huaqiao University (Natural Science), 2014, 35 (5): 552-557.
[26]李锦, 许绿丝, 李宝宁, 等. 改性酚醛基炭泡沫的表面结构及脱硫脱硝 [J].环境工程学报, 2012, 6 (5): 1637-1642. LI J, XU L S, LI B N, et al. Surface structure and simultaneous removal of SO2and NO of modified phenolic carbon foam [J]. Chinese J. Environ. Engine., 2012, 6 (5): 1637-1642.
[27]程辛. 尿素、丙烯酸和Co改性酚醛(炭)泡沫脱除SO2/NO的研究 [D]. 厦门: 华侨大学, 2014. CHENG X. Study on simultaneous removal of SO2and NO of phenolic (carbon) foam modified by urea, acrylic acid and Co [D]. Xiamen: Huaqiao University, 2014.
[28]李锦. 金属化合物/硝酸铵改性酚醛炭泡沫脱除SO2/NO的研究[D]. 厦门: 华侨大学, 2011. LI J. Study on simultaneous removal of SO2and NO of carbon foam modified by metal compound/ammonium nitrate [D]. Xiamen: Huaqiao University, 2011.
[29]ZDABROWSKI A, HUBICKI Z, PODKOŚ P, et al. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method [J]. Chemosphere, 2004, 56: 91-106.
[30]XIONG C, YAO C. Synthesis, characterization and application of triethylenetetramine modified polystyrene resin in removal of mercury, cadmium and lead from aqueous solutions [J]. Chem. Eng. J., 2009, 155: 844-850.
[31]KOLODYŃSKA D. The effect of the novel complexing agent in removal of heavy metal ions from waters and waste waters [J]. Chem. Eng. J., 2010, 165: 835-845.
[32]何炳林, 黄文强. 离子交换与吸附树脂 [M]. 上海: 上海科学教育出版社, 1995. HE B L, HUANG W Q. Ion Exchange and Adsorption Resin [M]. Shanghai: Shanghai Technical and Educational Press, 1995.
[33]LEE W, BAE G N. Removal elemental mercury (Hg(0)) by nanosized V2O5/TiO2catalysts [J]. Environ. Sci. Technol., 2009, 43 (5): 1522-1527.
[34]LIU C, CHEN L, LI J, et al. Enhancement of activity and sulfur resistance of CeO2supported on TiO2-SiO2for the selective catalytic reduction of NO by NH3[J]. Environ. Sci. Technol., 2012, 46 (11): 6182-6189.
[35]CHO C H, IHM S K. Development of new vanadium-based oxide catalysts for decomposition of chlorinated aromatic pollutants [J]. Environ. Sci. Technol., 2002, 36 (7): 1600-1606.
[36]GAO W, LIU Q, WU C, et al. Kinetics of mercury oxidation in the presence of hydrochloric acid and oxygen over a commercial SCR catalyst [J]. Chem. Eng. J., 2013, 220: 53-60.
[37]ESWARA S, STENGER H G. Understanding mercury conversion in selective catalytic reduction (SCR) catalyst [J]. Energy & Fuel, 2005, 19: 2328-2334.
[38]SANGHI R, VERMA P. Biomimetic synthesis and characterization of protein capped silver nanoparticles [J]. Bioresource Technol., 2009, 100: 501-504.
[39]LI L, LIU Z, LING Q, et al. Polystyrene-supported Cul-imidazole complex catalyst for aza-Michael reaction of imidazoles with α,β-unsaturated compounds [J]. J. Mol. Catal. A—Chem., 2007, 69: 29-40.
[40]OSTROVERKHOV N J, TIAN C S, SHEN Y R. Characterization of vibrational resonances of water-vapor interfaces by phase-sensitive sum-frequency spectroscopy [J]. Phys. Rev. Lett., 2008, 100: 096102.
[41]MONDAL B C, DAS D, DAS A K. Synthesis and characterization of a new resin functionalized with 2-naphthol-3,6-disulfonic acid and its application for the speciation of chromium in natural water [J]. Talanta, 2002, 56: 145-152.
[42]LOZANO-CASTELLÓ D, CAZORLA-AMORÓS D, LINARESSOLANO A, et al. Influence of pore structure and surface chemistry on electric double layer capacitance in non-aqueous electrolyte [J]. Carbon, 2003, 41: 1765-1775.
[43]CHENG X, LI S, ZHONG Z, WANG S, et al. Carbamodithioatebased dual functional fluorescent probe for Hg2+and S2-[J]. J. Fluoresc., 2014, 24: 1727-1733.
[44]CUI L, GUO X, WEI Q, et al. Removal of mercury and methylene blue from aqueous solution by xanthate functionlized magnetic grapheme oxide: sorption kinetic and uptake mechanism [J]. J. Colloid Interf. Sci., 2015, 439: 112-120.
[45]张青梅, 向仁军, 成应向. 巯基树脂的合成及对Hg2+的吸附特征[J]. 环境科学研究, 2010, 7 (23): 888-892. ZHANG Q M, XIANG R J, CHENG Y X. Synthesis of thiol resin and its adsorption properties for Hg2+[J]. Res. Environ. Sci., 2010, 7 (23): 888-892.
[46]TENG M, WANG H, LI F, et al. Thioether-functionalized mesoporous fiber membranes: sol-gel combined electrospun fabrication and their application for Hg2+removal [J]. J. Colloid Interf. Sci., 2011, 355: 23-28.
[47]LI G, SHEN B, LI Y, et al. Removal of element mercury by medicine residue derived biochars in presence of various gas compositions [J]. J. Hazard. Mater., 2015, 298: 162-169.
[48]LU X, JIANG J, SUN K, et al. Influence of the pore structure and surface chemical properties of activated carbon on the adsorption of mercury from aqueous solutions [J]. Mar. Pollut. Bull, 2014, 78 (1/2): 69-76.
[49]MIN H K, AHMAD T, KIM K Y, et al. Mercury adsorption characteristics of sulphur-impregnated activated carbon pellets for the flue gas condition of a cement-manufacturing process [J]. Adsorpt. Sci. Technol., 2015, 33 (3): 251-262. DOI: 10.1260/0263-6174.33.3.251.
[50]DUJARDIN M C, CAZE C, VROMAN I. Ion-exchange resins bearing thiol groups to remove mercury (Ⅰ): Synthesis and use of polymers prepared from thioester supported resin [J]. React. Funct. Polym., 2000, 43 (2000): 123-132.
[51]UKAWA N, TAKASHINA T, OSHIMA M, et al. Effect of salts on limestone dissolution rate in wet limestone flue gas desulfurization [J]. Environ. Prog., 1993, 12 (4): 294-299.
Hg2+removal from FGD system by thiol polystyrene resin
FU Kangli1, YAO Mingyu1, QIN Chuanguang2, CHENG Guangwen1, NIE Jianping1
(1National Energy R & D Center of Clean and High-efficiency Fossil-fired Power Generation Technology, Xi’an Thermal Power Research Institute Limited Company, Xi’an 710054, Shaanxi, China;2School of Natural and Applied Sciences, Northwestern Polytechnical University, Xi’an 710072, Shaanxi, China)
Abstract:Thiol polystyrene resin was prepared by two-step reaction with chloromethyl polystyrene resin as material, and then it was characterized by Fourier transform infrared spectroscopy, Brunauer-Emmett-teller, elemental and thermogravimetric analyses. The Hg2+removal performance of thiol polystyrene resin was also investigated in simulated flue gas containing Hg2+, desulfurization effluent and desulfurization slurry. It was found that thiol polystyrene resin possessed high thermal stability and could be used as an absorbent for Hg2+removal from flue gas, desulfurization effluent and desulfurization slurry. The thiol polystyrene resin showed Hg2+removal efficiencies above 90%, 100% and 100% in flue gas, desulfurization effluent and desulfurization slurry, respectively. The placation of thiol polystyrene resin in the wet flue gas desulfurization system could capture Hg2+in this system and avoid its entering to desulfurization gypsum, which could bring the mercury reemission. Moreover, thiol polystyrene resin captured Hg2+was regenerated successfully by 6 mol·L−1HCl and its regeneration rate with three times regeneration reached up 90.2%.
Key words:flue gas; coal combustion; waste water; mercury; thiol resin
中图分类号:X 701.7
文献标志码:A
文章编号:0438—1157(2016)06—2598—07
DOI:10.11949/j.issn.0438-1157.20151675
基金项目:中国博士后科学基金项目(2015M570849);国家科技支撑计划项目(2014BAA07B04)。
Corresponding author:FU Kangli, 406363513@qq.com;QIN Chuanguang, qinchg@nwpu.edu.cn

