7-羟基香豆素红外光谱的密度泛函理论研究
贾飞云,苏 宇,冉 鸣,朱 江,张 波*
1. 川北医学院化学教研室,四川 南充 637007 2. 四川师范大学化学与材料科学学院,四川 成都 610066
7-羟基香豆素红外光谱的密度泛函理论研究
贾飞云1,苏 宇1,冉 鸣2,朱 江1,张 波1*
1. 川北医学院化学教研室,四川 南充 637007 2. 四川师范大学化学与材料科学学院,四川 成都 610066
红外光谱是化合物结构鉴定的重要信息来源,对天然有机药物的分子结构及其生物活性研究意义重大。而随着理论计算方法更加合理、计算精度不断提高,理论计算在红外光谱模拟、振动模式归属指认等方面优势更加明显,对红外光谱解析等实验研究具有重要参考价值。本研究利用密度泛函理论(density functional theory, DFT)/B3LYP方法,在6-311G(d,p)基组水平对7-羟基香豆素进行了几何结构优化和红外光谱计算,得到稳定结构及全部振动模式。计算结果显示7-羟基香豆素红外光谱吸收峰主要分布于波数3 700~3 500,3 150~3 000,1 750~1 400,1 400~1 000,1 000~50 cm-1几个区域。除波数3 700~3 500,3 150~3 000 cm-1范围内振动相对独立,分别归属为O—H伸缩振动和芳环C—H伸缩振动外,其他几个区域均较为复杂,谱峰不同程度由多个振动模式叠加而成。最后,根据振动模式理论及振动图像分析,对所有振动模式进行了详细指认。并通过线性回归方法,利用相关系数值r研究了7-羟基香豆素红外光谱主要吸收峰波数理论计算值和实验数据的相关性。结果表明,计算值和实验值基本吻合,相关系数值等于0.998 5,相关性较好;采用密度泛函理论在该基组水平对7-羟基香豆素红外光谱的理论计算较为可靠。
红外光谱;密度泛函理论;7-羟基香豆素
引 言
香豆素类化合物是具有苯并α-吡喃酮母核天然产物的总称,结构上可以看成顺式邻羟基桂皮酸脱水而成的内酯化合物[1]。具有抗肿瘤、抗艾滋病、抗细胞增生、抗病毒、抗真菌、抗细菌、抗血管硬化、抗氧化、增强人体的免疫力等明显的生物活性[2-7]。天然香豆素中绝大多数在C7位均连接有含氧官能团,故7-羟基香豆素被认为是香豆素类化合物的基本母核。红外光谱作为化合物结构鉴定的重要信息来源[8],对7-羟基香豆素及其衍生物分子结构和生物活性研究意义重大。而随着理论计算方法更加合理、计算精度不断提高,理论计算在红外光谱模拟、振动模式归属指认等方面优势更加明显,对红外光谱解析等实验研究具有重要参考价值。本工作利用密度泛函理论研究了7-羟基香豆素的红外光谱,并对振动模式进行了详细指认,最后对主要吸收峰波数理论计算值与文献实验数据的相关性进行了研究,结果表明理论计算值与实验数据吻合较好。
1 计算方法
利用密度泛函理论(DFT)/B3LYP方法,在6-311G(d,p)基组水平优化了7-羟基香豆素几何结构,并以相同基组水平计算红外光谱,得到7-羟基香豆素稳定结构和全部振动模式。因理论计算未考虑非谐效应,故根据计算方法和基组水平,采用0.961 3[9]作为频率修正因子。所有计算均采用Gaussian09程序完成。
2 结果与讨论
2.1 几何构型


Fig.1 Optimized molecular structure of 7-Hydroxycoumarin
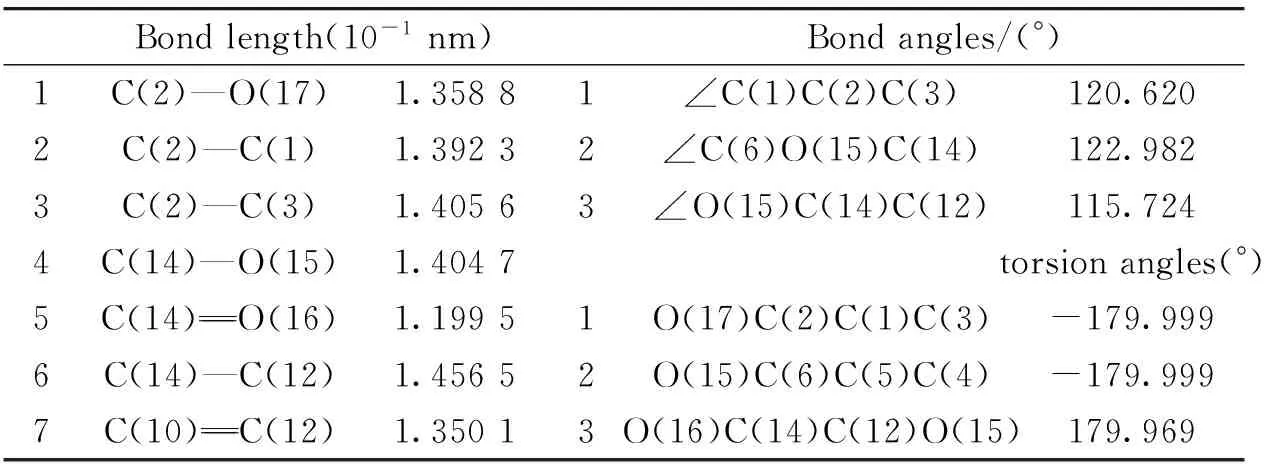
Table 1 Some geometrical parameters of 7-Hydroxycoumarin
2.2 红外光谱分析
图2表示在B3LYP/6—311G(d,p)水平理论计算得到的7-羟基香豆素红外光谱图。图3给出了实验测得的7-羟基香豆素的红外光谱图。由图2可知,7-羟基香豆素红外光谱吸收峰主要分布于波数3 700~3 500,3 150~3 000,1 750~1 400,1 400~1 000,1 000~50 cm-1几个区域。除波数3 700~3 500,3 150~3 000 cm-1范围内振动相对独立,分别归属为O—H伸缩振动和芳环C—H伸缩振动外,其他几个区域均较为复杂,谱峰不同程度由多个振动模式叠加而成。
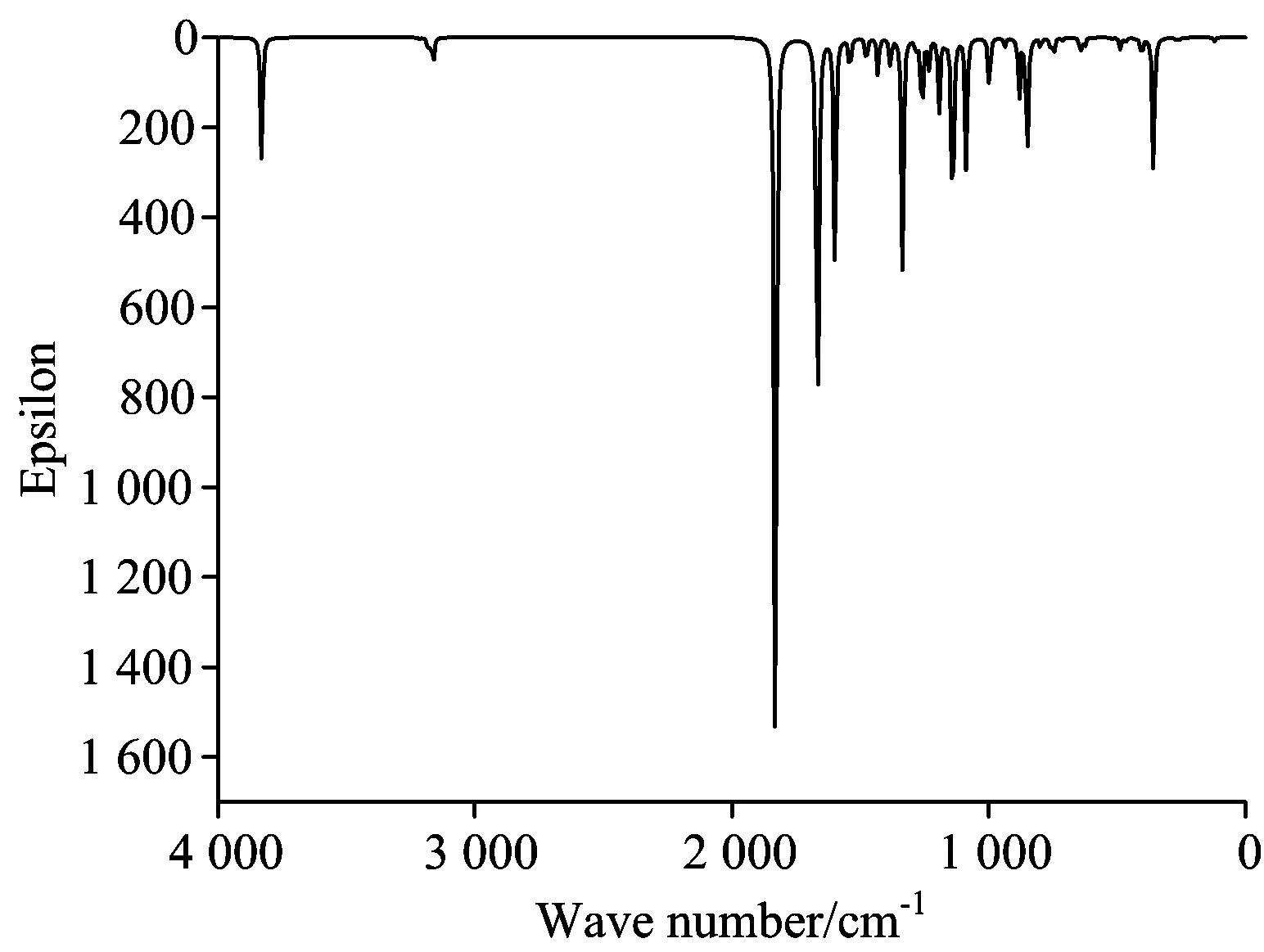
Fig.2 Calculated infrared spectum of 7-Hydroxycoumarin

Fig.3 Experimental infrared spectum of 7-Hydroxycoumarin*

为了更为直观地表示红外光谱理论计算数据与实验数据的相关性,以理论值为x轴、实验值为y轴,做出7-羟基香豆素红外光谱主要吸收峰波数的相关图,并进行了线性回归,相关系数值r=0.998 5。结果表明,7-羟基香豆素红外光谱主要吸收峰理论计算值与实验值相关性较好,r值为0.998 5。由此进一步表明,采用密度泛函理论在B3LYP/6-311G(d,p)基组水平对7-羟基香豆素红外光谱的理论计算较为可靠。

Fig.4 Correlation of calculated and experimental data for 7-Hydroxycoumarin

Table 2 Comparation of the theoretical vibrational modes and experimental IR for 7-Hydroxycoumarin
3 结 论
利用密度泛函理论DFT/B3LYP方法在6-311G(d,p)基组水平对7-羟基香豆素进行了结构优化和红外光谱计算,并根据振动模式理论图形分析,对所有振动模式进行了详细指认。最后通过线性回归方法,利用相关系数值r研究了7-羟基香豆素红外光谱主要吸收峰波数理论计算值和实验数据的相关性。结果表明,计算值和实验值基本吻合,相关性较好;采用密度泛函理论在该基组水平对7-羟基香豆素红外光谱的理论计算较为可靠。
[1] LUO Yong-ming(罗永明). Chemistry of Natural Medicine(天然药物化学). Wuhan:Huazhong University of Science & Technology Press(武汉:华中师范大学出版社), 2011. 80.
[2] Kong L L, Hu J F, Chen L H. Chinese Pharmacological Bulletin, 2012, 28(2): 165.
[3] Chen G, Xu G B. Chinese Traditional Patent Medicine, 2013, 35(6): 1288.
[4] Shikishima Y, Takaishi Y, Honda G, et al. Chemical and Pharmaceutical Bulletin, 2011, 49(7):877.
[5] Seema Singh, Shilpi Gupta, Bharat Singh, et al. J. Proteome Res., 2012, 11(6): 3259.
[6] Karthik S, Nagaprasad Puvvada, Prashanth Kumar B N, et al. ACS Appl. Mater. Interfaces, 2013, 5(11): 5232.
[7] Rajarajeshwari Thada, Shivashri Chockalingam, Ramesh Kumar Dhandapani, et al. J. Agric. Food Chem., 2013, 61(22): 5385.
[8] Koneni V Sashidhara, Manoj Kumar, Vikram Khedgikar, et al. J. Med. Chem., 2013, 56(1): 109.
[9] Jia Tingjian, Li Pengwei, Shang Zhiguo, et al. Chinese Journal of Light Scattering, 2007, 19(1): 1.
[10] HU Jie-han, ZHENG Xue-fang(胡皆汉,郑学仿). Practical Infrared Spectroscopy(实用红外光谱学). Beijing: Science Press(北京:科学出版社), 2011. 101.
*Corresponding author
The Study on Infrared Spectra of 7-Hydroxycoumarin by Density Functional Theory
JIA Fei-yun1, SU Yu1, RAN Ming2, ZHU Jiang1, ZHANG Bo1*
1. Teaching and Research Group of Chemistry, North Sichuan Medical College, Nanchong 637007, China
2. Department of Chemistry and Material Science, Sichuan Normal University, Chengdu 610066, China
Infrared spectroscopy is an important source of information for the identification of the compounds structure and it is great significant for biological activity research of natural and organic drug molecules. With the theoretical calculation method is more reasonable and calculation accuracy continues to improve, Theoretical calculate advantage is more obvious in the infrared spectrum simulation and vibration modes attributable identified. And it has important reference value for experimental study of infrared spectral analysis. Using density functional theory, geometry optimizations and frequencies calculation of 7-Hydroxycoumarin were performed at the level of B3LYP/6-311G(d,p), the stable structure and all vibration modes of 7-Hydroxycoumarin were attained. The results show that the infrared absorption peak of 7-hydroxycoumarin is mainly distributed in the several regions in wave number of 3 700~3 500, 3 150~3 000, 1 750~1 400, 1 400~1 000, 1 000~50 cm-1. In addition to the vibration in a wave number range of 3 700~3 500, 3 150~3 000 cm-1is relatively independent, and were attributed to OH stretching vibration and benzene ring CH stretching vibration, the other several vibration regions are more complex, the different degree of spectral peaks is composed of multiple vibration modes. Finally, based on the theoretical analysis of the vibration mode, the vibration modes of 7-Hydroxycoumarin molecule were assigned, and in order to discuss the reliability of theoretical calculation method, the correlation diagram of the main absorption peak of 7-hydroxyl group was drawn from the theoretical value ofXaxis and the experimental value ofYaxis, the correlation between experimental IR data and calculated IR data of 7-Hydroxycoumarin was analyzed through the linear regression method. Results show that they have good correlation, correlation coefficient values “r” equals 0.998 5,and the theory calculation of 7-Hydroxycoumarin IR by density functional theory at the base set level is reliable.
Infrared spectra; Density functional theory; 7-Hydroxycoumarin
Sep. 29, 2014; accepted Feb. 10, 2015)
2014-09-29,
2015-02-10
国家自然科学基金项目(21172025),四川省教育厅科研项目(14ZB0193),四川省中医药管理局中医药科学技术研究专项项目(2014K042)资助
贾飞云,1976年生, 川北医学院化学教研室副教授 e-mail: cloud7612@163.com *通讯联系人 e-mail: zhangbo6606@163.com
O657.6
A
10.3964/j.issn.1000-0593(2016)01-0060-04
- 光谱学与光谱分析的其它文章
- Determination of Component Contents of Blend Oil Based on Characteristics Peak Value Integration
- Identification of Haploid Maize Kernel Using NIR Spectroscopy in Reflectance and Transmittance Modes: A Comparative Study
- 基于光谱吸收法和荧光法的甲烷和二氧化硫检测系统的研究
- 基于TDLAS-WMS的痕量甲烷气体检测仪
- 推扫误差对计算光谱成像数据重构的影响分析
- ICP-MS用于云南南部四种特色蜂蜜的植物源鉴别分析

