迷迭香提取物对高脂膳食雌性果蝇致氧化应激的调控作用及机制
王 红,陈 纯,王轶菲,程立芳,蔡佳蓉,王 浩
(1. 天津科技大学食品工程与生物技术学院,天津 300457;2. 天津科技大学生物工程学院,天津 300457)
迷迭香提取物对高脂膳食雌性果蝇致氧化应激的调控作用及机制
王 红1, 2,陈 纯1,王轶菲1, 2,程立芳1, 2,蔡佳蓉1,王 浩1
(1. 天津科技大学食品工程与生物技术学院,天津 300457;2. 天津科技大学生物工程学院,天津 300457)
摘 要:以雌性果蝇为动物模型研究迷迭香提取物(ROE)对高脂膳食致氧化应激的调控作用及机制.收集2,d龄雌性果蝇,随机分为空白组、高脂组及分别饲喂高脂饲料添加迷迭香提取物0.2、0.5、1.5,mg/mL的剂量组,每3,d更换新鲜培养基,记录果蝇死亡数目,并计算其平均寿命和最高寿命;45,d后测定超氧化物歧化酶(SOD)、过氧化氢酶(CAT)等抗氧化酶活力和丙二醛(MDA)含量;实时荧光定量PCR(Real-time PCR)法测定相关基因mRNA表达水平.结果表明:饲喂迷迭香提取物的果蝇平均寿命与最高寿命明显延长;0.2,mg/mL ROE显著升高Mn-SOD活力(P<0.05),1.5,mg/mL ROE显著升高Cu-Zn-SOD、CAT活力(P<0.05),极显著升高Mn-SOD活力,降低MDA含量(P<0.01);0.5,mg/mL ROE 显著上调Cu-Zn-SOD、Mn-SOD、CAT mRNA表达水平(P<0.05),极显著上调Nrf2,mRNA表达水平,1.5,mg/mL ROE极显著上调Mn-SOD及Nrf2,mRNA表达水平(P<0.01).因此,ROE能有效延缓高脂导致的氧化损伤,这可能与上调抗氧化基因表达水平有关.
关键词:迷迭香提取物;高脂膳食;黑腹果蝇;抗氧化;酶活力;基因表达
迷迭香(Rosmarinus officinalis L.),为唇形科灌木,因其具有优良的抗氧化活性,广泛用于医药、食品及油脂保鲜等.迷迭香中含有鼠尾草酸、鼠尾草酚、迷迭香酸、迷迭香酚、迷迭香醌、咖啡酸、迷迭香二酚、熊果酸等多种活性成分.方晓璞等[1]研究了迷迭香天然抗氧化剂在核桃油中的应用,结果表明迷迭香具有较佳的抗氧化效果.Aruoma等[2]研究显示,鼠尾草酚、鼠尾草酸能有效阻止羟自由基的形成,抑制肝细胞微粒体脂质氧化[3].
高脂饮食可引起脂质过氧化损伤.抗氧化剂可以有效清除体内多余的自由基,延缓高脂导致的氧化损伤.研究显示[4],高脂膳食中分别添加0.5%,和5%,的迷迭香干粉,可以显著抑制C57BL/6J小鼠因高脂膳食引起的动脉血栓的形成.
果蝇常被作为研究衰老因素的模式生物[5].具有生命周期短,饲养简单的特点,且其衰老基因与人类具有很大的相似性[6].本文以Oregon K 野生型雌性黑腹果蝇(Drosophila melanogaster)为研究对象,探究迷迭香提取物对高脂导致氧化损伤的果蝇体内抗氧化酶活性及脂质过氧化物含量的影响,并采用定时荧光定量PCR(Real-time PCR)法测定其对抗氧化相关基因表达水平的影响.
1 材料与方法
1.1材料与仪器
Oregon K野生型黑腹果蝇(Drosophila melanogaster)由天津科技大学食品添加剂与营养调控研究室提供,迷迭香提取物(ROE)由天津尖峰天然产物研究开发有限公司提供.
超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、丙二醛(MDA)试剂盒,南京建成生物工程研究所;Trizol试剂、cDNA 合成试剂盒、SYBR green,宝生物工程(大连)有限公司.
UVmini-1240型紫外-可见分光光度计,日本岛津公司;HWS-850型智能恒温恒湿培养箱,宁波海曙赛福实验仪器厂;Real-time PCR仪,美国 Bio-Rad公司.
1.2方法
1.2.1果蝇培养
选用2,d龄雌性果蝇,于温度为(25±1)℃,湿度为50%~60%,的恒温恒湿培养箱内培养,每3,d更换新鲜培养基并记录果蝇生存状况.
基础培养基:蒸馏水1,000,mL,葡萄糖96,g,玉米粉96,g,酵母粉13.3,g,琼脂粉8,g,防腐剂(含1%,对羟基苯甲酸乙酯的75%,酒精)53.3,mL.
高脂培养基为普通培养基中添加10%,猪油,实验培养基在高脂培养基中分别添加0.2、0.5、1.5,mg/mL的迷迭香提取物.
1.2.2果蝇寿命实验
收集2,d龄雌性果蝇1,000只,分为5组,即正常组、高脂组和3个剂量组(迷迭香提取物质量浓度分别为0.2、0.5、1.5,mg/mL),每组200只,分别饲喂基础培养基、高脂培养基及添加不同质量浓度(0.2、0.5、1.5,mg/mL)迷迭香提取物的高脂培养基,置于培养箱中培养.每3,d更换新鲜培养基,同时记录果蝇生存状况,直至果蝇全部死亡,统计其平均寿命与最高寿命.
1.2.3果蝇抗氧化酶活力及MDA含量测定
收集2,d龄的雌性果蝇2,000只,每组400只,饲喂方法同寿命实验,45,d后,CO2麻醉后称质量,-80,℃备用.果蝇用生理盐水冰浴匀浆,4,℃、2,500 r/min离心10,min,取上清液测定蛋白质量浓度、Cu-Zn-SOD、Mn-SOD、CAT酶活力及MDA含量.
1.2.4果蝇Cu-Zn-SOD、Mn-SOD、CAT、MTH、Nrf2基因mRNA表达水平的测定
取45,d果蝇,Trizol法提取mRNA,反转录得cDNA,Real-time PCR法测定Cu-Zn-SOD、Mn-SOD、CAT、MTH和Nrf2,mRNA表达水平.果蝇抗氧化基因Real-time PCR引物[7]见表1.
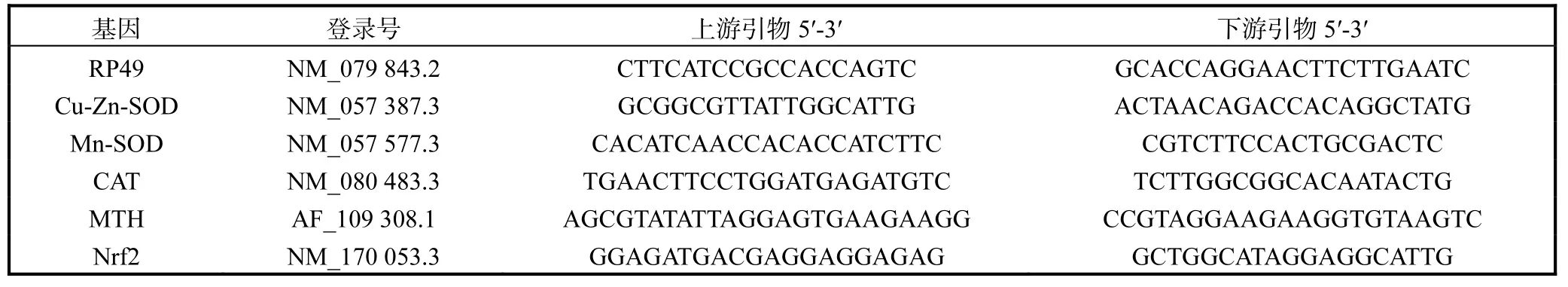
表1 果蝇抗氧化基因PCR引物Tab.1 PCR primers used to measure Drosophila melanogaster antioxidant genes
1.3数据处理
使用SPSS statistics17.0软件进行统计分析,以“平均值±标准差”形式表示,组间差异采用方差检验分析.#与##分别表示与正常组相比P<0.05、P<0.01;*与**分别表示与高脂组相比 P<0.05、P<0.01.
2 结果与分析
2.1迷迭香提取物主要活性成分分析
经HPLC-MS/MS检测,迷迭香提取物中主要活性成分分别为迷迭香酸1.5%、迷迭香酚7.2%、鼠尾草酚33.2%、鼠尾草酸30.3%、鼠尾草酸甲酯27.8%,具体结果见图1和表2.

图1 HPLC 分析迷迭香提取物及主要活性成分Fig.1 HPLC analysis of rosemary extract and its main active ingredients
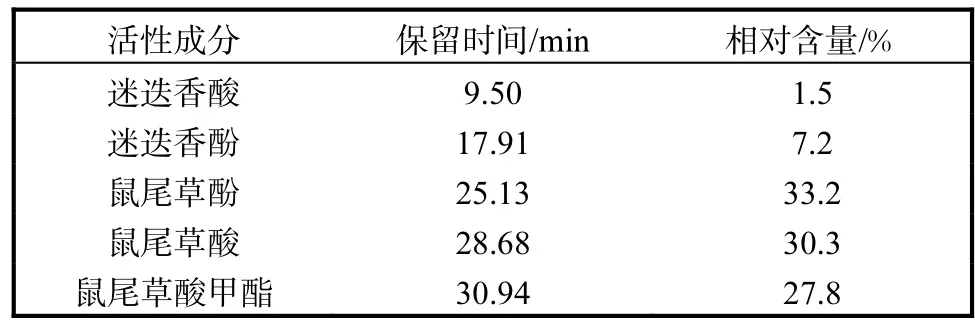
表2 迷迭香提取物主要活性成分及相对含量Tab.2 The main active ingredients of rosemary extract and its percentage compositions
2.2迷迭香提取物对雌性高脂果蝇寿命的影响
迷迭香提取物对雌性高脂果蝇寿命的影响,结果如图2所示.高脂膳食饲喂果蝇的平均寿命与最高寿命均明显低于正常组果蝇(P<0.05),而其体重间并无显著性差异(P>0.05).与高脂对照组比,饲喂迷迭香提取物的果蝇寿命显著延长(P<0.05),其中0.5、1.5,mg/mL剂量组果蝇的平均寿命与最高寿命分别延长了15.77%,、20.23%,和9.75%,、13.26%,呈现剂量依赖关系.具体结果见图2和表3.

图2 果蝇寿命曲线Fig.2 Lifespan curve of male Drosophila melanogasters
2.3迷迭香提取物对果蝇体内Mn-SOD、Cu-Zn-SOD、CAT酶活力及MDA含量的影响
饲喂迷迭香提取物后,果蝇体内相关抗氧化酶活力较正常组均有提高,过氧化产物含量均有下降,且呈现剂量依赖关系(表4).与对照组相比,0.2,mg/mL 组Cu-Zn-SOD、CAT活力升高,Mn-SOD活力显著升高(P<0.05),0.5,mg/mL组Mn-SOD活力显著升高(P<0.05),1.5,mg/mL组果蝇体内Cu-Zn-SOD、CAT活力显著升高(P<0.05),CAT活力极显著升高(P< 0.01),MDA含量显著降低(P<0.05).
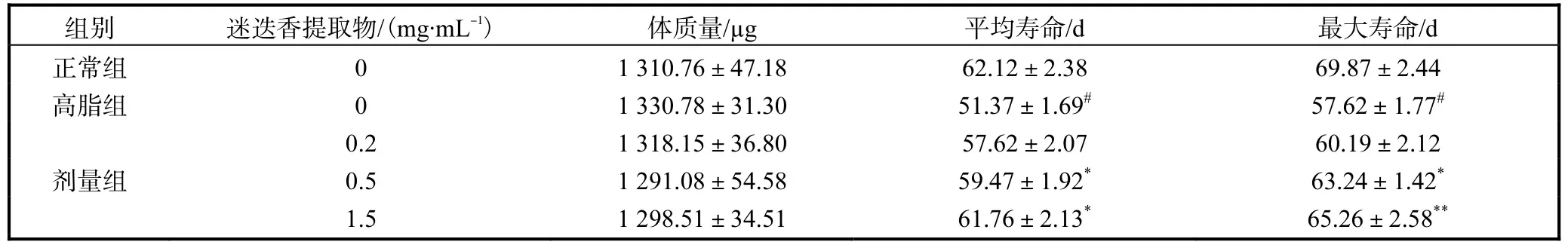
表3 迷迭香提取物对果蝇寿命的影响Tab.3 Effect of rosemary extract on the lifespan of Drosophila melanogasters
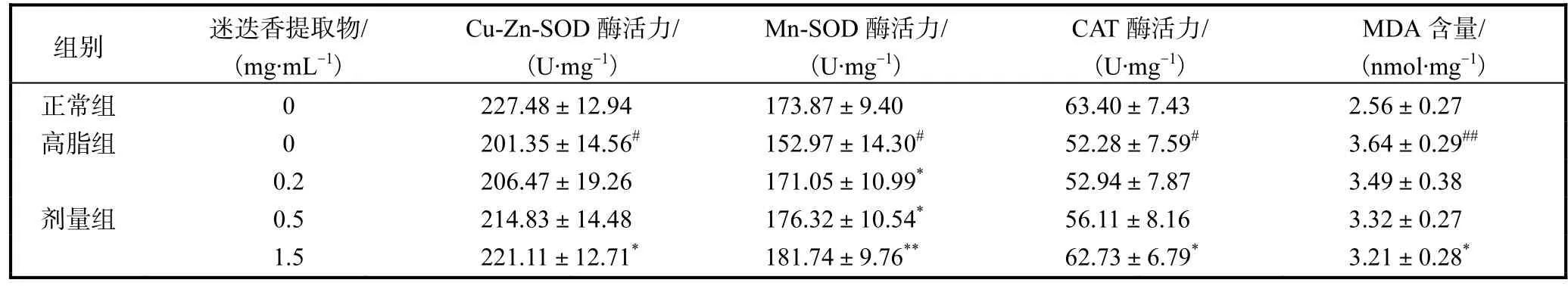
表4 迷迭香提取物对果蝇抗氧化酶活力及MDA含量的影响Tab.4 Effect of rosemary extract on the anti-oxidation enzyme activity and MDA content in Drosophila melanogasters
2.4Real-time PCR检测抗氧化基因Cu-Zn-SOD、Mn-SOD、CAT、MTH及Nrf2,mRNA表达水平
与高脂对照组相比,0.5,mg/mL组果蝇体内Cu-Zn-SOD、Mn-SOD、CAT mRNA表达水平显著上调(P<0.05),1.5,mg/mL Mn-SOD mRNA表达水平极显著上调(P<0.01),0.5和1.5,mg/mL组果蝇体内Nrf2,mRNA表达水平极显著上调(P<0.01),同时,MTH,mRNA表达水平呈下调趋势.具体结果见图3.


图3 迷迭香提取物对果蝇Cu-Zn-SOD、Mn-SOD、CAT、MTH、Nrf2,mRNA表达的影响Fig.3 Effect of rosemary extract on Cu-Zn-SOD,Mn-SOD,CAT,MTH and Nrf2,mRNA expression in Drosophila melanogasters
3 讨 论
Denham Harman提出的“衰老自由基学说”,认为衰老过程中的退行性变化是由于细胞正常代谢过程中产生的自由基的有害损伤造成的.低浓度自由基是机体执行正常生理功能所必需,但过量的自由基会对机体造成损伤[8].迷迭香是传统的天然香料,从中提取得到的迷迭香提取物,具有良好的抗氧化性能,Frankel等[9]研究了迷迭香提取物及其主要成分鼠尾草酸、鼠尾草酚、迷迭香酸等对不含VE的玉米油和玉米油-水乳状液氧化的抑制作用,发现迷迭香提取物、鼠尾草酚、鼠尾草酸具有显著的抗氧化活性.Richheimer等[10]研究发现,迷迭香提取物中鼠尾草酸能够有效抑制大豆油的氧化,其抗氧化能力数倍于BHA和BHT.
SOD、CAT是体内重要的抗氧化酶,广泛分布于各种生物体内.抗氧化酶活力的升高可显著减少自由基氧化损伤,延缓机体衰老[11].MDA是膜脂过氧化最重要的产物之一,具有细胞毒性,它的产生能加剧膜的损伤.本实验发现饲喂迷迭香提取物组果蝇较高脂模型组果蝇体内SOD、CAT活力显著升高,MDA含量显著下降,说明迷迭香提取物在体内发挥了抗氧化作用,使果蝇体内抗氧化酶活力升高,脂质过氧化产物含量下降.研究发现[12],迷迭香乙醇提取物能抑制四氧嘧啶导致的兔子血糖水平和血清MDA含量升高,并显著增强血清中抗氧化酶SOD和CAT活性.王虹等[13]也曾报道,迷迭香提取物能够升高D-半乳糖致衰老小鼠血清和脑中SOD活力,降低MDA含量并能延长小鼠耐缺氧时间.Sotelo-Felix等[14]研究了迷迭香甲醇提取物对CCl4氧化应激所致SD大鼠急性肝损伤的保护作用,结果显示迷迭香提取物在CCl4损伤发生24,h后能完全抑制CCl4氧化应激所导致的肝脂质体氧化和胆红素、谷丙转氨酶(ALT)活性升高.同样,Botsoglou等[15]以Wistar大鼠为研究动物,饲料中添加1%,的迷迭香干粉,6周末注射CCl4,结果显示,迷迭香能够抑制·CCl3自由基引发的氧化应激损伤.
Real-time PCR结果显示,饲喂迷迭香提取物后,果蝇体内抗氧化相关基因SOD、CAT mRNA表达水平显著上调,与对应抗氧化酶活力增强结果相一致;MTH是果蝇体内3号染色体上编码G蛋白偶联受体的基因,当MTH发生突变后,果蝇的平均寿命能够延长35%,且表现出更强的抵抗外源氧化应激[16].本实验测得MTH mRNA表达水平显著下调,与其体内过氧化物产量降低结果一致.Nrf2-keap1-ARE是机体抵御内外界氧化及化学应激的主要防御通路,调控抗氧化和Ⅱ相解毒酶的表达,组成一个强大的“抗氧化酶链”,起着重要的氧化防御功能[17]. 本研究发现,给予迷迭香提取物后,果蝇体内Nrf2 mRNA表达水平显著上调(P<0.05).Satoh等[18-19]也曾研究发现,鼠尾草酸在神经元HT22细胞中能够通过激活Nrf2/Keap1途径和ARE表达上调NQO1的表达.
因此,迷迭香提取物能有效降低果蝇体内氧化损伤,使果蝇体内抗氧化酶活力及其mRNA表达升高,脂质过氧化产物含量下降,有效调控高脂膳食导致的氧化应激损伤,从而延长果蝇寿命.参考文献:
[1] 方晓璞,解克伟,任春明,等. 迷迭香天然抗氧化剂的应用研究[J]. 中国油脂,2014(7):27-29.
[2] Aruoma O I,Halliwell B,Aeschbach R,et al. Antioxidant and pro-oxidant properties of active rosemary constituents:Carnosol and carnosic acid[J]. Xenobiotica,1992,22(2):257-268.
[3] Haraguchi H,Saito T,Okamura N,et al. Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis[J]. Planta Medica,1995,61(4):333-336.
[4] Naemura A,Ura M,Yamashita T,et al. Long-term intake of rosemary and common thyme herbs inhibits experimental thrombosis without prolongation of bleeding time[J]. Thrombosis Research,2008,122(4):517-522. [5] Minois N. How should we assess the impact of genetic changes on ageing in model species?[J]. Ageing Research Reviews,2006,5(1):52-59.
[6] Ji L L. Antioxidant enzyme response to exercise and aging[J]. Medicine and Science in Sports and Exercise,1993,25(2):225-231.
[7] 黄杰,王华丽,马娜,等. 迷迭香提取物对雌性果蝇寿命及抗氧化能力的影响[J]. 营养学报,2015,37(2):169-172.
[8] 陈瑾歆,陈建业. 自由基与衰老关系的研究进展[J].川北医学院学报,2004,19(1):207-209.
[9] Frankel E N,Huang S W,Aeschbach R,et al. Antioxidant activity of a rosemary extract and its constituents,carnosic acid,carnosol,and rosmarinic acid,in bulk oil and oil-in-water emulsion[J]. Journal of Agricultural and Food Chemistry,1996,44(1):131-135.
[10] Richheimer S L,Bernart M W,King G A,et al. Antioxidant activity of lipid-soluble phenolic diterpenes from rosemary[J]. Journal of the American Oil Chemists’ Society,1996,73(4):507-514.
[11] Gupta S C,Siddique H R,Saxena D K,et al. Hazardous effect of organophosphate compound,dichlorvos in transgenic Drosophila melanogaster (hsp70-lacZ):Induction of hsp70,anti-oxidant enzymes and inhibition of acetylcholinesterase[J]. Biochimica et Biophysica Acta (BBA)-General Subjects,2005,1725(1):81-92.
[12] Bakırel T,Bakırel U,Keleş O Ü,et al. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits[J]. Journal of Ethnopharmacology,2008,116(1):64-73.
[13] 王虹,刘红梅. 迷迭香提取物对亚急性衰老模型小鼠的抗衰老作用[J]. 中药药理与临床,2008,24(3):52-54.
[14] Sotelo-Felix J I,Martinez-Fong D,Muriel P,et al. Evaluation of the effectiveness of Rosmarinus officinalis (Lamiaceae) in the alleviation of carbon tetrachlorideinduced acute hepatotoxicity in the rat[J]. Journal of Ethnopharmacology,2002,81(2):145-154.
[15] Botsoglou N,Taitzoglou I,Zervos I,et al. Potential of long-term dietary administration of rosemary in improveing the antioxidant status of rat tissues following carbon tetrachloride intoxication[J]. Food and Chemical Toxicology,2010,48(3):944-950.
[16] Lin Y J,Seroude L,Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah[J]. Science,1998,282(5390):943-946.
[17] Kang M I,Kobayashi A,Wakabayashi N,et al. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes[J]. Proceedings of the National Academy of Sciences of the United States of America,2004,101(7):2046-2051.
[18] Satoh T,Kosaka K,Itoh K,et al. Carnosic acid,a catechol-type electrophilic compound,protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1 [J]. Journal of Neurochemistry,2008,104(4):1116-1131.
[19] Satoh T,Izumi M,Inukai Y,et al. Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners[J]. Neuroscience Letters,2008,434(3):260-265.
责任编辑:郎婧
The Antioxidant Effect of Rosemary Extract on High Fat Diet-induced Damage in Female Drosophila melanogaster and its Regulatory Mechanism
WANG Hong1, 2,CHEN Chun1,WANG Yifei1, 2,CHENG Lifang1, 2,CAI Jiarong1,WANG Hao1
(1.College of Food Engineering and Biotechnology,Tianjin University of Science & Technology,Tianjin 300457,China;2.College of Biotechnology,Tianjin University of Science & Technology,Tianjin 300457,China)
Abstract:To study the effect of rosemary extract(ROE)on anti-aging and antioxidation in high-fat dietary Drosophila melanogaster,Drosophila melanogasters were divided into three groups:the normal group:the high fat diet group(HF diet)and the experimental groups fed with different doses of rosemary extract(0.2,0.5,1.5,mg/mL).The medium was replaced every three days and the number of the dead Drosophila melanogasters was recorded immediately,so the mean lifespan and maximum lifespan were calculated.The variation of superoxide dismutase(SOD),catalase(CAT)and malondialdehyde(MDA),as well as the mRNA expression levels of their encoding genes were determined after 45,days by Real-time PCR.It revealed that the lifespan of Drosophila melanogasters treated with resomary extract was extended significantly compared with the normal group. 0.2,mg/mL ROE could increase the enzyme activity of Mn-SOD significantly(P<0.05),and 1.5,mg/mL ROE increased the enzyme activity of Cu-Zn-SOD,Mn-SOD and CAT significantly(P<0.05)accompanied by a decrease of MDA content(P<0.01);with 0.5,mg/mL ROE,the mRNA expression levels of Cu-Zn-SOD,Mn-SOD,CAT and Nrf2 were up-regulated(P<0.05);with 1.5,mg/mL ROE,the mRNA expression levels of Mn-SOD and Nrf2,were up-regulated very significantly(P<0.01).It is concluded that the effect of rosemary extract on extending lifespan is likely to be related with the up-regulation of the gene expression level of endogenous antioxidant enzymes.
Key words:rosemary extract;high fat diet;Drosophila melanogaster;antioxidant;enzyme activity;gene expression
中图分类号:TS201.4
文献标志码:A
文章编号:1672-6510(2016)02-0031-05
收稿日期:2015-07-06;修回日期:2015-09-29
基金项目:国家自然科学基金青年科学基金资助项目(31201322)
作者简介:王 红(1990—),女,山东人,硕士研究生;通信作者:王 浩,副教授,wanghao@tust.edu.cn.
DOI:10.13364/j.issn.1672-6510.20150088

