CdS光化学修饰介孔TiO2及其增强的可见光催化活性
袁淑筠,孙丰强,龙培英,刘波雄
(华南师范大学化学与环境学院,广东广州 510006)
CdS光化学修饰介孔TiO2及其增强的可见光催化活性
袁淑筠,孙丰强,龙培英,刘波雄
(华南师范大学化学与环境学院,广东广州510006)
摘要:【目的】提高介孔TiO2材料的光催化活性。【方法】采用蒸发诱导自组装法(EISA),以四氯化钛和钛酸丁酯为钛源,嵌段共聚物P123(EO20PO70EO20)为模板剂,制备介孔TiO2。用光化学修饰法将CdS掺进介孔TiO2中,合成对可见光有较好响应的复合材料,并利用X射线衍射(XRD)、透射电镜(TEM)、原子吸收分光光度法(AAS)和光催化等手段对样品进行表征。【结果】XRD和TEM结果表明成功合成有序的六方介孔材料;AAS确定复合材料中Cd的含量为0.96 mg/g;光催化于500 W氙灯下以2×10-5mol/L次甲基蓝(MB)为模型污染物,结果显示CdS/TiO2复合材料的可见光催化活性明显提高。【结论】光化学修饰法制备的介孔CdS/TiO2复合材料可增强其可见光催化活性。
关键词:介孔CdS/TiO2光化学光催化可见光EISA
0Introduction
【Research significance】Mesoporous TiO2has better adsorption property and higher catalytic activity than non-mesoporous materials due to its high specific surface area.It is widely used for effective decomposition of organic pollutants in water or air under UV light irradiation.However,limited by titania’s low quantum efficiency and high band gap (3.0~3.2 eV,located at the ultraviolet (UV) wavelength range),very few visible light is available.To depress the recombination of photogenerated electron-hole pairs in photocatalytic processes,ingredient or structure modification have thus become an appealing challenge for developing photocatalytic technologies.【Previous research progress】Accordingly,semiconductor[1-5],transition metal[6-9],noble metal[10-12]or ion[13-17]doped mesoporous TiO2and photosensitized[18]mesoporous TiO2have been developed.The couple of TiO2with narrow band gap materials such as CdS,CdSe,PbS,etc.,was found to be effective for enhancing its visible light activity due to the fact that the photogenerated electrons from CdS could be injected into the conduction bands of TiO2,resulting in the photocatalytic reaction[19-23].Meanwhile,CdS/TiO2composites have been extensively investigated for their applications in solar energy cells,catalysis,water purification and electrochromic devices[24].CdS/TiO2composite nano-materials were studied frequently in the sol-gel[25-27],photoelectrochemical[24,28-30],coprecipitation[31-35]system.A self-assembly method was also used to prepared macroporous CdS/TiO2films materials[36]or solar cells[35].【Research breakthrough point】To date,the studies of CdS doped mesoporous TiO2compounds synthesized by photochemical modification technology have not yet been established.Nevertheless,compared to the photochemical method[37-40],hereinbefore synthesis methods often require a long time and multiple-step procedures with the problem of high costs.For instance,as the most widely used method of mesoporous TiO2modification,the sol-gel method has to take alkoxide type,water,temperature,gel formation time and catalyst into account[8-9,16,41-42].And the coprecipitation method has to bath with a great deal of organic solvent to control its hydrolysis rate[31-35].【Key issues to be resolved】By changing the light intensity and illumination time at room temperature to control the intensity of reaction,photochemical modification of nano-materials is more feasible.In this present work,using P123 as a template agent,mesoporous TiO2was prepared using the EISA method.Applying a vacuum-aided photochemical reduction technique,CdS was incorporated into its framework.The mesoporous CdS/TiO2composite was obtained with UV-light irradiation.And its physical and photochemical performances were studied.The preparation of mesoporous CdS/TiO2is schematically shown in Fig.1.
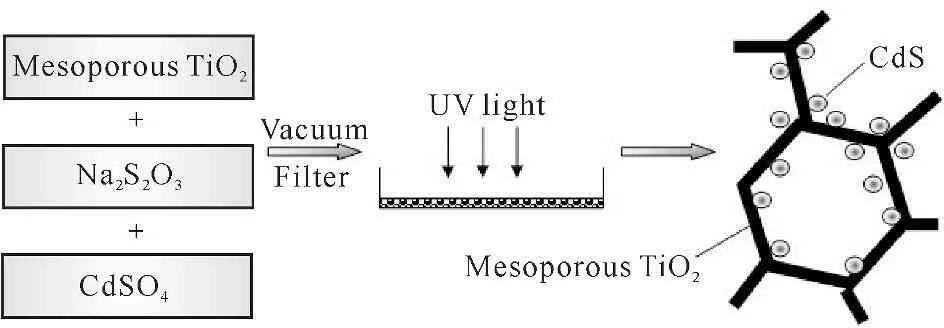
Fig.1A schematic procedure for preparation of mesoporous CdS/TiO2
1Materials and methods
1.1Synthesis of mesoporous TiO2
A typical synthesis went as follows:First,1 g block copolymer P123(EO20PO70EO20,Aldrich) was dissolved into 20 mL ethanol,and then 1 mL TiCl4and 3 mL Ti(OC4H9)4were dissolved into this solution,respectively.Here,Ti precursor particles were fully adsorbed on the surface of P123 micelle[43].Second,the resulting mixed solution was aged in an dish(with a lip made of paper )with a low humidity at 40℃ for 12 h,to evaporate the solvent and form a mesostructured copolymer/Ti hybrid transparent gel.Third,the gel was baked at 100℃ for 30 min.In this process,the micelle which adhesion metal ions inside the sol-film self-assembly arranged in the network structure[44].Last,sol-film was calcined in air with a ramp of 5℃/min to 350℃ and remained there for 70 min in non-air atmosphere,to remove the template and obtain crystallined mesoporous TiO2(sample S1).
1.2Photochemical modification of Mesoporous TiO2
An aqueous solution of 80 mL containing 0.15 mol/L CdSO4and 0.0168 mol Na2S2O3was prepared using deionized water,then vacuumed 5 min in order to eliminate the air from mesoporous and made the solution into the pores.Then,the sample was positioned so that radiation intensity received was 0.84 mW/cm2at λ=254 nm,and the diameter of the illumination region was approximately 15 mm.After 24 h,the excess CdSO4and Na2S2O3were washed away with 2% HNO3,and various methods were used to determine the properties of mesoporous CdS/TiO2(sample S2).
1.3Photocatalysis
A 500 W Xenon lamp was positioned inside a cylindrical quartzose vessel and surrounded by a circulating water jacket to cool the lamp.0.05 g of photocatalyst was suspended in a 200 mL aqueous solution of 2×10-5mol/L methylene blue(MB).Prior to irradiation,the suspensions were magnetically stirred for 30 min to ensure establishment of an adsorption/desorption equilibrium among the photocatalyst,MB and atmospheric oxygen.At room temperature and under normal pressure,5 mL of the suspensions were collected every 20 min,and then centrifuged to separate the photocatalyst particles.The degradations of MB were analyzed by a UV-vis spectrophotometer(752,Shanghai Jinghua Technology Instrument Co.,Itd)and the absorption peak at 665 nm was monitored.
1.4Characterization
Low-angle XRD measurement (0.6~3°,40 kV/20 mA,0.25°/min) was made on a Rigaku D/MAX-2200 X-ray diffractometer with Cu Kαradiation,while wide-angle XRD measurement (20~80°,30 kV/20 mA,3.6°/min) was made on a XRD-2000 X-ray diffractometer.Crystal size was measured from XRD peak broadening using the Scherrer equation.Direct TEM (JEM-2010HR,operated at 200 kV) photograph was also used to determine the structure of sample S1.
Sample S2 solution was prepared by melt in 10 mL concentrated H2SO4(150℃,1.5 h),then a TSA-986 flame atomic spectrophotometer(λ=228.9 nm) was used to determin its cadmium content.
2Results and discussion
2.1Characterized by XRD and TEM
Low-angle X-ray diffraction (LXRD,Fig.2b) shown that sample S1 obtained a peak at 1.18°,from Scherrer equation,its crystallite size is near by 9 nm,belonging to mesoporous materials; and the height and narrow diffraction peak sufficiently prove that the mesoporous TiO2sample’s pore structure with good short-range order.The size measured by XRD was found to correspond closely with that measured by direct TEM imaging(Fig.2a) for these powder.Observing reveats that the mesoporous TiO2is prepared with mesoporous ordered distribution,continuous pore,and hexagonal arrays.Wide-angle X-ray diffraction (WXRD) patterns of the prepared sample were shown in Fig.2c.The five Bragg diffraction peaks are found at 20~60°,corresponding to the anatase (101),(004),(200),(105) and (211) crystal planes,respectively.WXRD result of mesoporous sample S2 is similarity to sample S1,but there are some miscellaneous weaker peaks at the range of 20~30°,which may be CdS diffraction peaks because of the small amount CdS doping.However,CdS diffraction peaks and TiO2strong diffraction peaks are adjacent or overlapping.Therefore,it is difficult to tell them apart.
2.2Characterized by AAS
Measurement of cadmium content of sample S2,which has a dosage of 0.012 mol Cd, determines that the incorporation amount of Cd is 0.96 mg/g,that is,n(Ti)∶n(Cd)=1.4×10-4(R=0.99696).
2.3Formed mechanism

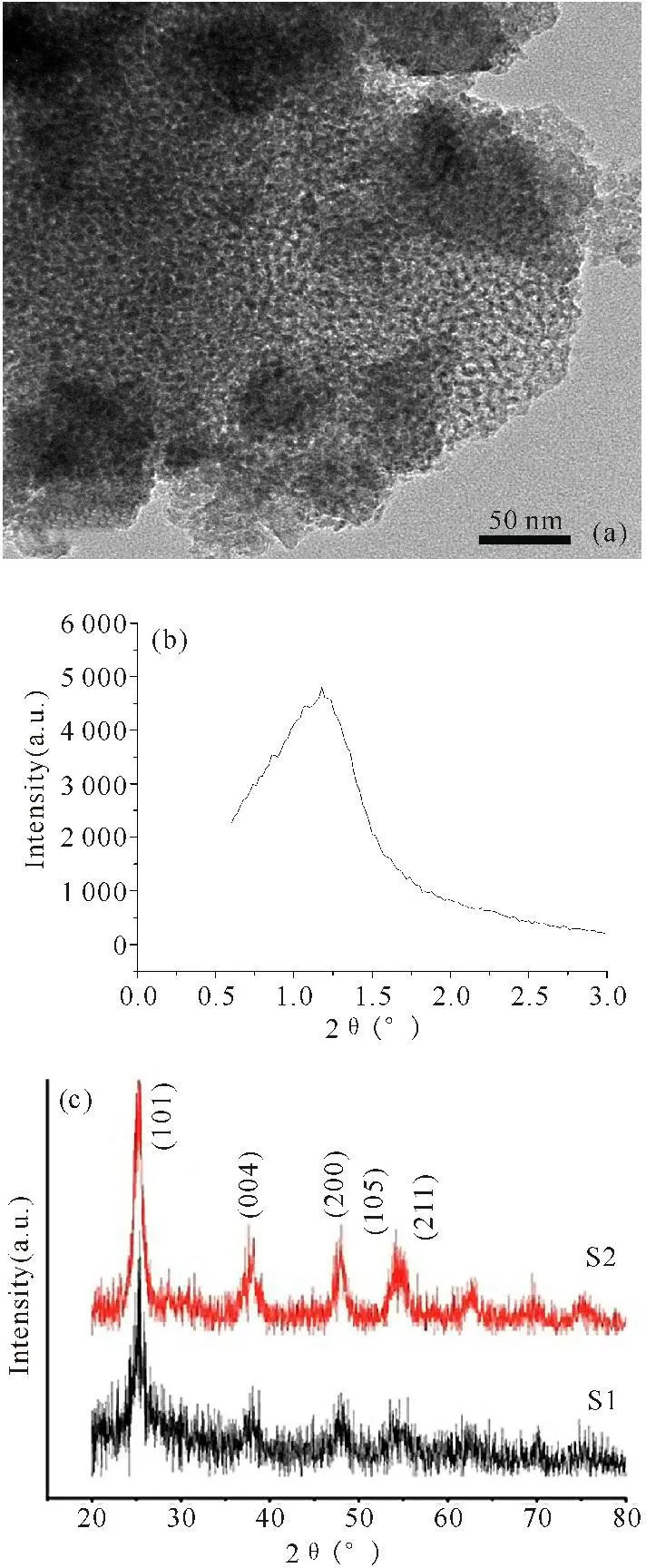
(a)TEM photography of sample S1;(b)low-angle XRD of sample S1;(c)Wide-angle XRD figure of the samples S1 and S2
Fig.2Characterizations of mesoporous TiO2(S1) and mesoporous CdS/TiO2(S2)
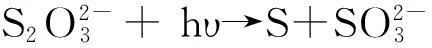
(1)


(2)
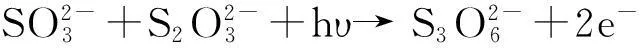
(3)
And then,CdS particles are formed in the pores.
Cd2++S+2e-=CdS.
(4)
2.4Enhanced visible-light photocatalytic activity
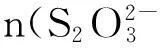

Fig.3Photocatalytic perforence of mesoporous TiO2before and after doping CdS to its framework
2.5Photocatalysis mechanism
As shown in Fig.4,mesoporous TiO2can be coupled by interparticle electron transfer from irradiated CdS nanocrystals to its conduction band.Upon UV excitation,an electron of anatase TiO2(3.2 eV band gap) may be promoted from the valence band to the conduction band (ECB) leaving behind a beneficial hole in the valence band (EVB)[30].When coupling CdS with TiO2,photogenerated electrons (ECB)flowed toward CdS and accumulated at CdS and formed Schottky barrier between CdS and TiO2(References[45-46]).
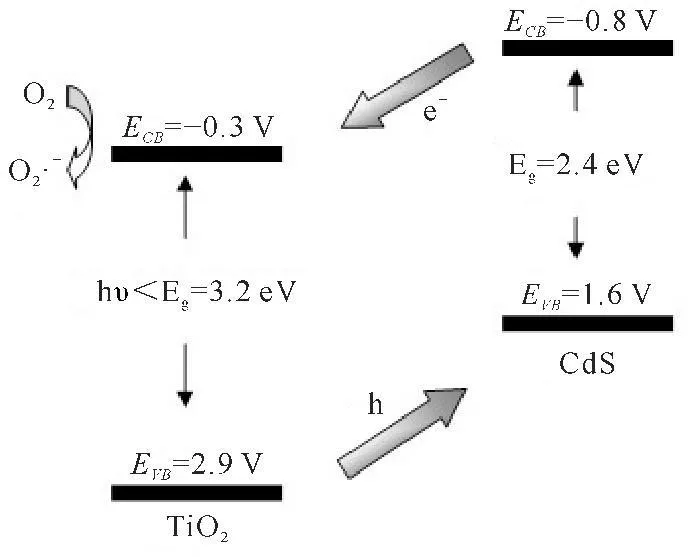
Fig.4Schematic diagram illustrating charge injection from excited CdS into TiO2
CdS/TiO2+hυ→CdS(h+)/TiO2(e-).
(5)
The electrons are then scavenged by molecular oxygen O2to yield the superoxide radical anion O2·-in oxygen-equilibrated media.
e-+ O2→O2·-.
(6)
These new formed intermediates can interreact to produce hydroxyl radical OH·.

(7)
H2O+h+→OH·+H+.
(8)
The OH· radical is a powerful oxidizing agent capable of degrading most pollutants.
OH·+MB→degradation products.
(9)
3Conclusion
The mesoporous CdS/TiO2composite material with enhanced visible-light photocatalytic activity can be prepared by photochemical modification technique.Due to its good chemical stability and non-toxic,we can foresee that it will not trigger unnecessary responses,in other words,it may be generate to low foreign objects.The reaction region and its strength can be controlled by light.It is obviously,with moderate price,the material is readily available with the best operability,strong coverage,and high refractive index[40].Therefore,the materials have good prospects in water or soil pollution,dust-proof paints and coatings,and life sciences field.
References:
[1]HE C X,TIAN B Z,ZHANG J L.Thermally stable
SiO2-doped mesoporous anatase TiO2with large surface area and excellent photocatalytic activity[J].Journal of Colloid and Interface Science,2010,344(2):382-389.
[2]JING D,GUO L.WS2sensitized mesoporous TiO2for efficient photocatalytic hydrogen production from water under visible light irradiation[J].Catalysis Communications,2007,8(5):795-799.
[3]LI L,LI Y J,MA Y,et al.Preparation and photocatalytic behaviors of nanoporous polyoxotungstate-anatase TiO2composites[J].Journal of Rare Earths,2007,25(1):68-73.
[4]BANERJEE A N.The design,fabrication,and photocatalytic utility of nanostructured semiconductors:Focus on TiO2-based nanostructures[J].Nanotechnology,Science and Applications,2011,4:35-65.
[5]KIM H,KIM J,KIM W,et al.Enhanced photocatalytic and photoelectrochemical activity in the ternary hybrid of CdS/TiO2/WO3through the cascadal electron transfer[J].The Journal of Physical Chemistry C,2011,115(19):9797-9805.
[6]MALWADKAR S S,GHOLAP R S,AWATE S V,et al.Physico-chemical,photo-catalytic and O2-adsorption properties of TiO2nanotubes coated with gold nanoparticles[J].Journal of Photochemistry and Photobiology A Chemistry,2009,203(1):24-31.
[7]CHAN C C,CHANG C C,HSU W C,et al.Photocatalytic activities of Pd-loaded mesoporous TiO2thin films[J].Chemical Engineering Journal,2009,152(2/3):492-497.
[8]LEE A C,LIN R H,YANG C Y,et al.Preparations and characterization of novel photocatalysts with mesoporous titanium dioxide (TiO2) via a sol-gel method[J].Materials Chemistry and Physics,2008,109(2/3):275-280.
[9]KORZHAK A V,ERMOKHINA N I,STROYUK A L,et al.Photocatalytic hydrogen evolution over mesoporous TiO2/metal nanocomposites[J].Journal of Photochemistry and Photobiology A:Chemistry,2008,198(2/3):126-134.
[10]SONG C X,WANG D B,XU Y H,et al.Preparation of Ag-TiO2hollow structures with enhanced photocatalytic activity[J].Materials Letters,2011,65(5):908-910.
[11]TANAKA A,SAKAGUCHI S,HASHIMOTO K,et
al.Preparation of Au/TiO2with metal cocatalysts exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation under irradiation of visible light[J].ACS Catalysis,2013,3(1):79-85.
[12]PRIMO A,CONCEPCION P,CORMA A.Synergy between the metal nanoparticles and the support for the hydrogenation of functionalized carboxylic acids to diols on Ru/TiO2[J].Chemical Communications,2011,47(12):3613-3615.
[13]HUANG S,YANG L Z,LI F Y.Effect of double elements Co-doping on nano-TiO2photocatalysis of rhodamine B solution[J].Advanced Materials Research,2013,798:25-29.
[14]KIM D,TSUCHIYA H,FUJIMOTO S,et al.Nitrog-
en-doped TiO2mesosponge layers formed by anodization of nitrogen-containing Ti alloys[J].Journal of Solid State Electrochemistry,2011,16(1):89-92.
[15]SHI Y D,GUO Q,XIE Y S.The preparation of C,N,S Tri-doped TiO2and visible-light photo-degradation of methylene blue and dyes[J].Advanced Materials Research,2011,287:1735-1743.
[16]SREETHAWONG T,LAEHSALEE S,CHAVADEJ
S.Comparative investigation of mesoporous- and non-mesoporous-assembled TiO2nanocrystals for photocatalytic H2production over N-doped TiO2under visible light irradiation[J].International Journal of Hydrogen Energy,2008,33(21):5947-5957.
[17]HOU Y D,WANG X C,WU L,et al.N-doped SiO2/TiO2mesoporous nanoparticles with enhanced photocatalytic activity under visible-light irradiation[J].Chemosphere,2008,72(3):414-421.
[18]LUCARELLI L,NADTOCHENKO V,KIWI J.Environmental photochemistry:Quantitative adsorption and FTIR studies during the TiO2-photocatalyzed degradation of orangeⅡ[J].Langmuir,2000,16(3):1102-1108.
[19]WU L,YU J C,FU X Z.Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2nanocrystals under visible light irradiation[J].Journal of Molecular Catalysis A:Chemical,2006,224(1/2):25-32.
[20]SUN W T, YU Y, PAN H Y, et al.CdS quantum dots sensitized TiO2nanotube-array photoelectrodes[J].Journal of the American Chemical Society,2008,130(4):1124-1125.
[21]ROBERT D.Photosensitization of TiO2by MxOyand MxSynanoparticles for heterogeneous photocatalysis applications[J].Catalysis Today,2007,122(1/2):20-26.
[22]ZHANGX W,LEI L C,ZHANG J L,et al.A novel CdS/S-TiO2nanotubes photocatalyst with high visible light activity[J].Separation and Purification Technology,2009,66(2):417-421.
[23]JANG J S,KIM H G,BORSE P H,et al.Simultaneous hydrogen production and decomposition of H2S dissolved in alkaline water over CdS-TiO2composite photocatalysts under visible light irradiation[J].International Journal of Hydrogen Energy,2007,32(18):4786-4791.
[24]JIAH M,XU H,HU Y,et al.TiO2@CdS core-shell nanorods films:Fabrication and dramatically enhanced photoelectrochemical properties[J].Electrochemistry Communications,2007,9(3):354-360.
[25]SO W W,KIM K J,MOON S J.Photo-production of hydrogen over the CdS-TiO2nano-composite particulate films treated with TiCl4[J].International Journal of Hydrogen Energy,2004,29(3):229-234.
[26]WANGC Y,SHANG H M,TAO Y,et al.Properties and morphology of CdS compounded TiO2visible-light photocatalytic nanofilms coated on glass surface[J].Separation and Purification Technology,2003,32(1/2/3):357-362.
[27]KUMAR A,JAIN A K.Photophysics and photochemistry of colloidal CdS-TiO2coupled semiconductors-photocatalytic oxidation of indole[J].Journal of Molecular Catalysis A:Chemical,2001,165(1/2):265-273.
[28]HAOE C,YANG B,REN H,et al.Fabrication of composite film comprising TiO2/CdS and polyelectrolytes based on ionic attraction[J].Materials Science and Engineering C,1999,10(1/2):119-122.
[29]QIAN X M,QIN D Q,SONG Q,et al.Surface photovoltage spectra and photoelectrochemical properties of semiconductor-sensitized nanostructured TiO2electrodes[J].Thin Solid Films,2001,385:152-161.
[30]CHEN S G,PAULOSE M,RUAN C M,et al.Electrochemically synthesized CdS nanoparticle-modified TiO2nanotube-array photoelectrodes:Preparation,characterization and application to photoelectrochemical cells[J].Journal of Photochemistry and Photobiology A:Chemistry,2006,177(2/3):177-184.
[31]NIITSOO O,SARKAR S K,PEJOUX C,et al.Chemical bath deposited CdS/CdSe-sensitized porous TiO2solar cells[J].Journal of Photochemistry and Photobiology A:Chemistry,2006,181(2/3):306-313.
[33]MANE R S,YOON M Y,CHUNG H,et al.Co-deposition of TiO2/CdS films electrode for photo-electrochemical cells [J].Solar Energy,2007,81:290-293.
[34]KANG M G,HAN H E,KIM K J.Enhanced photodecomposition of 4-chlorophenol in aqueous solution by deposition of CdS on TiO2[J].Journal of Photochemistry and Photobiology A:Chemistry,1999,125(1/2/3):119-125.
[35]WIJAYANTHA K G U,PETER L M,OTLEY L C.Fabrication of CdS quantum dot sensitized solar cells via a pressing route[J].Solar Energy Materials and Solar Cells,2004,83(4):363-369.
[36]SINGH R S,RANGARI V K,SANAGAPALLI S,et al.Nano-structured CdTe,CdS and TiO2for thin film solar cell applications[J].Solar Energy Materials and Solar Cells,2004,82(1/2):315-330.
[38]SUN L,LAI Y K,WANG C L,et al.Ultrasound aided photochemical synthesis of Ag loaded TiO2nanotube arrays to enhance photocatalytic activity[J].Journal of Hazardous Materials,2009,171:(1/2/3):1045-1051.
[39]PODDER J,KOBAYASHI R,ICHIMURA M.Photochemical deposition of CuS thin films from aqueous solutions[J].Thin Solid Films,2005,472(1):71-75.
[40]SUNG-SUH H M,CHOI J R,HAH H J,et al.Comparison of Ag deposition effects on the photocatalytic activity of nanoparticulate TiO2under visible and UV light irradiation[J].Journal of Photochemistry and Photobiology A:Chemistry, 2004,163(1/2):37-44.
[41]BESSEKHOUAD Y,ROBERT D,WEBER J V.Synthesis of photocatalytic TiO2nanoparticles:Optimization of the preparation conditions[J].Journal of Photochemistry and Photobiology A:Chemistry,2003,157(1):47-53.
[42]YU J G,ZHAO X J.Effect of surface treatment on the photocatalytic activity and hydrophilic property of the sol-gel derived TiO2thin films[J].Materials Research Bulletin,2001,36(1/2):97-107.
[43]BECK J S,VAURTLI J C,ROTH W J,et al.A new family of mesoporous molecular sieves prepared with liquid cyrstal templates[J].Journal of the American Chemical Society,1992,114(27):10834-10843.
[44]YIN J B,ZHAO X P.Enhanced electrorheological activity of mesoporous Cr-doped TiO2from activated[J].The Journal of Physical Chemistry B,2006,110(26):12916-12925.
[45]ANANDAN S,SATHISH KUMAR P,PUGAZHEN-
THIRAN N,et al.Effect of loaded silver nanoparticles on TiO2for photocatalytic degradation of acid red 88[J].Solar Energy Materials and Solar Cells,2008,92(7):929-937.
[46]SUBRAMANIAN V,WOLF E,KAMAT P V.Semiconductor-metal composite nanostructures.To what extent do metal nanoparticles improve the photocatalytic activity of TiO2films?[J].The Journal of Physical Chemistry B,2001,105(46):11439-11446.
(责任编辑:陆雁)
Photochemical Modification with CdS and Enhanced Visible-light Photocatalytic Activity of Mesoporous TiO2*
YUAN Shujun,SUN Fengqiang**,LONG Peiying,LIU Boxiong
(School of Chemistry and Environment,South China Normal University,Guangzhou,Guangdong,510006,China)
Abstract:【Objective】To improve the photocatalytic activity of mesoporous TiO2.【Methods】Mesoporous TiO2 was prepared by the method of evaporation-induced self-assembly (EISA),while TiCl4 and Ti(OC4H9)4 as titanium sources,and block copolymer P123(EO20PO70EO20)as the template.Using the photochemical modification technology,composite material was prepared with good response of visible light by doping CdS to its framework.The obtained samples were characterized by X-ray diffraction (XRD),transmission electronic micrograph (TEM),atomic absorption spectrophotometer(AAS)and photocatalytic.【Results】The results of XRD and TEM proved the synthesis of ordered hexagonal mesoporous materials.AAS determined the composite material content of 0.96 mg/g Cd.In 500 W xenon lamp,2×10-5mol/L methylene blue(MB) as model pollutant,the photocatalytic results showed that the photocatalytic activity of the mesoporous CdS/TiO2 composite was further improved.【Conclusion】The mesoporous CdS/TiO2 composite material with enhanced visible-light photocatalytic activity can be prepared by photochemical modification technique.
Key words:mesoporous CdS/TiO2,photochemical,photocatalytic,visible light,EISA
收稿日期:2016-01-08
作者简介:袁淑筠(1988-),女,化学分析工程师,主要从事高纯特种气体研究。
中图分类号:O643.3
文献标识码:A
文章编号:1005-9164(2016)02-0167-07
修回日期:2016-02-03
*国家自然科学基金项目(批准号:21571068)资助。
**通讯作者:孙丰强(1974-),男,教授,主要从事多孔材料、光催化等方面的研究,E-mail:fqsun@scnu.edu.cn。
广西科学Guangxi Sciences 2016,23(2):167~173
网络优先数字出版时间:2015-05-12
网络优先数字出版地址:http://www.cnki.net/kcms/detail/45.1206.G3.20160512.0944.008.html

