磁共振血管壁成像技术现状及进展
李赟铎,周赜辰,李睿*,苑纯
磁共振血管壁成像技术现状及进展
李赟铎1,周赜辰1,李睿1*,苑纯2
[摘要]磁共振血管壁成像是利用磁共振原理抑制血管内流动血液信号,获取血管壁等静态组织图像的一种成像方法。由于可以对血管壁进行直接成像,这种方法可以用于评估动脉粥样硬化斑块的形态、成分,进而确定斑块的风险程度。血管壁成像技术的核心问题在于如何有效抑制流动血液的信号,本文就目前磁共振血管壁成像技术的现状及进展做简要的总结回顾。
[关键词]磁共振成像;心血管疾病;动脉粥样硬化斑块
作者单位:1. 清华大学医学院生物医学工程系,生物医学影像研究中心,北京 100084 2. 美国华盛顿大学放射学系血管成像实验室,西雅图
接受日期:2015-12-07
李赟铎, 周赜辰, 李睿, 等. 磁共振血管壁成像技术现状及进展. 磁共振成像,2016, 7(2): 142–148.
2Department of Radiology University of Washington, Box 357115, 1959 NE Pacific Ave, Seattle, WA 98195, USA
*Correspondence to: Li R, E-mail: leerui@tsinghua.edu.cn
Received 29 Otc 2015, Accepted 7 Dec 2015
ACKNOWLEDGMENTS This work was part of Fund Project of Beijing Municipal Science and Technology Commission(No. Z131100005213001).
因血管高危斑块所引发的心脑血管疾病已经成为危害人类健康的头号杀手。基于影像学手段的血管斑块监测,对于心脑血管疾病的预测、分期和预后评估都有着非常重要的意义。好的影像学评估方法应能够充分满足临床诊治的需要,结合动脉粥样硬化相关疾病的临床实践,其应尽可能满足以下3点要求[1]:(1)具有非侵入性以保证可以进行短期和长期的研究;(2)能够提供斑块在不同时期的形态学、组织成分和炎症反应的定量信息;(3)能够被病理学的金标准所验证。
磁共振成像设备是综合了物理、电子、材料、计算机、数学、医学等学科高新技术的现代化仪器。磁共振成像具有高软组织对比度、多对比度成像、任意截面成像、无电离辐射等诸多优势,在血管壁成像方面,磁共振成像可以满足上述3个条件,具有广阔的发展和应用空间。磁共振血管壁成像技术是基于磁共振物理原理,通过抑制血管内流动血液的信号来获得血管壁等静态组织信息的一种方法,能够对动脉粥样硬化斑块的形态和成分进行评估。以下将简要综述磁共振血管壁成像技术的发展现状、前沿进展和临床应用。
1 磁共振血管壁成像技术
血管壁成像技术的核心问题在于如何有效抑制流动血液的信号,从而准确识别血管腔-壁交界,评估动脉粥样硬化斑块的形态和成分。笔者针对磁共振血管壁成像发展历史上的一些重要技术作简要综述。
1.1传统的二维血管壁成像技术
传统的二维血管壁成像技术包括饱和带技术、双反转恢复技术以及四反转恢复技术。饱和带技术[2-3]是通过在血流流入方向施加饱和带来实现血流抑制的目的,该技术是最为“古老”的磁共振血管壁成像技术,但其血流抑制效果差容易出现血流伪影,现在已较少在临床上应用;双反转恢复技术[4]通过分别施加一个非选择性180度反转脉冲和一个选择性180度反转脉冲来实现血流抑制,该方法是目前最常用的血流抑制方法,但由于恢复时间T1较长且只能单层采集,采集效率很低。为解决这一问题,Song HK等[5]提出了利用多层选择反转脉冲来提高采集效率,Yarnykh V等[6]随后又提出了增加反转脉冲层厚同时覆盖多层的技术,但其血流抑制效果会受到一定的影响;而四反转恢复技术[7]通过施加两组双反转脉冲实现血流抑制,该技术对于血液T1值的波动不敏感,可以用于对比增强磁共振血管壁成像,但其原理和双反转恢复技术类似,采集效率很低。由于以上技术都基于血流流动方向与成像平面大体垂直这一假设,因此它们均依赖于流入效应来达到血流抑制的效果,无法进行层面内的血流抑制,基本上都只用于二维成像。与二维成像方式相比,三维成像具有采集效率和信噪比较高,以及可以实现各向同性分辨率采集等优势,近年来,研究人员提出了若干适合三维成像的血管壁成像方法。
1.2运动敏感驱动平衡(motion sensitized driven equilibrium, MSDE)技术
MSDE被广泛应用于磁共振血管壁成像中,该技术的原理主要是依靠MSDE准备脉冲内设置的梯度场各阶矩,使血流散相,从而达到血流抑制的目的,血液流动模式越复杂、流动速度越快,则越容易通过该技术达到抑制效果。该技术最早于2007年被不同的两个研究组先后提出[8-9],分别被用于3 T主动脉和颈动脉成像上。2010年通过引入双聚相脉冲针对MSDE准备脉冲的涡流响应及B0和B1特性进行了优化,优化后的技术称为iMSDE[10](improved MSDE),并结合散相梯度回波(spoiled gradient echo, SPGR)采集方式,得到三维各向同性分辨率血管壁图像,该技术被称为3DMERGE技术[11],对管壁增厚程度(斑块尺寸)的测量更为准确。最近,Obara M等人[12]通过在iMSDE前设置一对双极性梯度波形,对涡流响应又进行了进一步改善,从而得到了信号强度更为均匀的脑组织图像。目前,MSDE技术可以实现在很短的时间内(0~18 ms)达到大范围抑制血流的效果,同时对于主磁场B0和发射场B1的不均匀性也具备一定的抵抗能力。但这种方法在预脉冲当中采用了T2准备脉冲和双极梯度,从而导致图像信噪比下降并使图像的对比度带有一定的T2和扩散加权。
1.3T2IR技术
2010年,Liu CY等[13]提出了一类只依赖于纵向弛豫时间T1和横向弛豫时间T2的选择性血流抑制方法(T2-prepared Inversion Recovery, T2IR),结合二维快速自旋回波(turbo spin echo, TSE)作为数据采集模块,在1.5 T下被用于主动脉的大范围成像。2011年在采集方面,利用平衡稳态自由进动(balanced steady state free precession, bSSFP)替换了TSE,序列的采集效率得以改进,被用于1.5 T下肢腘动脉的成像[14]。T2IR还可以与相位敏感技术结合,以牺牲采集效率为代价进一步改善了管腔和管壁之间的对比度,被用于3 T下三维下肢动脉管壁的成像[15]。T2IR技术表面上回避了血液流动问题,但由于特异性选择血液信号需要较长的T2准备脉冲时间(≥40 ms),使得其无法覆盖流速较慢或极快的血液。此外,B0和B1场在成像区域内存在不均匀性,有可能导致T2准备脉冲失效,从而对血流抑制的效果造成影响。
1.4DANTE技术
最近有研究人员针对三维黑血预脉冲提出了变延迟进动定制激发(delays alternating with nutation for tailored excitation, DANTE)的血流抑制方法[16],通过连续的小角度激发脉冲结合散相梯度,使得处于运动和静止的物质产生不同的稳态信号,从而达到抑制血液信号的目的,该方法对于B0和B1的不均匀性不敏感。相对于MSDE方法,DANTE的优势在于,其对静态组织信号的保护比较好。但是DANTE的问题在于,如果要达到较好的血流抑制效果,需要反复施加DANTE的血流抑制小单元,使得整个准备模块的时间较长。同时,该方法对于梯度系统的要求也较高,需要梯度场能够在短时间内攀升到相对比较大的梯度强度。目前基于该方法已经建立起检测斑块内出血(intra plaque hemorrhage, IPH)的三维快速成像序列[17]。DANTE对于流速较慢的脑脊液(cerebral spinal fluid, CSF)也能起到比较好的信号抑制作用,可以为颈部脊髓成像[18]和颅内管壁成像[19]提供更好的对比度。
1.5SNAP技术
此外,针对斑块特定危险成分的检测如IPH,也引起了磁共振成像领域的广泛关注。高铁血红蛋白作为一种内源性对比剂,它将导致纵向弛豫常数T1的缩短,从而在T1加权图像上产生高信号。因此,高铁血红蛋白的存在促进了磁共振对IPH的识别,目前最为经典的IPH检测序列是基于反转准备脉冲的快速梯度回波(magnetization prepared rapid gradient echo, MPRAGE)序列[20],它既可以显示出IPH,也可以达到抑制管腔内血液信号的作用[21]。2010年,Wang J等人[22]设计出体选择相位敏感反转(slab-selective phase-sensitive inversion-recovery, SPI)序列,该技术降低了对血液T1值估计和序列参数设置准确性的要求,提高了管壁管腔的对比度以及IPH和正常管壁之间的对比度。通过进一步优化采集方式和成像参数,Wang J等人[23]又于2013年提出非增强血管造影和IPH同时成像(simultaneous noncontrast angiography and intraPlaque hemorrhage, SNAP)序列,该技术利用一次采集,就可以同时得到磁共振血管造影的信息以及IPH的分布信息,避免了采集效率上的损失。
1.6变角度多自旋回波序列
基于自旋回波序列的各种改进构成了血管壁成像方法的另一大类,为了提高采集效率,一般都采用带有回波链的快速自旋回波进行成像,这种序列当中存在大量的180度回聚脉冲,一方面会使采集效率变低,另一方面会产生过高的特定吸收率(specific absorption rate, SAR)。针对这一问题,一系列基于拓展相位图(extended phase graph,EPG)方法设计的变角度硬脉冲方法[24-26]应运而生,可以使快速自旋回波在高场下能够完成三维大范围成像采集。另一方面,变角度的回聚脉冲对于抑制血流也会产生更好的效果[27-28],这是由于变角度回聚脉冲会产生多条回波通路,使得分布在回聚脉冲前后的散相梯度对运动变得更为敏感,这一现象也能够通过类似DANTE的血流抑制原理来解释。此外有研究人员还通过在第一个180度回聚脉冲前后各引入一个单极梯度,进一步改善变角度回聚TSE序列的血流抑制效果[29]。这一系列改进使得TSE序列可以应用于从颅内动脉至下肢动脉的全身各部位血管床的黑血管壁成像[29-32]。该序列虽然保证了管壁信号具有足够高的SNR,但其采集效率相对于梯度回波序列而言较低。
伴随着磁共振软硬件技术的迅速发展,磁共振血管壁成像技术已日趋成熟,成像空间维度由二维发展到三维,成像范围不断扩大,血流抑制效果不断优化,对于管壁斑块成分的识别和定量分析也更加准确。血管壁成像技术的发展历程详见图1。

图1 血管壁成像技术的发展历程Fig. 1 The development of vessel wall imaging techniques
2 临床应用
在临床上,磁共振血管壁成像技术被用于多个血管床成像,针对不同血管床的结构和血流,研究人员开发了不同的技术,以满足相应的临床应用需求。
颈动脉因其所处位置较为表浅,并且尺寸与磁共振成像的分辨率较为匹配,因此针对颈动脉血管壁已建立起较为成熟的磁共振动脉粥样硬化斑块风险评估体系[33-35]。临床上,研究人员通过多对比度成像的方法,可以识别血管斑块的成分,如斑块内出血(intra-plaque hemorrhage, IPH)、钙化(calcification, CA)、脂质核(lipid rich necrotic core, LRNC)、纤维帽(fibrous cap, FC)等,进而达到对血管斑块定量分析的目的。目前采用的二维成像序列包括T1和T2加权的TSE序列,以及三维飞行时间(time of flight, TOF)序列。以上3个序列与质子密度加权的基准序列配合,可以用来识别钙化和脂质核。此外,利用钆对比剂增强T1加权图像,可以使脂质核的评估更为准确,同时对比剂增强也有利于识别及测量纤维帽。不同斑块成分所对应的图像强度特性见表1。
除颈动脉以外,也有大量针对颅内血管床管壁成像的研究,通过多对比成像的方式来进行颅内斑块成分的识别[36-37]。颅内血管床由于走形迂曲,且血管内径较细,对磁共振血管壁成像技术提出了诸多挑战。最近,有研究者将变角度TSE序列和DANTE配合使用,应用于大范围颅内外血管壁成像,成像质量和血流抑制效果都显著优于单独使用变角度TSE序列[38]。目前颅内血管壁成像技术的分辨率已经可以观测到大脑中动脉[39-40],文献报道的最高的三维成像空间分辨率达到0.4~0.5 mm(各向同性)[31]。
近年来,也有研究开始将血管壁成像应用于冠状动脉的评估。与颈动脉和颅内动脉相比,冠状动脉管壁面临着更多的技术挑战,包括心脏搏动、呼吸所造成的运动伪影,以及冠脉管壁较细等,都对成像的时间和空间分辨率提出了一定要求。早期的研究尝试通过二维TSE成像并要求受试者屏气[41]或使用导航门控[42]的方式,对冠脉进行管壁成像。为了实现快速采集,三维螺旋采集[43]和放射状采集[44]技术,也被用于三维冠脉管壁成像。以上技术也逐步开始应用于冠脉外向重构[45-50](outward remodeling)、冠脉斑块[51-54]和对比剂增强成像[55-58]的研究,但成像质量和稳定性都有待提高。近年来,有研究者提出多时相冠脉管壁成像[59-60](multiphase acquisitions)的技术,与以往只采集心动周期单个特定时相的图像不同,多时相管壁成像在一个心动周期内,选择多个时间点进行采集,允许图像判读人员从多幅图像选择质量最优的进行分析,这样使得总体成像的质量和稳定性得到提升。

表1 多对比度磁共振斑块成分区分标准[34]Tab. 1 Criteria for the identification of plaque components in MR plaque imaging[34]
3 问题及展望
传统的多对比度血管壁成像技术,在技术层面还存在一些问题亟待解决和优化:(1)目前还需要通过扫描多个序列才能获取血管壁的较为完整的信息,这就会带来诸如扫描时间较长、因病人在序列间隙移动而导致序列之间的图像错配、以及临床上图像判读复杂等问题;(2)受限于线圈覆盖范围等技术问题,传统的血管壁成像技术的成像范围较小,难以对诸如颅内外血管床等大范围血管床进行全面评估;(3)目前的血管壁成像技术成像速度较慢,单次检查至少需要15~20 min,限制了其在临床上的应用。针对这些问题,在今后的研究中,磁共振血管壁成像技术还可以进一步发展。
近年来,研究人员在已有血管壁成像技术的基础上,又提出了一些新的成像方案。2014年,Fan Z等人[61]开发了MATCH(multi-contrast atherosclerosis characterization)技术,实现了在5 min之内采集多对比度的2D图像。通过在一个重复时间(repetition time,TR)中多次采集,MATCH可以获取到T1、T2加权,以及灰血的图像,通过解读这些图像,可以在一个成像序列内分辨出斑块内出血、钙化和脂质核等斑块成分信息。该技术目前只实现了2D成像,并且覆盖范围仅限于颈动脉。
为了对颅内外血管同时成像,在临床上全面评估颅内外血管病变,清华大学生物医学影像研究中心利用自主研发的36通道神经血管线圈,采用3D-MERGE、VISTA(volumetric isotropic TSE acquisition)序列和SNAP序列,实现了可覆盖颈动脉直至颅内的大范围多对比度3D黑血成像[62](图2)。该方法可以在15 min之内完成大范围多对比度的三维血管壁图像,其较长的扫描时间在一定程度上限制大范围血管壁成像技术在临床上的应用。
通过数据降采,在图像重建层面实现快速成像,也是未来磁共振血管壁成像领域的一个重要发展方向。近年来,有研究尝试将压缩感知和3DMERGE序列结合,在不影响血流抑制效率和成像质量的情况下,提高了成像速度[63-65]。Gong E等人[66]利用多对比度不同序列图像中可共享的信息,优化了压缩感知结合部分并行成像,提出了可应用于血管壁多对比度成像的应用可共享数据的并行成像及压缩感知的重建方法(parallelimaging and compressed sensing reconstruction of multicontrast imaging using sharablE information,PROMISE),该方法对于序列之间病人的运动更为不敏感,提高了管壁斑块多对比度图像的重建质量。Zhou Z等人[67]开发了一种基于自支撑定制k空间估计的并行成像(self-supporting tailored k-space estimation for parallel imaging reconstruction,STEP)方法,进一步提升了重建质量。
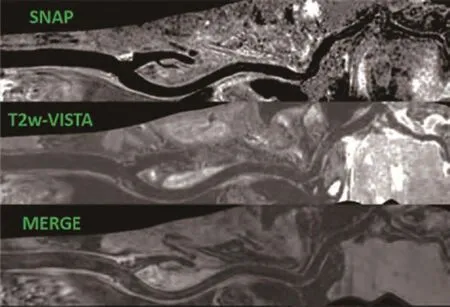
图2 经曲面重建后的大范围多对比度黑血成像,覆盖范围从颈总动脉至大脑中动脉。从上至下依次为SNAP、T2w-VISTA和MERGEFig. 2 Large-coverage multi-contrast black blood imaging (after curved reconstruction), which covers an area from common carotid artery to middle cerebral artery. Different imaging sequences are SNAP(upper panel), T2-VISTA(middle panel) and MERGE(lower panel), respectively.
4 总结
综合以上讨论,磁共振血管壁成像可以提供精细的空间分辨率和斑块成分的定量分析,有潜力成为临床评估动脉粥样硬化致病风险的重要手段。当前,磁共振黑血成像技术还面临一些挑战:第一,磁共振黑血成像技术虽然对于颈动脉管壁成像效果较好,但是在其他动脉血管壁成像,如冠状动脉成像方面,仍存在一定局限性[68];第二,其成像速度较慢[69],这成为该技术向临床推广应用的一大瓶颈。如何在短时间内获得大范围、高质量的、包含斑块各成分信息的图像,将成为磁共振血管壁成像领域未来的发展方向。
参考文献[References]
[1]Yuan C, Kerwin WS, Yarnykh VL, et al. MRI of atherosclerosis in clinical trials. NMR Biomed, 2006, 19(6): 636-654.
[2]Edelman RR, Atkinson DJ, Silver MS, et al. Frodo pulse sequences: a new means of eliminating motion, flow, and wraparound artifacts. Radiology, 1988, 166(1): 231-236.
[3]Felmlee JP, Ehman RL. Spatial presaturation: a method for suppressing flow artifacts and improving depiction of vascular anatomy in MR imaging. Radiology, 1987, 164(2): 559-564.
[4]Edelman RR, Chien D, Kim D. Fast selective black blood MR imaging. Radiology, 1991, 181(3): 655-660.
[5]Song HK, Wright AC, Wolf RL, et al. Multislice double inversion pulse sequence for efficient black-blood MRI. Magn Reson Med, 2002, 47(3): 616-620.
[6]Yarnykh VL, Yuan C. Multislice double inversion-recovery black-blood imaging with simultaneous slice reinversion. J Magn Reson Imaging, 2003, 17(4): 478-483.
[7]Yarnykh VL, Yuan C. T1-insensitive flow suppression using quadruple inversion-recovery. Magn Reson Med, 2002, 48(5): 899-905.
[8]Koktzoglou I, Li D. Diffusion-prepared segmented steady-state free precession: application to 3D black-blood cardiovascular magnetic resonance of the thoracic aorta and carotid artery walls. J Cardiovasc Magn Reson, 2007, 9(1): 33-42.
[9]Wang J, Yarnykh VL, Hatsukami T, et al. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn Reson Med, 2007, 58(5): 973-981.
[10]Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized drivenequilibrium (iMSDE) sequence. J Magn Reson Imaging, 2010,31(5): 1256-1263.
[11]Balu N, Yarnykh VL, Chu B, et al. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med, 2011, 65(3): 627-637.
[12]Obara M, Kuroda K, Wang J, et al. Comparison between two types of improved motion-sensitized driven-equilibrium (iMSDE) for intracranial black-blood imaging at 3.0 tesla. J Magn Reson Imaging, 2014, 40(4): 824-831.
[13]Liu CY, Bley TA, Wieben O, et al. Flow-independent T2-prepared inversion recovery black-blood MR imaging. J Magn Reson Imaging, 2010, 31(1): 248-254.
[14]Kawaji K, Nguyen TD, Zou Z, et al. Three-dimensional flowindependent balanced steady-state free precession vessel wall MRI of the popliteal artery: preliminary experience andcomparison with flow-dependent black-blood techniques. J Magn Reson Imaging, 2011, 34(3): 696-701.
[15]Xie J, Bi X, Fan Z, et al. 3D flow-independent peripheral vessel wall imaging using T2-prepared phase-sensitive inversionrecovery steady-state free precession. J Magn Reson Imaging,2010, 32(2): 399-408.
[16]Li L, Miller KL, Jezzard P. DANTE-prepared pulse trains: a novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med,2012, 68(5): 1423-1438.
[17]Li L, Chai JT, Biasiolli L, et al. Black-blood multicontrast imaging of carotid arteries with DANTE-prepared 2D and 3D MR imaging. Radiology, 2014, 273(2): 560-569.
[18]Li L, Kong Y, Zaitsu Y, et al. Structural imaging of the cervical spinal cord with suppressed CSF signal using DANTE pulse trains. Magn Reson Med, 2015, 74(4): 971-977.
[19]Wang JN, Helle M, Zhou ZC, et al. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn Reson Med, 2015, 13(3): 25667
[20]Mugler JP, Brookeman JR. Three-dimensional magnetizationprepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med, 1990, 15(1): 152-157.
[21]Moody AR, Pollock JG, O'Connor AR, et al. Lower-limb deep venous thrombosis: direct MR imaging of the thrombus. Radiology, 1998, 209(2): 349-355.
[22]Wang J, Ferguson MS, Balu N, et al. Improved carotid intraplaque hemorrhage imaging using a slab-selective phasesensitive inversion-recovery (SPI) sequence. Magn Reson Med,2010, 64(5): 1332-1340.
[23]Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med,2013, 69(2): 337-345.
[24]Hennig J, Weigel M, Scheffler K. Calculation of flip angles for echo trains with predefined amplitudes with the extended phase graph (EPG)-algorithm: principles and applications to hyperecho and TRAPS sequences. Magn Reson Med, 2004,51(1): 68-80.
[25]Busse RF, Hariharan H, Vu A, et al. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med, 2006, 55(5): 1030-1037.
[26]Park J, Mugler J, Horger W, et al. Optimized T1-weighted contrast for single-slab 3D turbo spin-echo imaging with long echo trains: application to whole-brain imaging. Magn Reson Med, 2007, 58(5): 982-992.
[27]Storey P, Atanasova IP, Lim RP, et al. Tailoring the flow sensitivity of fast spin-echo sequences for noncontrast peripheral MR angiography. Magn Reson Med, 2010, 64(4): 1098-1108.
[28]Busse RF. Flow sensitivity of CPMG sequences with variable flip refocusing and implications for CSF signal uniformity in 3D-FSE imaging. In Proceedings of the 14th Annual Meeting of ISMRM 2006, 2430.
[29]Fan Z, Zhang Z, Chung YC, et al. Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). J Magn Reson Imaging, 2010,31(3): 645-654.
[30]Mihai G, Chung YC, Merchant A, et al. T1-weighted-SPACE dark blood whole body magnetic resonance angiography (DBWBMRA): initial experience. J Magn Reson Imaging, 2010,31(2): 502-509.
[31]Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 T esla. J Magn Reson Imaging, 2011, 34(1): 22-30.
[32]Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on highspatial-resolution MR images. Radiology, 2014, 271(2): 534-542.
[33]Kerwin WS, Canton G. Advanced techniques for MRI of atherosclerotic plaque. J Magn Reson Imaging, 2009, 20(4): 217-225.
[34]Kerwin WS. Carotid artery disease and stroke: assessing risk with vessel wall MRI. ISRN Cardiol, 2012, 2012(2012): 180710.
[35]Kerwin WS, Hatsukami T, Yuan C, et al. MRI of carotid atherosclerosis. AJR Am J Roentgenol, 2013, 200(3): 304-313.
[36]Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke, 2013, 44(1): 287-292.
[37]Ryu CW, Kwak HS, Jahng GH, et al. High-resolution MRI of intracranial atherosclerotic disease. Neurointervention, 2014,9(1): 9-20.
[38]Xie Y, Yang Q, Xie G, et al. Improved black-blood imaging using DANTE-SPACE for simultaneous carotid and intracranial vessel wall evaluation. Magn Reson Med, 2015, 17(1): 1-2.
[39]Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis, 2010, 212(2): 507-511.
[40]Li ML, Xu WH, Song L, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3 T. Atherosclerosis, 2009, 204(2): 447-452.
[41]Fayad Z, Fuster V, Fallon J, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation, 2000, 102(5): 506-510.
[42]Botnar R, Stuber M, Kissinger K, et al. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation, 2000, 102(21): 2582-2597.
[43]Botnar R, Kim W, Bornert P, et al. 3D coronary vessel wall imaging utilising a local inversion technique with spiral image acquisition. Magn Reson Med, 2001, 46(5): 848-854.
[44]Katoh M, Spuentrup E, Buecker A, et al. MRI of coronary vessel walls using radial k-space sampling and steady state free rpeces- sion imaging. AJR Am J Roentgenol, 2006, 186(6): s401-s406.
[45]Kim W, Stuber M, Bornert P, et al. Three-dimensional blackblood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation, 2002, 106(3):296-299.
[46]Fernandes JL, Serrano CV Jr, Blotta MH, et al. Regression of coro- nary artery outward remodeling in patients with non-ST-segment acute coronary syndromes: a longitudinal study using noninvasive magnetic resonance imaging. Am Heart J, 2006,152(6): 1123-1132.
[47]Miao C, Chen S, Macedo R, et al. Positive remodelling of the coro- nary arteries detected by MRI in an asymptomatic population: the multi-ethnic study of atherosclerosis (MESA). J Am Coll Cardiol, 2009, 53(18): 1708-1715.
[48]Terashima M, Nguyen P, Rubin G, et al. Right coronary wall CMR in the older asymptomatic advance cohort: positive remodelling and associations with type 2 diabetes. J Cardiovasc Magn Reson, 2010, 12(1): 75-81.
[49]Kim W, Astrup S, Stuber M, et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation, 2007, 115(2): 228-235.
[50]Scott A, Keegan J, Mohiaddin R, et al. Noninvasive detection of coronary artery wall thickening with age in healthy subjects using high resolution MRI with beat-to-beat respiratory motion correction. J Magn Reson Imaging, 2011, 34(4): 824-830.
[51]Finn A, Nakona M, Narula J, et al. Concept of vulnerable/ unstable plaque. Arterioscler Thromb Vasc Biol, 2010, 30(7): 1282-1292.
[52]Maintz D, Ozgun M, Hoffmeier A, et al. Selective coronary plaque visualisation and differentiation by contrast-enhanced inversion prepared MRI. Eur Heart J. 2006, 27(14): 1732-1736.
[53]Kawasaki T, Koga S, Noguchi T, et al. Characterization of hyper- intense plaque with noncontrast T1-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc Imaging, 2009, 2(6): 720-728.
[54]Noguchi T, Kawasaki T, Tanaka A, et al. High-intensity signals in coronary plaques on non-contrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol, 2014, 63(10): 989-999.
[55]Yeon S, Sabir A, Clouse M, et al. Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multi-slice computed tomography and quantitative coronary angiography. J Am Coll Cardiol, 2007,50(5): 441-447.
[56]Ibrahim T, Makowski M, Jankauskas A, et al. Serial contrastenhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC Cardiovasc Imaging, 2009, 2(5): 580-588.
[57]Schneeweis C, Schnackenburg B, Stuber M, et al. Delayed contrast-enhanced MRI of the coronary artery wall in takayasu arteritis. PLoS One, 2012, 7(12): e50655.
[58]Hussain T, Fenton M, Peel SA, et al. Detection and grading of coronary allograft vasculopathy in children with contrastenhanced magnetic resonance imaging of the coronary vessel wall. Circ Cardiovasc Imaging, 2013, 6(1): 91-98.
[59]Abd-Elmoniem K, Gharib A, Pettigrew R. Coronary vessel wall 3 T MR imaging with time-resolved acquisition of phasesensitive dual inversion recovery (TRAPD) technique: initial results in patients with risk factors for coronary artery disease. Radiology, 2012, 265(3): 715-723.
[60]Abd-Elmoniem K, Weiss R, Stuber M. Phase-sensitive blackblood coronary vessel wall imaging. Magn Reson Med, 2010,63(4): 1021-1030.
[61]Fan Z, Yu W, Xie Y, et al. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. J Cardiovasc Magn Reson, 2014, 16(1): 53-64.
[62]Zhou Z, Li R, Zhao X, et al. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson, 2015, 17(1): 41-51.
[63]Makhijani MK, Balu N, Yamada K, et al. Accelerated 3D MERGE carotid imaging using compressed sensing with a hidden markov tree model. Magn Reson Med, 2012, 36(5): 1194-1202.
[64]Li B, Dong L, Chen B, et al. Turbo fast three-dimensional carotid artery black-blood MRI by combining three-dimensional MERGE sequence with compressed sensing. Magn Reson Med,2013, 70(5): 1347-1352.
[65]Li B, Li H, Li J, et al. Relaxation enhanced compressed sensing three-dimensional black-blood vessel wall MR imaging: preliminary studies. Magn Reson Imaging, 2015, 33(7): 932-938.
[66]Gong E, Huang F, Ying K, et al. Promise: parallel-imaging and compressed-sensing reconstruction of multicontrast imaging using sharable information. Magn Reson Med, 2015, 73(2): 523-535.
[67]Zhou Z, Wang J, Balu N, et al. STEP: self-supporting tailored kspace estimation for parallel imaging reconstruction. Magn Reson Med, 2015, 11(3): 25663.
[68]Yuan C, Zhao XH. MR imaging of vulnerable plaque: consensus and challenges. Chin J Magn Reson Imaging, 2010,1(6): 429-431.苑纯, 赵锡海. 易损斑块磁共振成像:共识与挑战. 磁共振成像, 2010, 1(6): 429-431.
[69]Zhang ZQ, He Y, Dai QY, et al. Accuracy of MR imaging to identify the coronary artery plaque: comparison with intravascular ultrasound. Chin J Magn Reson Imaging, 2010,1(2): 94-97.张兆琪, 贺毅, 戴沁怡, 等. 磁共振黑血序列冠状动脉管壁成像评价粥样硬化斑块初步研究结果:与血管内超声对照研究. 磁共振成像, 2010, 1(2): 94-97.
Current status and progress in magnetic resonance vessel wall imaging
LI Yun-duo1, ZHOU Ze-chen1, LI Rui1*, YUAN Chun21Center for Biomedical Imaging Research, Department of Biomedical Engineering,Medical School, Tsinghua University, Beijing 100084, China
Key wordsMagnetic resonance imaging; Cardiovascular diseases; Atherosclerotic plaque
AbstractMR vessel wall imaging (MRVWI) acquires the information of vessel wall by suppressing the signal of flowing blood in lumen area. MRVWI techniques can visualize vessel wall directly and evaluate plaque vulnerability by measuring morphology and components of plaque. The main target of MRVWI techniques is to suppress the signal of the flowing blood. In this survey, we will briefly review the current status and progress in MRVWI techniques.
基金项目:北京市科学技术委员会基金项目(编号:Z131100005213001)
通讯作者:李睿,E-mail: leerui@tsinghua.edu.cn
收稿日期:2015-10-29
中图分类号:R445.2;R543
文献标识码:A
DOI:10.12015/issn.1674-8034.2016.02.012

