The structure,tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics☆
Yao Dou ,Buning Zhang ,Ming He ,Guoqiang Yin ,*,Yingde Cui,3
1 School of Materials Science and Engineering,Northwestern Polytechnical University,Xi'an 710072,China
2 Green Chemical Engineering Institute,Zhongkai University of Agriculture and Engineering,Guangzhou 510225,China
3 Guangzhou Vocational College of Science and Technology,Guangzhou 510550,China
1.Introduction
There is currently a surge in research on biodegradable materials obtained from renewable materials such as proteins,polysaccharides and lipids[1,2].Application of biodegradable materials is considered as the most effective solution for a number of the environmental pollution problems caused by synthetic polymers.Feather is one of the most important potential resources to produce biodegradable materials.More than 90% of feather is a protein called keratin,which is abundantly available in the poultry industry[3].It is estimated that 3-4 billion lbs of feather are generated as the by-product of the poultry industry in the United States[4],and more than 1.5 billion lbs in China[5].Although feather is cheap,renewable,biodegradable and abundantly available,it is almost useless in industry applications.Except for applications in animal feed,duvet and down coat,feather is mostly disposed in the land fills as the solid wastes,causing environmental and economic issues[4].In addition,the abandoned duvet and down coat could result in a secondary pollution.To solve the solid waste pollution,several research groups are working on developing potential materials from feather.
The use of hydrolyzed feather keratin solution to produce films by casting(wet process)has been reported[1,6-9].However,due to the high cost of preparation from the solution,most of the investigations on the protein-based films are focused on the thermo-mechanical processing(dry process),such as hot-pressing and extrusion[10-12].As native chicken feathers are non-thermoplastic,some studies have been done to convert native feathers into films by thermal processingviachemical modifications,such as plasticization,grafting or crosslink.The poultry feather fiber was hot-pressed into films at 160°C using a variable amount of glycerol(15%-50%)as a plasticizer.The values of film tensile strength and elongation at break were 6-15 MPa and 8%-50%,respectively[10].Native chicken feathers were cyanoethylated using acrylonitrile and sodium carbonate.Cyanoethylated feathers showed a melting peak at167°C and could be hot-pressed into thermoplastic films at180°C using glycerol(20%)as the plasticizer[13].Poultry feathers were partly hydrolyzed by the alkaline agent and cross-linked by citric acid,and then hot-pressed into films plasticized with glycerol(20%).Cross-linking with a citric acid concentration of 2%resulted in the strength and elongation values of 5.2 MPa and 13.5%,respectively[14].In addition,S-sulfo keratin powder was extracted from wool by using sodium disulfite and sodium dodecyl sulfate(SDS).The powder was then mixed with ethanol/water and hot-pressed into the films with tensile strength of 27.7 MPa and elongation at break of 4.7%.The film supported the adhesion and proliferation of fibroblast cell,and the film swelling was 53.4%at pH 7[15].
Above reports show that the films developed from the native feather using a chemical modification by hot-pressing are opaque and nonuniform and still contain some feather fibers[4,13].The type and extent of grafting may also decrease the biodegradability of the feather.Besides,the hot-pressed S-sulfo keratin films showed a relatively low flexibility and high moisture sensitivity.At the best of our knowledge,little information is available on the thermal processing of the hydrolyzed keratin films.The aim of this work is to produce plasticized hydrolyzed feather keratin films by hot-pressing.The film properties were controlled by addition of glycerol as an effective plasticizer for protein-based materials(glycerol has low molecular weight and can easily diffuse into the protein molecule to interact with polar groups).Furthermore,the hydrolyzed feather keratin was extracted from the chicken feathers using a reducing agent,sodium sulfide,which is cheaper and more effective than other reducing agents[16].
This work is focused on the evaluation of the processability,thermal properties,structure,tensile properties,micromorphology,water resistance and water vapor permeability(WVP)of the HFK films produced by hot-pressing.
2.Materials and Methods
2.1.Materials
The chicken feathers used in this study were collected from farmer's markets.Urea,sodiumsul fide nonahydrate(Na2S·9H2O),sodiumdodecyl sulfate(SDS),hydrochloric acid and glycerol were purchased from a local company(Guangzhou Chemical Reagent Factory,Guangzhou,China).
2.2.Extraction of the hydrolyzed feather keratin(HFK)
HFK was extracted by an amended technique reported in[16].The sterilized chicken feathers were soaked in 7 mol·L-1urea solution(1:15,mass ratio)at 50 °C for 24 h.Then,Na2S·9H2O(40 wt%of the feather mass)and SDS(1 wt%of the feather mass)were added to the mixture and extraction was carried out at 50°C for 30 min under continuous stirring.The extracted solution was then centrifuged at 1000 r·min-1for 15 min,and pH of the supernatant was adjusted to 4.7(isoelectric point of keratin)using a 1 mol·L-1hydrochloric acid solution to obtain the HFK,followed by vacuum-drying at room temperature for 24 h.The protein content calculated after nitrogen analysis was 96.51 wt%(dry matter basis),using Kjeldahl nitrogen determination method with the conversion factor,6.25(K-314,BUCHI).
2.3.HFK film preparation
The HFK powder was mixed with water(15 wt%)and glycerol(20 wt%-40 wt%).The amount of the each reagent was chosen based on the weight of the HFK.To obtain a uniform HFK/glycerol mixture,glycerol was first dissolved in water.The mixture was ground using a mortar and then was added into a kitchen mixer to ensure the uniform mixing.The mixture was stored at 25°C for 24 h and then was compress molded into the films in a press at 130°C for 6 min under 10 MPa of pressure.The compression molding was controlled and wateredcooled to 40°C in 10 min to obtain a semitransparent smooth brown film.
2.4.Characterization
The effect of water and glycerol thermal processing was assessed using a DSC(METTLER Instruments 910s).Aluminum pans were used as the sample containers.The DSC analysis of the HFK powder or feather was performed from 25 °C to 260 °C at a heating rate of 10 °C · min-1[17].The HFK/glycerol mixtures and films were first heated from 25 to 105 °C at a heating rate of 20 °C · min-1,holding for 5 min,and then cooled from 100 to-60 °C at a rate of 20 °C · min-1.The second heating step was from-60 °C to 260 °C at a heating rate of 10°C·min-1.
FTIR spectra of the samples were analyzed using a FTIR spectrometer(spectrum 100,Perkin-Elmer)with ATR attachment.An average of 8 scans was used for the analysis.
The tensile strength(σb)and breaking elongation(εb)of the samples were measured on a universal testing machine(CMT6503,Shenzhen MTS Test Machine Company Ltd.,China)according to ASTM standard D638 with a rate of 10 mm·min-1.HFK films were conditioned at 25°C and 55%relative humidity for 24 h before tensile testing.Films were cut to size of 75 mm×10 mm.Thickness of the samples was measured by a micrometer and ranged from 0.20 to 0.40 mm for the various conditions studied.The results were averaged from three samples.
Micrographs of the films were obtained using a Quanta 400 SEM(Oxford,England).To perform the SEM tests,the samples were coated with a thin layer of gold.All the samples were examined using an accelerating voltage of 20 kV.
The water resistance of the HFK film was expressed by the swelling and solubility.The rectangular specimens(40 mm×10 mm)were preconditioned by drying in an air oven at 70°C for 12 h,followed by a cooling in a desiccator for a few minutes and immediate weighing(W1).The preconditioned specimens were immersed in distilled water at(25± 1)°C for 24 h under shaking.The specimens were removed,wiped with a filter paper to remove the water on the surface of the films and immediately weighed(W2).The as-prepared wet specimens were dried again in an oven at 70°C for 24 h followed by a cooling in a desiccator and immediate weighing(W3).Each film was tested 3 times.The swelling and solubility of the films were calculated using the following Eqs.(1)-(2):

Water vapor permeability(WVP)of the films was measured using a water vapor transmittance tester(Perme W3/030,Labthink Ltd,CHN)at 38°C with a gradient of 90%relative humidity(RH)to 0%RH(dry air)across the film.
3.Results and Discussion
3.1.Thermal properties
Thermal analysis of the HFK powder depends on the moisture content(0,5.5 wt%and 9.8 wt%),see Fig.1.All of the four curves show two endothermic peaks.A broad,low-temperature peak was observed at around 100°C,which is attributed to the evaporation of bound water in the protein structure(could be regarded as the glass transition—Tg),as previously reported.The second peak is observed at around 210-240°C,which is assigned to the crystalline melting[15,18-20].Following extraction with sodium sulfide,the temperature and the area under the peak of the crystalline melting peak decreased significantly.This could be due to the transition of the α-form crystallites to β-pleated sheet structures,similar to those in wool[21].The unpredictable behavior in all of the four curves at temperatures above the melting temperature corresponds to the degradation of the keratin[17].The degradation was observed as a color change from gray to black with a release of a small from the oven,indicating degradation and a chemical modification accompanying the physical changes.

Fig.1.DSC curves of the HFK and feather showing denaturation peak around 100°C and crystalline melting peak at 220-240°C.The moisture contents of the HFK powder were 0,5.5 wt%and 9.8 wt%,whereas that of the native feather was 15.2 wt%.
Chicken feather containing 15.2%moisture showed theTgat 69.4°C.As the moisture content decreased from 9.8%to 0%,theTgof the HFK power clearly increased from78.1 °C to 124.6 °C.The observed variation trend may be due to the drying effect on the HFK,which is similar to that of wool and human hair[15,19].Water is a plasticizer,which can weaken or break the interaction in the keratin matrix through forming hydrogen-bond with polar amino acid residues.The hydrogen-bond can be disrupted by drying the keratin powder in a vacuum oven with an increase in theTgaccordingly.As a plasticizer,water can improve the mobility of the native and hydrolyzed keratin molecules,which makes the keratin thermoplastic.
The thermal transition behaviors of the HFK/glycerol mixtures and films were studied by DSC.The mixtures with glycerol content of(10-40)wt%were coded as GL-10,GL-20,GL-30 and GL-40,respectively.The GL-10-GL-40 mixtures were compressed at 130°C for 6 min to obtain the films,which were coded as FGL-10-FGL-40,respectively.The DSC curves of the GL-10-GL-40 mixtures are shown in Fig.2(a)The DSC curves of the mixtures of GL-series exhibited three phase transitions.An inconspicuous low-temperature broad peak ataround 110°C was attributed to the glass transition behavior of the HFK/glycerol mixture.The glass transition temperature(Tg)decreases by increasing the glycerol content.The moisture evaporation peak associated with the GL-30 and GL-40 is more intense than those of the GL-10 and GL-20.This might be due to the hydrophilic nature of glycerol.The second peak was observed at around 180°C that may be due to the glycerolrich domains(T1)where glycerol is loosely bound to the proteins.A small peak at around 230°C might be due to the protein-rich domains(T2)where glycerol forms a hydrogen-bound structure twined with the proteins.The phase transition behaviors are similar to the previously reported ones in the investigations on the thermal properties of the glycerol-plasticized soy protein and starch[22].As the glycerol content increases,the temperature decreases and the peak range becomes wider in the protein-rich domains,which suggests that the glycerol disrupt the crystalline structure of keratin.The change is likely due to the hydrogen bonds formed between the glycerol and the keratin molecules,which weakens the internal hydrogen bonds,van der Waals force,or ionic interactions that hold the protein chains together[23].The broad endothermic peak aboveT2is considered to be the thermal degradation of keratin and the evaporation of glycerol.
A slow and gradual moisture evaporation peak is shown in the curves of FGL-series films(Fig.2(b))at around 60°C.This might be due to the moisture reduction of the feather keratin film in the hot pressing process,after which only one phase transition could be observed before the thermal degradation of keratin.Besides,the transition temperature is higher thanT1but lower thanT2discussed in the mixtures of the GL-series.This suggests a better compatibility between the glycerol-rich domains and the protein-rich ones.Moreover,the crystalline melting peak disappears.These results indicate that the high temperature and pressure could improve the compatibility between the glycerol and the protein.This might be because the hotpressing process could strengthen the interaction between the glycerol and the polargroups in the keratin,and this increases the free volume in keratin matrix.In addition,to obtain a film without any powdery part,15 wt% of water was needed to add to the HFK/glycerol mixture.This indicated that water as the plasticizer cooperates with glycerol would enhance the mobility of the keratin molecules.
3.2.FT-IR analysis
FTIR analysis can be used as an effective method to confirm the generation of the hydrogen bonds in the proteins,as previously reported[24].Fig.3(a)shows the IR spectra of the FGL-40 film and the GL-40 mixture.The absorption peaks at1109,1042 and 994 cm-1are assigned to the characteristic peaks of the glycerol and the absorptions peaks at 1633 and 1537 cm-1attributed to amide I(C=O stretching)and amide II(N-H bending and C-H stretching)bands of the HFK,respectively.The intensity of the characteristic peaks shows noticeable changes after hot-pressing.The absorption peak intensity of amide I band becomes stronger in the FGL-40 film,while that of the glycerol molecules becomes weaker than those of the GL-40 mixture.This indicates that the O-H groups of the glycerol form strong hydrogen bonds with N-H and C=O groups of the HFK,when the GL-40 mixture is hot pressed into a FGL-40 film at 130°C.

Fig.2.DSC curves of(a)the GL-10-GL-40 mixtures and(b)the FGL-10-FGL-40 films.

Fig.3.(a)FTIR spectra of the FGL-40 film and the GL-40 mixture;(b)amide II region spectra of(A)the HFK powder,(B)the FGL-20 film,(C)the FGL-30 film and(D)the FGL-40 film,respectively.
This change in bonding was further confirmed by changes in amide II band of FTIR spectrum(Fig.3(b)).The amide II band originates in the N-H bending and C-N stretching vibrations,which is very sensitive to the environment of the N-H group.Therefore,the amide II band can be used to infer changes to the environment of the N-H groups.This reflects the alterations of the hydrogen bond environment in the peptide chains[25].It is observed that the amide II peak positions shifts to higher wave numbers in all curves of the HFK/glycerol film,when compared to that of the HFK powder.This shift trend could be attributed to the formation of the new hydrogen bonds between N-H groups of the HFK and O-H groups of the glycerol,thus disrupting and weakening the internal hydrogen bonds of the proteins.
3.3.Effect of glycerol content on the tensile properties of the HFK films
Glycerol is usually used as a plasticizer for the protein film to improve its ductility.The effect of the glycerol content on the tensile properties of HFK films is presented in Table 1.The films that contain less than 20%glycerol are fragile and uneven.When the glycerol content is greater than 40%,some glycerol diffuses out of the films within a few minutes.In this condition,the film surfaces absorb the moisture easily and are felt“oily”.With an increase of glycerol content,the tensile strength shows that it reaches a maximum of10.5 MPa for the film containing 20%glycerol,followed by a gradual decrease to 5.7 MPa for glycerol content of 40%.However,the elongation at break increases from 40.5%to 63.8%in the range from 20%to 35%of the glycerol content,followed by a sharp decrease to 43.6%for the glycerol content of 40%.This variation tendency is because the glycerol forms hydrogen bonds with hydrophilic groups in the HFK chains,which could break the hydrogen bonds between the protein chains and could add to the freevolume between them.The mobility of the HFK chain is improved with an increase of the glycerol content[26,27].The tensile strength is higher than that of the HFK films made by casting(2.0-7.6 MPa,11.9%-31.9%)[1]and the hot-pressed feather films that are partly hydrolyzed(5.9 MPa,31.7%)[14].
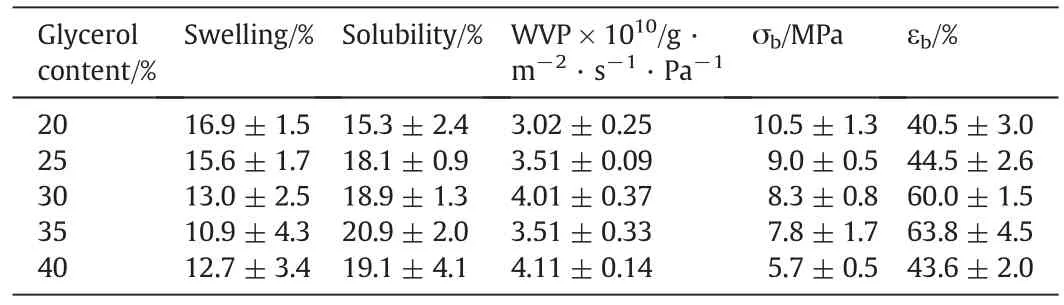
Table 1Effect of the glycerol content on the tensile properties,swelling,solubility and water vapor permeability of the hot-pressed HFK film
3.4.Micro structure of the HFK films
Fig.4(a,b and c)shows the SEM images of the surface morphology of the HFK films made with 0%,10%and 20%glycerol,respectively.A very dense structure can be observed in all films.However,the surfaces of the films that contain glycerol are more homogeneous and smoother than those of the film without glycerol.Homogeneity and density can also be observed in the SEM image(Fig.4(d))of the fractured surface micro structure prepared by liquid nitrogen freezing of the HFK film containing 40%glycerol.Some cracks can be observed in this fractured surface structure of the film,which might be the reason for the sequential decrease in its elongation at break.
3.5.Water resistance of the HFK films
Water stability is an important property of the protein-based films.For instance,in tissue engineering applications and agricultural fields,film integrity and stability in the humid environment are required for a relatively longer time.The water resistance of the films is expressed by the swelling and solubility,see Table 1.It is observed that the solubility of the films is just below 21%,which shows a higher stability in water,when compared to the plasticized HFK films prepared by casting(30.7%-50.7%)[1].This is probably due to the high temperature and pressure,which promotes the formation of the cross-links between the protein molecules.Increasing the glycerol concentration in the films results in a remarkable increase in the film water solubility.This indicates that a portion of the glycerol and some peptide chains with low molecular weights in films is dissolved in water.The results also indicate that the addition of glycerol prevents the formation of the cross-linking in the protein matrix.
The film swelling is also relatively lower than that of the HFK films prepared by casting(110%-207%)[1].This reveals that the extent of the cross-linking in the hot-pressed HFK film is deeper than that in the films prepared by casting.The results also indicate that high temperature and pressure improve the cross-linking density in the protein matrix.The obtained HFK films remain intact after immersion in water for 24 h.Therefore,it is shown that the hot-pressing process improves the water resistance of the HFK films.

Fig.4.SEM images of the HFK films:surface of the HFK film produced with(a)no glycerol;(b)10%glycerol;(c)20%glycerol.(d)Fractured surface of the HFK film containing 40%glycerol.
3.6.Water vapor permeability(WVP)
The effect of glycerol content on the WVP of the hot-pressed HFK films is shown in Table 1.The WVP values of the hot-pressed HFK films are in the range of 3.02×10-10g·m-2·s-1·Pa-1to 4.11×10-10g·m-2·s-1·Pa-1,which is lower than those reported for plasticized HFK films prepared by casting[9].The WVP is influenced by the hydrophilicity, flaws of a material and the internal tortuosity in the structure[28].It is observed that the film WVP increases as the glycerol content increases.This is likely due to the increase in moisture content of the films when high hydrophilic plasticizer-glycerol is used.On the other hand,it is well known that the water molecules have the high plasticizing effect in the protein films[23].Under the plasticization of the water and glycerol together,the increase in the free volume of the HFK system results in a protein network with less density,which consequently increases the water diffusion in the protein matrix.
4.Conclusions
Glycerol-plasticized and hydrolyzed feather keratin films are successfully produced by the hot-pressing process.It is shown that the overall properties of the films can be controlled by altering the ratio of the HFK and the glycerol in the mixed system.Thermal analysis showed that water and glycerol addition are necessary to obtain homogeneous thermoplastic HFK films.It is also shown that the glass transition temperature of the HFK/glycerol mixtures decreased as the glycerol content increased.This might be due to the hydrogen bond formation between the glycerol and the keratin molecule and weakening the internal interactions in the protein matrix.Two glass transition temperatures were noticed in the HFK/glycerol mixtures,while only one transition temperature in the HFK films is observed.This suggests that the high temperature and pressure improves the compatibility between glycerol and the protein.The glycerol content also significantly affects the tensile properties,water resistance,micro structure and water barrier property of the HFK film.As a result,the obtained HFK films show reliable tensile properties and thermo-stability,which can be useful in agricultural,packaging and other potential applications.These findings provide an opportunity to enhance the potential value of the waste keratin resources.
[1]G.Rocha Plácido Moore,S.Maria Martelli,C.Gandolfo,P.José do Amaral Sobral,J.Borges Laurindo,Influence of the glycerol concentration on some physical properties of feather keratin films,Food Hydrocoll.20(7)(2006)975-982.
[2]A.N.Mauri,M.C.Anon,Effect of solution pH on solubility and some structural properties of soybean protein isolate films,J.Sci.Food Agric.86(7)(2006)1064-1072.
[3]A.Lasekan,F.Abu Bakar,D.Hashim,Potential of chicken by-products as sources of useful biological resources,Waste Manage.33(3)(2013)552-565.
[4]E.Jin,N.Reddy,Z.Zhu,Y.Yang,Graft polymerization of native chicken feathers for thermoplastic applications,J.Agric.Food Chem.59(5)(2011)1729-1738.
[5]L.Zhao,H.Zhou,J.Hua,New progress in utilization of feather fibers,China Leather40(5)(2011)36-40.
[6]L.Cui,J.Gong,X.Fan,P.Wang,Q.Wang,Y.Qiu,Transglutaminase-modified wool keratin film and its potential application in tissue engineering,Eng.Life Sci.13(2)(2013)149-155.
[7]T.Tanabe,N.Okitsu,A.Tachibana,K.Yamauchi,Preparation and characterization of keratin-chitosan composite film,Biomaterials23(3)(2002)817-825.
[8]S.M.Martelli,G.Moore,S.S.Paes,C.Gandolfo,J.B.Laurindo,In fluence of plasticizers on the water sorption isotherms and water vapor permeability of chicken feather keratin films,LWT Food Sci.Technol.39(3)(2006)292-301.
[9]S.Maria Martelli,G.Rocha Placido Moore,J.Borges Laurindo,Mechanical properties,water vapor permeability and water affinity of feather keratin films plasticized with sorbitol,J.Polym.Environ.14(3)(2006)215-222.
[10]J.R.Barone,W.F.Schmidt,C.F.E.Liebner,Thermally processed keratin films,J.Appl.Polym.Sci.97(4)(2005)1644-1651.
[11]J.R.Barone,W.F.Schmidt,N.T.Gregoire,Extrusion of feather keratin,J.Appl.Polym.Sci.100(2)(2006)1432-1442.
[12]J.F.Martucci,R.A.Ruseckaite,Tensile properties,barrier properties,and biodegradation in soil of compression-molded gelatin-dialdehyde starch films,J.Appl.Polym.Sci.112(4)(2009)2166-2178.
[13]N.Reddy,C.Hu,K.Yan,Y.Yang,Thermoplastic films from cyanoethylated chicken feathers,Mater.Sci.Eng.C31(8)(2011)1706-1710.
[14]N.Reddy,L.Chen,Y.Yang,Biothermoplastics from hydrolyzed and citric acid crosslinked chicken feathers,Mater.Sci.Eng.C Mater.Biol.Appl.33(3)(2013)1203-1208.
[15]K.Katoh,M.Shibayama,T.Tanabe,K.Yamauchi,Preparation and physicochemical properties of compression-molded keratin films,Biomaterials25(12)(2004)2265-2272.
[16]A.J.Poole,R.E.Lyons,J.S.Church,Dissolving feather keratin using sodium sulfide for bio-polymer applications,J.Polym.Environ.19(4)(2011)995-1004.
[17]E.Senoz,R.P.Wool,C.W.J.McChalicher,C.K.Hong,Physical and chemical changes in feather keratin during pyrolysis,Polym.Degrad.Stab.97(3)(2012)297-307.
[18]J.R.Barone,W.F.Schmidt,Effect of formic acid exposure on keratin fiber derived from poultry feather biomass,Bioresour.Technol.97(2)(2006)233-242.
[19]F.J.Wortmann,M.Stapels,R.Elliott,L.Chandra,The effect of water on the glass transition of human hair,Biopolymers81(5)(2006)371-375.
[20]M.Spei,R.Holzem,Thermoanalytical investigations of extended and annealed keratins,Colloid Polym.Sci.265(1987)965-970.
[21]C.Tonin,A.Aluigi,M.B.Songia,C.D'Arrigo,M.Mormino,C.Vineis,Thermoanalytical characterisation of modified keratin fibres,J.Therm.Anal.Calorim.77(3)(2004)987-996.
[22]P.Chen,L.Zhang,New evidences of glass transitions and microstructures of soy protein plasticized with glycerol,Macromol.Biosci.5(3)(2005)237-245.
[23]C.J.R.Verbeek,L.E.van den Berg,Extrusion processing and properties of proteinbased thermoplastics,Macromol.Mater.Eng.295(1)(2010)10-21.
[24]Q.X.Wu,L.N.Zhang,Properties and structure of soy protein isolate-ethylene glycol sheets obtained by compression molding,Ind.Eng.Chem.Res.40(8)(2001)1879-1883.
[25]A.Ullah,T.Vasanthan,D.Bressler,A.L.Elias,J.Wu,Bioplastics from feather quill,Biomacromolecules12(10)(2011)3826-3832.
[26]L.Zhang,P.Chen,J.Huang,G.Yang,L.S.Zheng,Ways of strengthening biodegradable soy-dreg plastics,J.Appl.Polym.Sci.88(2)(2003)422-427.
[27]P.Chen,H.Tian,L.Zhang,P.R.Chang,Structure and properties of soy protein plastics with epsilon-caprolactone/glycerolas binary plasticizers,Ind.Eng.Chem.Res.47(23)(2008)9389-9395.
[28]M.Pereda,M.I.Aranguren,N.E.Marcovich,Effect of crosslinking on the properties of sodium caseinate films,J.Appl.Polym.Sci.116(1)(2010)18-26.
 Chinese Journal of Chemical Engineering2016年3期
Chinese Journal of Chemical Engineering2016年3期
- Chinese Journal of Chemical Engineering的其它文章
- Influence of synthesis parameters on the properties of LiFePO4/C cathode material☆
- Controlled release and enhanced antibacterial activity of salicylic acid by hydrogen bonding with chitosan☆
- Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb(II)and Cd(II)☆
- Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion☆
- Performance evaluation of a modified step-feed anaerobic/anoxic/oxic process for organic and nutrient removal
- Biodiesel synthesis via metal oxides and metal chlorides catalysis from marine alga Melanothamnus afaqhusainii
