Biodiesel synthesis via metal oxides and metal chlorides catalysis from marine alga Melanothamnus afaqhusainii
Abdul Majeed Khan,Noureen Fatima
Research Laboratory of Bioenergy,Department of Chemistry,Federal Urdu University of Arts,Science and Technology,Gulshan-e-Iqbal Campus,University Road,Karachi-75300,Pakistan
1.Introduction
Energy is the basic human survival need.The current energy requirements are fulfilled by the consumption of fossil fuels(coal,petroleum and natural gas)which are being depleted with the passage of time[1].In addition,the burning of fossil fuels emits toxic and greenhouse gases which are resulting in global warming and pollution[2-4].The limited supply and the non-renewable mode of fossil fuels have urged to search for the viable alternatives of energy that can overcome these issues[1,5,6].Biodiesel is the green alternative of petrodiesel that emits a lesser amount of pollutants like CO,CO2,SO2and un-burnt hydrocarbons.It is the mixture of fatty acid alkyl esters produced by the transesterification of edible or non-edible oils with the short chain alcohols[7-10].
Transesterification takes place in the presence of homogeneous or heterogeneous catalysts.The homogeneous catalysts are highly reactive and provide single phase for the reactants,but the separation of catalyst and byproducts from biodiesel is difficult.On the other hand,heterogeneous catalysts are also very reactive,non-corrosive,avoid soap formation and easily separated at the end of the reaction.There are different kinds of heterogeneous catalysts,including the metal oxides,solid bases,solid acids and enzymes[11-15].Various metal oxides such as BaO,SrO,CaO,ZnO,TiO2,ZrO2,CeO2,MgO,CaO/SiO2,BaO/SiO2and MgO/SiO2have been reported for biodiesel synthesis[16-19].The metalchlorides like ZnCl2,AlCl3,SnCl2and several transition metal complexes of Sn2+,Zn2+,Pb2+and Hg2+have also been reported to be the reactive heterogeneous Lewis acid catalysts for the transesterification reaction[9,20,21].
The use of non-edible oils is preferred as the source of biodiesel production,because the consumption of vegetable oils or animal fats for this purpose can cause food starvation[22].Various species of marine macro-algae and micro-algae have been investigated for biodiesel synthesis[23-26].In this article,marine macroalgaMelanothamnus afaqhusainiihas been utilized for biodiesel production.M.afaqhusainiibelongs to the class Rhodophyta which is reddish brown in color with height up to 80 cm.This alga has a small disk like holdfast with small rhizoidal projections and it is strongly attached to the rocks[27,28].A number of metals including Mg,Fe,Mn,Cu,Ni,Zn,Cr,Pb,Co and Cd are reported fromM.afaqhusainii[29].The fatty acid composition of this alga has also been reported[30].This research article represents the conversion of marine red macroalgaM.afaqhusainiioil to biodiesel in the presence of a number of metal oxides and metal chlorides as catalysts.
2.Experimental
2.1.Materials
Different reagents that have been used for the synthesis of biodiesel and its analysis including dichloromethane,ethyl acetate,n-hexane,silica gel,methanol,ethanol,petroleum ether,chloroform,toluene,diethyl ether,acetic acid,acetone,CaO,MgO,Al2O3,HgO,PbO,PbO2,TiO2,MnO2,Fe2O3,ZnO,CaCl2,MgCl2,AlCl3,HgCl2,PbCl2,SnCl2,TiCl4,MnCl2,FeCl3,ZnCl2,conc.H2SO4,iodine crystals,vanillin,ceric sulfate,ninhydrin,silica gel and K2Cr2O7.All these chemicals were of analytical grade.
2.2.Collection and pre-treatment
The marine red macroalgaM.afaqhusainiiwas collected from the Bullejicoast,Karachi(Fig.1).The alga was identified by Prof.Dr.Mustafa Shameel(late)and its voucher specimen(KUH-SW-R1461)was deposited in the herbarium of the Department of Botany,University of Karachi.It was dried,crushed and then ground by the chopper of Black and Decker Company(FX3508)to obtain the powdered alga(12 kg).The alga was soaked in dichloromethane:ethyl acetate:n-hexane(1:2:3)for three weeks.The solvent was separated using a rotary evaporator under reduced pressure to obtain the concentrated oily contents.The oil was purified by passing it through the column chromatography using silica gel as stationary phase whilen-hexane as mobile phase.

Fig.1.Pictorial representation of marine red alga Melanothamnus afaqhusainii.
2.3.Transesterification
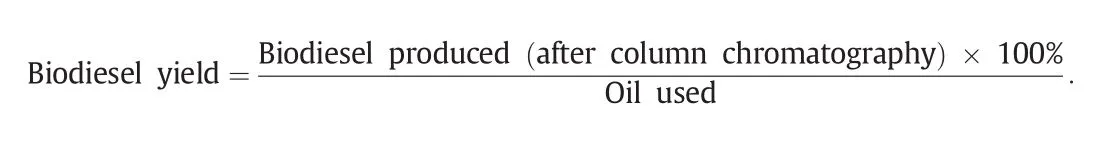
Algal oil(5 g),methanol(10 ml)and CaO(0.5 g)were taken in an Erlenmeyer flask.The mixture was stirred and re fluxed on the hot plate stirrer(Lab Tech® Daihan Labtech Co.,Ltd)at 100-110°C for 18 h.The reaction was monitored with the TLC examination each over 6 h ofbiodieselproduction using petroleum ether:chloroform:toluene(7:2:1)as mobile phase and iodine crystals/silica gel as visualizing agent.When the reaction completed,two distinct layers were observed,the upper layer contains biodieseli.e.fatty acid methyl ester(FAME)while the lower layer contains glycerol,un-reacted oil and catalyst.Both the layers were separated by separating funnel.Similar method was used to synthesize FAME using a number of other metal oxides,including MgO,Al2O3,HgO,PbO,PbO2,TiO2,MnO2,Fe2O3,ZnO and metal chlorides CaCl2,MgCl2,AlCl3,HgCl2,PbCl2,SnCl2,TiCl4,MnCl2,FeCl3and ZnCl2.The biodiesel was purified by column chromatography using the same solvent system as used for TLC examination.The percentage yield of the biodiesel was calculated using the formula:
2.4.Reusability of catalysts
After the completion of the transesterification reactions with different heterogeneous catalysts including metaloxides and metalchlorides,the reaction mixtures were kept at room temperature for 1 h to cool down the contents.Then,it was filtered to separate out the solid catalysts from the product.The recovered catalyst on the filter paper(85-90%of original used catalyst)was washed three times with the mixture ofn-hexane:chloroform(1:1,20 ml)to remove oily contents and biodiesel traces.The catalysts were dried for 1 h at 100°C in the oven.The mass of the catalysts so recovered was found to be 5-10%less than the originally taken catalysts.These catalysts were re-used for the transesterification of algal oil to check their catalytic activity.
2.5.Screening of mobile phases and visualizing agents for TLC
The TLC of biodiesel was run with different combinations of polar and non-polar mobile phases to check the best system that separates the products efficiently.Mobile phases used in the analysis were petroleum ether,petroleum ether:chloroform:toluene(8:1:1 and 7:2:1),n-hexane:diethyl ether:acetic acid(8:2:0.1),n-hexane:ethyl acetate:acetic acid(9:1:0.1),n-hexane:diethyl ether:acetone(8:2:0.2),petroleum ether:ethyl acetate:acetone(9:0.5:0.5)and petroleum ether:diethyl ether:chloroform(4.5:4.5:1).The analytical grade petroleum ether having boiling point range 40-60°C was used as mobile phase for column and thin layer chromatography.
After running the TLC in these mobile phases,it was visualized by a number of spraying reagents like H2SO4(10%)in ethanol,iodine crystals/silica gel,vanillin(15 g)in ethanol(250 ml)and conc.H2SO4(2.5 ml),H2SO4(10%)in ceric sulfate,FeCl3in methanol:H2O(1:1),ninhydrin(0.2 g)in ethanol(100 ml),K2Cr2O7(0.5%)in conc.H2SO4(0.2 ml)and UV-lamp(254 nm)in order to select the best one for biodiesel identification.
2.6.Analysis
The oiland biodiesel were characterized by a numberof fuelproperties that include density,dynamic viscosity,kinematics viscosity,acid value,cloud point and pour point.These properties were compared to the ASTM standard limits of biodiesel.In addition,the oil and biodiesel were also analyzed by thin layer chromatography.
3.Results and Discussion
The dry weight of marine macroalgaM.afaqhusainiiproduced 1.3%oily content that was purified by the column chromatography.The oil was transesterified to biodieseli.e.fatty acid methyl ester(FAME)by mechanical stirring using a series of metal oxides and metal chlorides as catalysts at 100-110°C.The reactions were continued for 18 h to achieve the maximum biodiesel yield.The reaction progress was checked after the intervals of 6 h.Metal oxides were found to be more reactive than the corresponding metal chlorides.Generally,metal oxides act as basis,whereas metal chlorides act as Lewis acids.The mechanism showed that the metal oxides formed the complexes both with the methanol and the carbonyl group of a triglyceride molecule[31].This complex formation favored the transesterification reaction.CaO gave the highest yield(80%)of biodiesel after 6 h at 100-110°C.It is due to the highest basic character ofCaO.The reactivity of metaloxides decreased in the order of CaO>MgO>PbO2>PbO>ZnO>TiO2whereas,Al2O3,HgO,MnO2and Fe2O3were found to be non-reactive at the experimental conditions followed.All the metal oxides were easily recovered after completion of the reactions and re-used for catalysis.A number of solvent systems were investigated to select the best mobile phase for TLC examination of biodiesel.Petroleum ether:chloroform:toluene(7:2:1)was selected as the most suitable mobile phase.Furthermore,different visualizing agents were also used to sort out the reagents that give the most prominent spot of biodiesel.The investigation showed that iodine crystals/silica gel is the suitable candidate to identify the biodiesel.The Rf value of FAME was calculated to be 0.35(Table 1,Figs.2-4).
The metal chlorides are less reactive than metal oxides due to their acidic behavior.However,they also produced a significant yield of biodieseli.e.60%,53%and 42%biodiesel was produced using ZnCl2,TiCl4and HgCl2respectively after 6 h at 100-110°C.The mechanism ofreaction showed that the acidic site(free metal orbitals)of metal chloride interacts the oxygen atom of the carbonyl group of triglycerides to make the complex,which activated the reactive site(carbonyl carbon)of triglyceride that enhanced the rate of attack of methanol to form the desired product[21].The reactive transition metal chlorides like HgCl2,TiCl4and ZnCl2made colored complexes while the other complexes of metal chlorides were found to be colorless.The overall reactivity order of the metalchlorides as observed by the TLC examination was observed to be ZnCl2>TiCl4>HgCl2>AlCl3>SnCl2while CaCl2,MgCl2,PbCl2,MnCl2and FeCl3were found to be non-reactive for this reaction at the operating conditions.The separation of few metal chlorides was slightly difficult as they were miscible in the un-reacted alcoholic
phase.The product was confirmed by the same TLC examination system as used for metal oxide catalysts(Table 1,Figs.2,5 and 6).
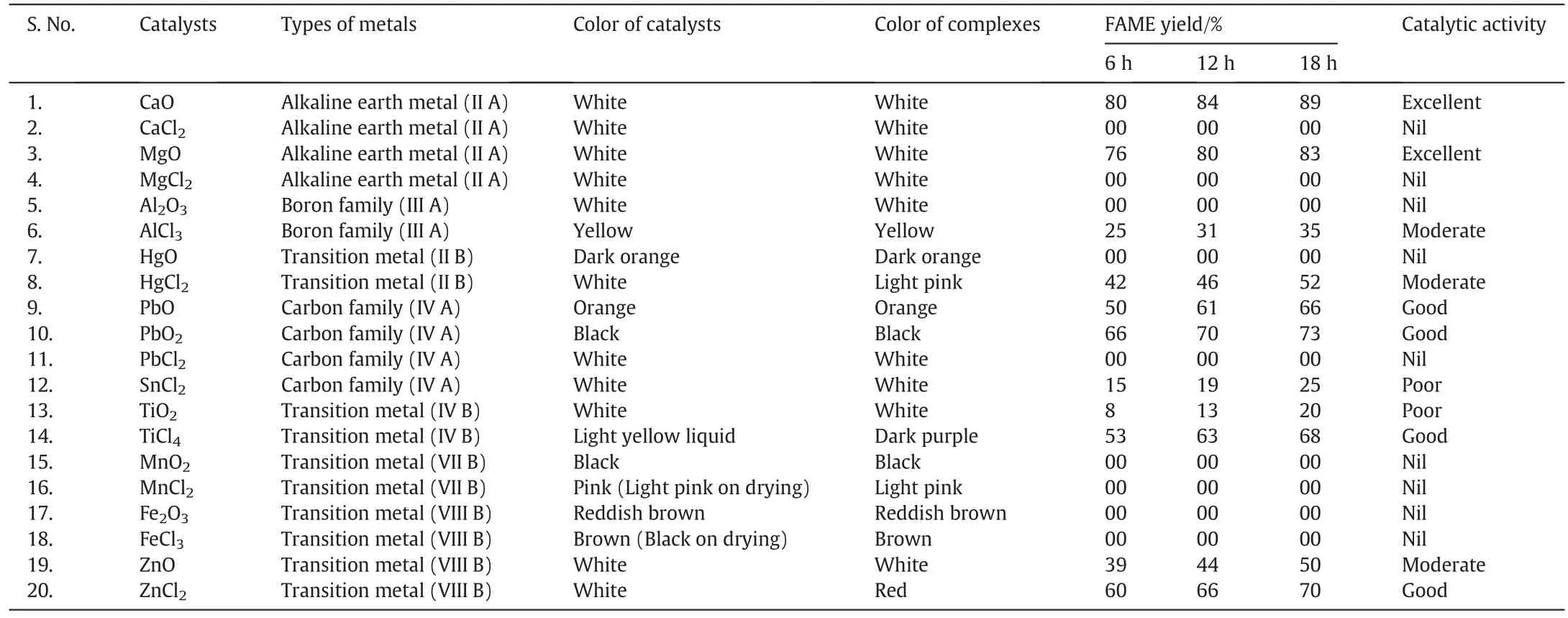
Table 1Comparison of metal oxides and metal chlorides for the catalysis of biodiesel production
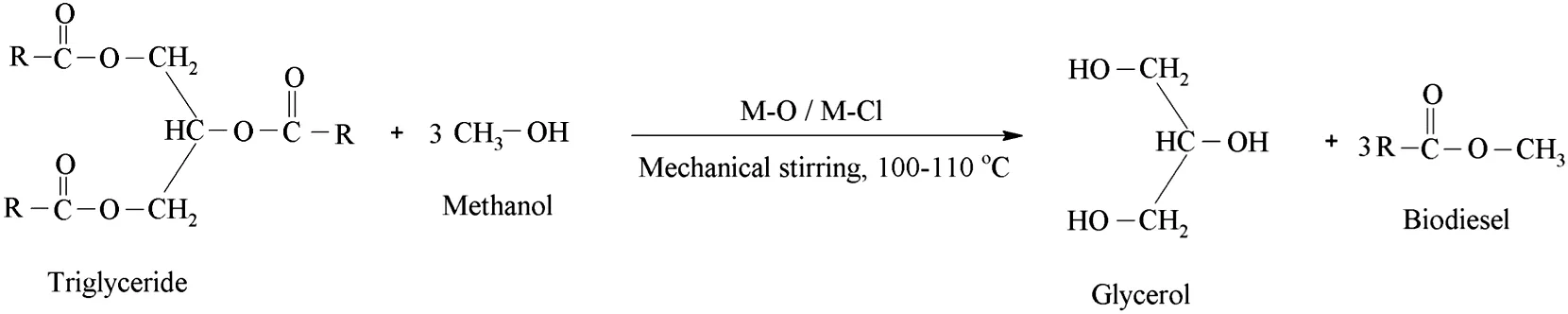
Fig.2.General chemical reaction for biodiesel production using metal oxide and metal chloride catalysts.

Fig.3.Mechanism of biodiesel production using metal oxides.

Fig.4.TLC examination of FAME catalyzed by different metal oxides.
In most of the cases,the catalysts for biodiesel synthesis are purchased from the market that increases the production cost of biodiesel synthesis on large scale.Therefore,if the already used catalysts will be re-used in the next coming batch of biodiesel synthesis then the cost of the biodiesel production will be reduced to a significant level.The re-usability of these catalysts has been confirmed experimentally and all the metal oxides(CaO,MgO,PbO2,PbO,ZnO and TiO2)and metal chlorides(ZnCl2,TiCl4,HgCl2,AlCl3and SnCl2)showed the same reactivity towards the next coming batches of biodiesel synthesis as for the previous one.These catalysts were found to be highly stable even at high temperature as it is evident from their calcination at 800-1000°C for the preparation of nanoparticles.
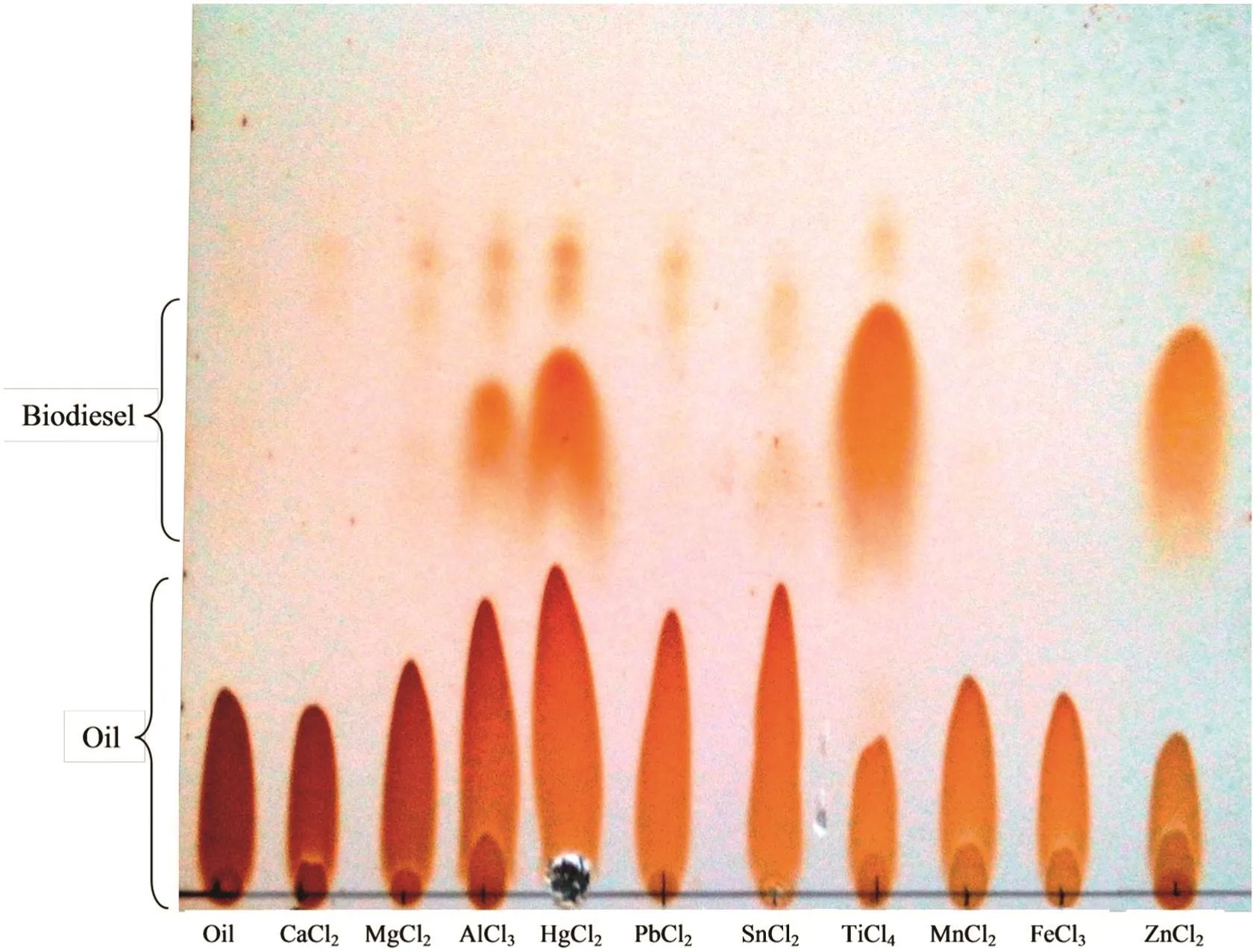
Fig.6.TLC examination of FAME catalyzed by different metal chlorides.
The TLC examination system to analyze biodiesel was finalized after screening a wide variety of mobile phases and TLC spraying reagents and techniques.When only petroleum ether as mobile phase was used for this purpose,it did not separate the biodiesel from fatty acids.The presence of acetone in most of the cases absorbed moisture that badly disturbed the separation.Petroleum ether:diethyl ether:chloroform(4.5:4.5:1)displayed the spots of fatty acids and biodiesel but showed overlapping with each other.Petroleum ether:chloroform:toulene(7:2:1)was selected as the best mobile phase system because it separated the biodiesel spot from other spots very clearly(Table 2).The UV lamp at 254 nm was only helpful to detect the conjugated fatty acids,but biodiesel spots could not be visualized.The presence of H2SO4in few spraying reagents damaged the TLC strip due to the moisture content.The iodine crystals/silica gel was found to be the most effective spraying reagent for biodiesel identification on laboratory scale as well as on a commercial scale.The iodine crystals not only detected the fatty acids,but also the biodiesel spots were very clear while the silica gel was added in order to absorb the moisture during the TLC examination(Table 3).
The fuel properties like density,dynamic viscosity,kinematics viscosity,cloud point,pour point and acid value of oil were found to be higher than ASTM standard limits of biodiesel.However,after the transesterification of oil to biodiesel(FAME),these properties decreased and found to be in accordance with the ASTM standard limits for biodiesel which provided the strong evidence of biodiesel production(Table 4).
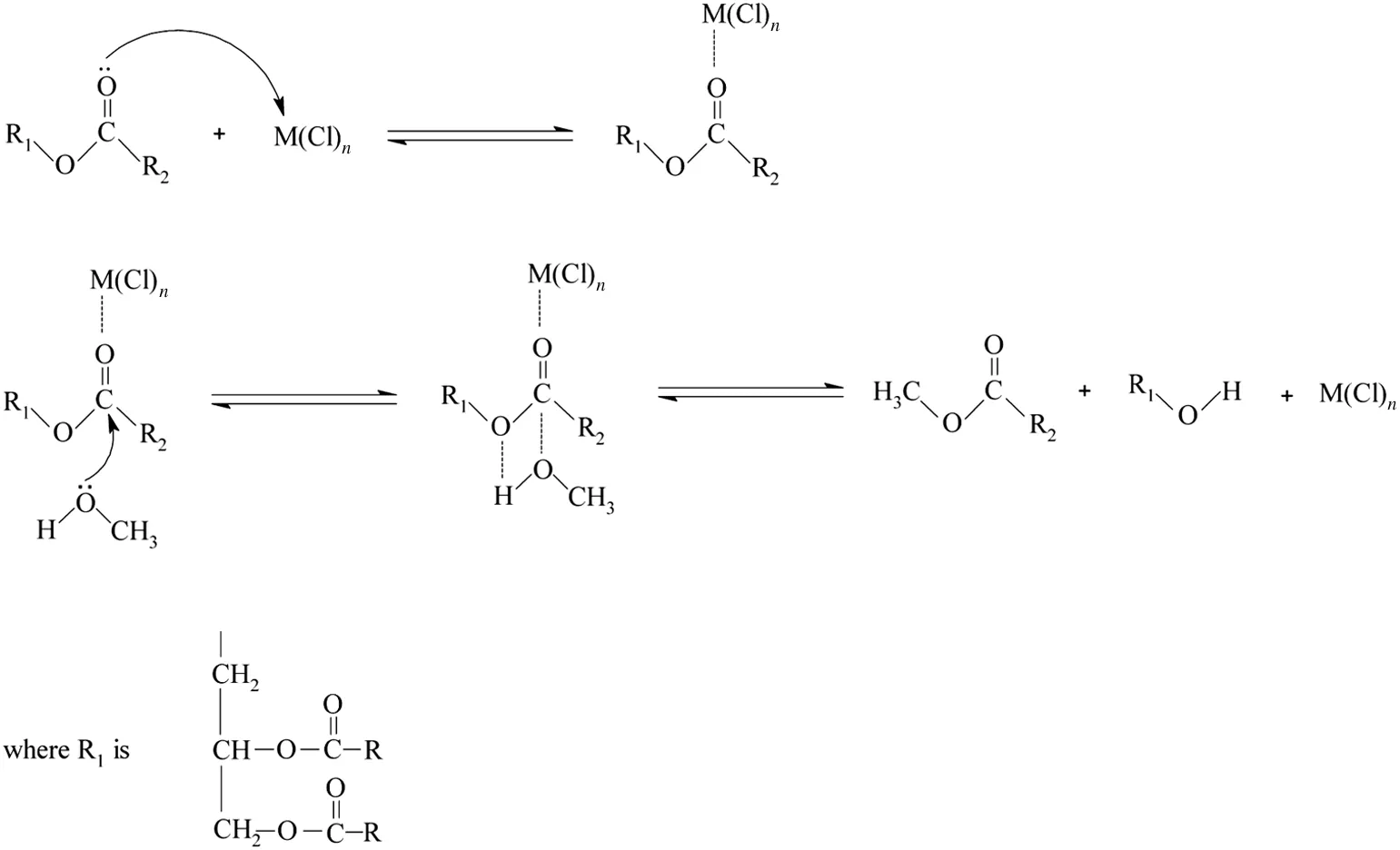
Fig.5.Mechanism of transesterification of triglycerides catalyzed by metal chlorides.

Table 2Screening of mobile phases for thin layer chromatography(TLC)of biodiesel

Table 3Screening of TLC visualizing spraying reagents and techniques
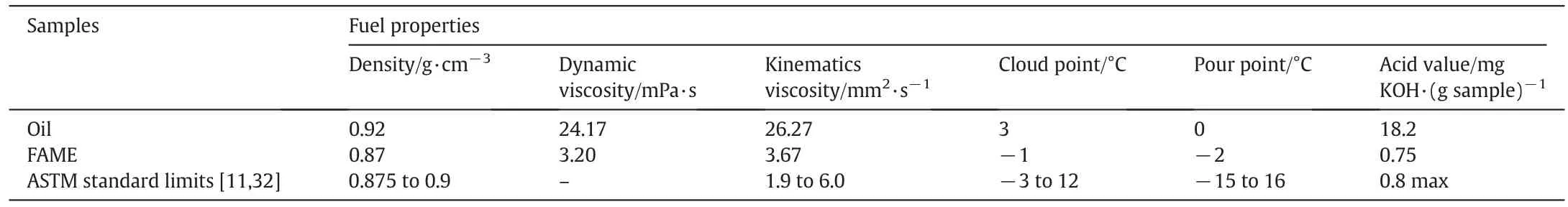
Table 4Fuel properties of biodiesel in comparison with the oil and ASTM standard limits
4.Conclusions
Biodiesel was synthesized from the oily content of marine red macro-algaM.afaqhusainiiusing various heterogeneous catalysts including metal oxides and metal chlorides.The metal oxides produced higher yield of biodiesel and thus showed more reactivity as compared to the metalchlorides.The reactivity order of metaloxides was found to be CaO>MgO>PbO2>PbO>ZnO>TiO2and that of metal chlorides was ZnCl2>TiCl4>HgCl2>AlCl3>SnCl2.All the catalysts were easily recovered atthe end of reactions and re-used in the nextcoming batch of biodiesel synthesis where they showed similar reactivity and stability.In addition,petroleum ether:chloroform:toluene(7:2:1)was selected as the best mobile phase and iodine vapors/silica gel as visualizing agent for biodiesel.This system displayed clear spots of both fatty acids and biodiesel.The fuel properties of biodiesel such as density,kinematics viscosity,cloud point,pour point and acid value matched the criteria of ASTM standard limits of biodiesel.This research article will be helpful to synthesize the biodiesel on a commercial scale from any cheapest source like waste cooking oil,animal fats,and jatropha oil.In fact,this research will be valuable to overcome the energy crisis,global warming,pollution,biodiversity and instability of global economy.
Acknowledgments
The authors are greatly thankful to the Higher Education Commission of Pakistan for providing the Ph.D.scholarship(117-3083-PS7-208,50018488)to Noureen Fatima under Indigenous Ph.D.5000 Fellowship Program.
[1]B.Sanjay,Heterogeneous catalyst derived from natural resources for biodiesel production:A review,Res.J.Chem.Sci.3(6)(2013)95-101.
[2]A.M.Khan,S.Naz,Biopower generation from kitchen wastewater using a bioreactor,Water Environ.Res.86(1)(2014)3-12.
[3]A.M.Khan,M.Obaid,R.Sultana,Production of biodiesel from marine algae to mitigate environmental pollution,J.Chem.Soc.Pak.37(3)(2015)612-620.
[4]A.M.Khan,Electricity generation by microbial fuel cells,Adv.Nat.Appl.Sci.3(2)(2009)279-286.
[5]A.M.Khan,M.M.Ali,S.Naz,M.Sohail,Generation of bioenergy by the aerobic fermentation of domestic wastewater,J.Chem.Soc.Pak.32(2)(2010)209-214.
[6]A.M.Khan,Ataullah,A.Shaheen,I.Ahmad,F.Malik,H.A.Shahid,Correlation of COD and BOD of domestic wastewater with the power output of bioreactor,J.Chem.Soc.Pak.33(2)(2011)269-274.
[7]A.M.Khan,S.Khaliq,R.Sadiq,Investigation of waste banana peels and radish leaves for their biofuels potential,Bull.Chem.Soc.Ethiop.29(2)(2015)239-245.
[8]A.M.Khan,A.Shaikh,I.Khan,S.Kanwal,Comparative production of biodiesel from waste chicken fats and cooking oil,J.Biofuels5(1)(2014)32-40.
[9]F.R.Abreu,D.G.Lima,E.H.Hamú,S.Einloft,J.C.Rubim,P.A.Z.Suarez,New metal catalysts for soybean oil transesterification,J.Am.Oil Chem.Soc.80(6)(2003)601-604.
[10]A.M.Khan,S.Ali,Production of biodiesel and bioethanol from the legumes ofLeucaena leucocephala,J.Biofuels5(2)(2014)76-82.
[11]S.G.Chopade,K.S.Kulkarni,A.D.Kulkarni,N.S.Topare,Solid heterogeneous catalysts for production of biodiesel from trans-esterification of triglycerides with methanol:A review,Acta Chim.Pharm.Indica2(1)(2012)8-14.
[12]L.T.Thanh,K.Okitsu,L.V.Boi,Y.Maeda,Catalytic technologies for biodiesel fuel production and utilization of glycerol:A review,Catalysts2(2012)191-222.
[13]H.Muthu,V.SathyaSelvabala,T.K.Varathachary,D.K.Selvaraj,J.Nandagopal,S.Subramanian,Synthesis of biodiesel from neem oil using sulfated zirconia via transesterification,Braz.J.Chem.Eng.27(4)(2010)601-608.
[14]S.T.Jiang,F.J.Zhang,L.J.Pan,Sodium phosphate as a solid catalyst for biodiesel preparation,Braz.J.Chem.Eng.27(1)(2010)137-144.
[15]A.A.Refaat,Biodiesel production using solid metal oxide catalysts,Int.J.Environ.Sci.Technol.8(1)(2011)203-221.
[16]B.Salamatinia,I.Hashemizadeh,A.Z.Abdullah,Alkaline earth metal oxide catalysts for biodiesel production from palm oil:Elucidation of process behaviors and modeling using response surface methodology,Iran.J.Chem.Chem.Eng.32(1)(2013)113-126.
[17]S.J.Yoo,H.S.Lee,B.Veriansyah,J.Kim,J.D.Kim,Y.W.Lee,Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts,Bioresour.Technol.101(2010)8686-8689.
[18]Venkatesh,S.Z.M.Shamshuddin,M.Shyamsundar,V.T.Vasanth,Biodiesel synthesis fromPongamia pinnataoil over modified CeO2catalysts,J.Mex.Chem.Soc.58(4)(2014)378-385.
[19]M.Mohadesi,Z.Hojabri,G.Moradi,Biodiesel production using alkali earth metal oxides catalysts synthesized by sol-gel method,Biofuel Res.J.1(2014)30-33.
[20]N.U.Soriano,R.Venditti Jr.,D.S.Argyropoulos,Biodiesel synthesis via homogeneous Lewis acid-catalyzed transesterification,Fuel88(2009)560-565.
[21]A.Casas,M.J.Ramos,J.F.Rodríguez,Á.Pérez,Tin compounds as Lewis acid catalysts for esterification and transesterification of acid vegetable oils,Fuel Process.Technol.106(2013)321-325.
[22]A.Talebian-Kiakalaieh,N.A.S.Amin,H.Mazaheri,A review on novel processes of biodiesel production from waste cooking oil,Appl.Energy104(2013)683-710.
[23]D.M.El Maghraby,E.M.Fakhry,Lipid content and fatty acid composition of Mediterranean macro-algae as dynamic factors for biodiesel production,Oceanologia57(2015)86-92.
[24]A.M.Khan,M.S.Hussain,Production of biofuels from marine macroalgaeMelanothamnus afaqhusainiiandUlva fasciata,J.Chem.Soc.Pak.37(2)(2015)371-379.
[25]A.Sánchez,R.Maceiras,A.Cancela,M.Rodríguez,In fluence ofn-hexane onin situtransesterification of marine macroalgae,Energies5(2012)243-257.
[26]T.M.Mata,A.A.Martins,N.S.Caetano,Microalgae for biodiesel production and other applications:A review,Renew.Sust.Energ.Rev.14(2010)217-232.
[27]S.Afaq-Husain,M.Shameel,Further investigations on the red algaMelanothamnus afaqhusainii(Ceramiales)from the coast of Pakistan,Pak.J.Bot.32(1)(2000)15-26.
[28]A.M.Khan,Phytochemical and s ructural studies on the chemical constituents ofTaxus wallichiana,Tanacetum gracile,Jolyna laminarioidesand other marine algae(Ph.D.Thesis)H.E.J Research Institute of Chemistry,University of Karachi,Pakistan,2000(Retrieved from http://eprints.hec.gov.pk/979/1/711.html.htm).
[29]R.Qari,S.A.Siddiqui,A comparative study of heavy metal concentrations in red seaweeds from different coastal areas of Karachi,Arabian sea,Indian J.Mar.Sci.39(1)(2010)27-42.
[30]L.Shehnaz,Comparative phycochemical investigations on a variety of marine algae from Karachi coast(Ph.D.Thesis)Department of Botany,University of Karachi,Pakistan,2003(Retrieved from http://prr.hec.gov.pk/Thesis/1010.pdf).
[31]A.P.S.Chouhan,A.K.Sarma,Modern heterogeneous catalysts for biodiesel production:A comprehensive review,Renew.Sust.Energ.Rev.15(2011)4378-4399.
[32]A.U.Ofoefule,C.N.Ibeto,I.E.Ugwuamoke,Determination of optimum catalyst concentration for biodiesel yield from coconut(Cocos nucifera)oil,Int.Res.J.Pure Appl.Chem.3(4)(2013)357-365.
 Chinese Journal of Chemical Engineering2016年3期
Chinese Journal of Chemical Engineering2016年3期
- Chinese Journal of Chemical Engineering的其它文章
- Influence of synthesis parameters on the properties of LiFePO4/C cathode material☆
- Controlled release and enhanced antibacterial activity of salicylic acid by hydrogen bonding with chitosan☆
- The structure,tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics☆
- Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb(II)and Cd(II)☆
- Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion☆
- Performance evaluation of a modified step-feed anaerobic/anoxic/oxic process for organic and nutrient removal
