Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb(II)and Cd(II)☆
Huizhu Zhou,Huanhuan Hou,Lei Dai*,Yuehua Li,Jing Zhu,Ling Wang*
College of Chemical Engineering,North China University of Science and Technology,Tangshan 063009,China
Hebei Province Key Laboratory of Inorganic and Non-metal Materials,Tangshan 063009,China
1.Introduction
Among the electro-analytical systems,the electrochemical stripping analysis is recognized as a powerful and low cost technique for measuring trace heavy metal ions due to its high sensitivity and selectivity[1-3],especially suitable for on-site analysis of environmental samples.Conventionally,mercury film electrodes were used as working electrodes due to the superior electro-analytical performance of mercury,such as,its wide operational potential window and high over potential for hydrogen evolution reaction over other materials.However,mercury toxicity and difficulties associated with its handling,storage and disposal cause safety concern[1].So,researchers have always sought for new electrode materials that can potentially replace toxic mercury in electro-analysis.The bismuth film electrode was introduced as an efficient replacement for mercury counterparts in 2000[4,5],because Bi is of relatively low toxicity,able to alloy with many metals and has a reasonably wide potential window.The cathodic part of the operational potential window of the bismuth film electrode is very similar to that of mercury.Since then,a lot of research works have been carried out on Bi film electrode preparation and its application[6-17].Svancara has reviewed recent advance in anodic stripping voltammetry with Bimodified carbon paste electrodes[18,19].By now,bismuth based electrodes have been presented in different configurations,that is,bismuth film electrode,bismuth bulk electrode[6-16],and bismuth powder modified carbon paste electrode[17].These electrodes show a good reproducibility and sensitivity and have been successfully used for measuring various metal ions and other species associated with environmental monitoring.Among these electrodes,Bi film electrode is advantageous.Usually,Bi film electrodes were prepared byin-situorex-situmethod with non-porous structure.Recently,it was found that compared to non-porous Bi film electrode,the porous structured Bi film electrodes which are of high surface area and high activity can improve detection performance for the determination of trace heavy metals.This has led to an interest in preparation of micro-and nanostructured electrodes[20-22].Based on hydrated aluminum oxide template technology,nano-structured Bi film electrode was prepared on a glassy carbon electrode[21].When the electrode was used for the detection of Pb(II)and Cd(II),the current signals were over 50 times higher than these obtained from a conventional Bi electrode.The polystyrene sphere templates were also used to elaborate highly porous bismuth film electrodes on a gold electrode surface[22].The porous Bi film with controlled porosity showed an increased internal electroactive area,which led to high sensitivity and low detection limits for Pb(II)and Cd(II)analysis by anodic stripping voltammetry.
Recently,electro-deposition at elevated current density,where hydrogen evolution is pronounced,has been shown to be an effective method to prepare high surface area metals directly(that is,hydrogen bubbles dynamic template technology)and porous Ag,Pt electrodes have been prepared[23,24].Obviously,the technology can be also used for preparation of porous Bi film electrode.
The aim of this paper is to prepare Bi film electrodes on glassy carbon electrode surface using constant current electro-deposition with hydrogen bubble dynamic template.The performance of the prepared Bi film for analyzing Pb(II)and Cd(II)by square wave stripping voltammetry was examined.
2.Experimental
All chemicals were of analytical-grade and were used without further purification.All solutions were prepared with ultrapure distilled water.Plating bath consisted of 0.7 mol·L-1Bi(NO3)3in 1.5 mol·L-1HNO3.0.1 mol·L-1sodium acetate/acetate acid buffer(pH 4.5)served as the supporting electrolyte for Pb(II)and Cd(II)detection.
The Bi film electrodes were prepared on the surface of glassy carbon electrode by constant current electrochemical deposition of Bi from a 0.7 mol·L-1Bi(NO3)3in 1.5 mol·L-1HNO3in a two-electrode cell at room temperature.Glassy carbon electrode and platinum disc electrode were used as cathode and anode.The H2bubbles produced during electroplating Bi were used as dynamic templates.In order to obtain Bi film with different porosity,different current densities were applied for variable time but with the same quantities of electricity.After electro-deposition the electrodes were carefully rinsed by sonic vibration in distilled water for the following measurements.
The electrode surface morphologies were characterized by scanning electron microscope(KYKY2800).The square-wave stripping analysis was performed using the electrochemical workstation(LK3200A,China)in a three-electrode cell.The prepared Bi film electrode was used as working electrode,saturated calomel electrode(SCE)as reference electrode and platinum disc electrode as counter electrode.
The prepared Bi film electrodes were used to analyze Pb(II)and Cd(II)by square wave stripping voltammetry.The analysis process can be divided into three steps.Firstly,the tested solutions containing different contents of Pb(II)and Cd(II)with 0.1 mol/L sodium acetate/acetate acid buffer(pH 4.5)as supporting electrolyte were deoxygenated by purging with pure argon for 30 min[25];secondly,a certain deposited potential of-1.0 V(vs.saturated Hg/Hg2Cl2)was applied to the working electrode for some time under stirring in order to convert the heavy metal ions on the Bi film into metal;then stirring was stopped and after 15 s equilibration time,the anodic square-wave stripping votammogram was recorded from-1.0 V to-0.4 V with an amplitude of 20 mV,a frequency of 5 Hz and a potential step of 5 mV.Prior to the next measurement,a cleaning step at-0.4 V for 60 s was performed.Peak currents were registered as analytical signals.
3.Results and Discussion
3.1.Preparation and characterization of the prepared Bi film
The mechanism of formation of the Bi film by hydrogen dynamic template technology has been described in literature[26].Brie fly,at low current density,because of the low nucleation and deposition rate of Bi on glassy electrode surface,non-porous Bi film was obtained.With the increase of deposited current density,the nucleation and deposition rate of Bi on glassy electrode surface were greatly increased,and at the same time,a large number of hydrogen bubbles were evolved and as a result,these hydrogen bubbles played dynamic template roles in the formation of the porous structured Bi film[26].
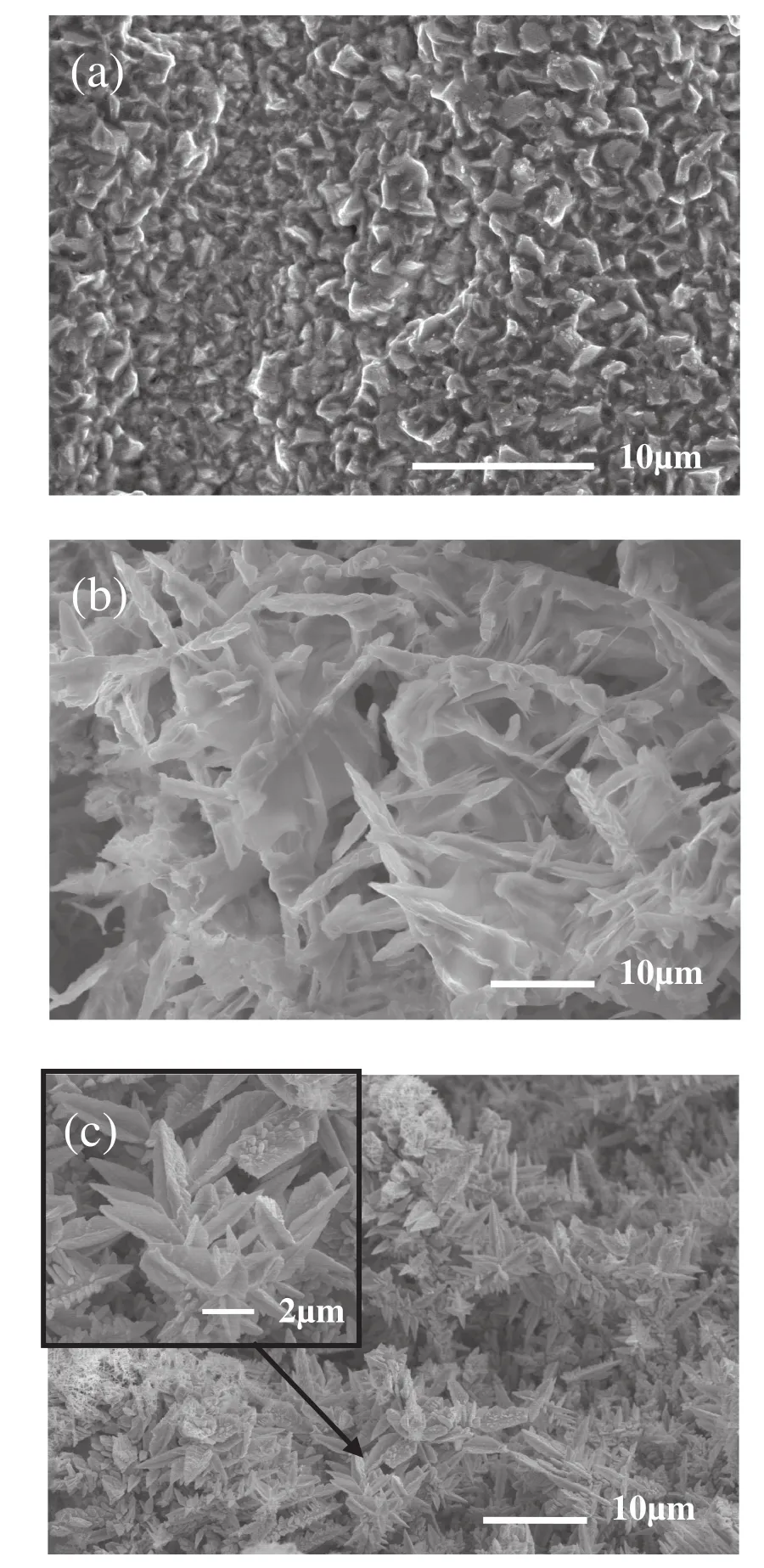
Fig.1.SEM photos of Bi film electrodes elctrochemically deposited through a H2 dynamic templates on glassy carbon electrode surface with different deposition currentdensity but same deposition charge of 3.6 C·cm-2(a)207;(b)525 and(c)605 mA·cm-2.
Bi film electrodes with different structure were obtained by applying a currentdensity of207,525 or605 mA·cm-2for17.5,6.9 and 6 s(with electricity quantities equal to 3.6 C·cm-2),which were marked as electrode1,electrode2 and electrode3,respectively.It should be noted that the quantities of electricity is same for Bi electro-deposition,however,current efficiency was different,resulting in different mass of metal Bi.Fig.1 displays SEM photos of the Bi film surface for electrode1,electrode2 and electrode3,respectively.We can see from Fig.1 that the cathodic current density is found to have a strong effect on the morphology of Bi film.Along with the increase of the applied current density,the cathode polarization and the speed of hydrogen evolution increase and the obtained Bi film has a higher porosity and ultimately a higher electroactive surface area.When a low current density is applied(electrode1),because the current density is too low to make hydrogen evolution,only Bi3+deposition takes place on the glassy carbon surface and a non-porous Bi film is obtained(Fig.1(a)).With the increase of current density,besides Bi deposition,hydrogen ion reduction occurs as well,so a Bi film with pores begin to form by attached hydrogen bubbles(Fig.1(b))(electrode2).Compared to electrode1,the specific area of electrode 2 obviously increases.On furtherincreasing applied current density,because of the increase of hydrogen evolution,very branchy dendrites and small agglomerates of Bi grains are formed on glassy carbon electrode surface(Fig.1(c))(electrode3).Obviously,the Bi film electrode is of porous structure.The inset of Fig.1(c)is the local enlarged image(×5000)from a dendritic structure.We can find from the magnified view in Fig.1(c)that the single Bidendrite is assembled by many small dendrites with length of 4-7 μm and width of 2-5 μm.These dendrites with one end tightly grow in the center of the big dendrite.It is obvious that electrode3 has bigger specific area due to the formation of three-dimensional structure.When current density is more than 620 mA·cm-2,the deposited Bi film begins to partially fall down from glassy carbon electrode surface during washing due to low adhesive force.
3.2.Analytical performance of the Bi films
In order to demonstrate the improvement of electrochemical performance,the three different Bi film electrodes were tested for Pb(II)and Cd(II)analysis by square-wave stripping method under the conditions described above.
Fig.2 illustrates the voltammograms of the Bi film electrodes1-3 for successive additions of Cd(II)and Pb(II)in the concentration range of5-50 μg·L-1at-1.0 V deposition potential.The Bi film electrodes(nonporous and porous)exhibit well-developed and separated stripping peaks.The characteristic peaks of Cd(II)and Pb(II)are observed at-0.84 and-0.64 V,respectively.The stripping peak currents increase with the increase of Pb(II)and Cd(II)concentrations.It should be indicated that the stripping potentials of Pb(II)and Cd(II)are not identical for the 3 electrodes,which is attributed to surface difference of Bi film electrode.In fact,the stripping potentials of Pb(II)and Cd(II)also shift for the same electrode when the deposited amount is different[21].
3.3.The influence of deposition time
To test the deposition time effect on the stripping peak currents,a solution containing 40 μg·L-1Pb(II)and Cd(II)in 0.1 mol·L-1acetic acid/sodium acetate buffer with pH 4.5 was analyzed by square wave stripping voltammetry using electrode3 and result was shown in Fig.4.The stripping peak currents linearly increase with deposition time varying from60 to 200 s,indicating that the accumulating quantity of Pb and Cd is directly proportional to deposition time(Fig.3).
3.4.The performance comparison of three Bi film electrodes
The comparison of calibration curves of three Bi film electrodes for determination of Cd(II)and Pb(II)was shown in Fig.4(a)and(b).As demonstrated in Fig.4(a)and(b),the stripping peak currents of the non-porous Bi electrode1 are the linear function of Cd(II)and Pb(II)concentrations with a sensitivity of 0.0673 μA·L·μg-1(R20.998)for Pb(II)and 0.0225 μA·L·μg-1(R20.987)for Cd(II).The stripping peak currents of the electrode2 also depend linearly on analyte concentrations but with a higher sensitivity of 0.145 μA·L·μg-1(R20.995)for Pb(II)and 0.0601 μA·L·μg-1(R20.999)for Cd(II).The relationship between currents of the electrode3 and analyte concentrations has not only higher sensitivity (0.23 μA·L·μg-1for Pb(II)and 0.083 μA·L·μg-1for Cd(II))but also good linear behavior for Pb(II)(R20.999)and Cd(II)(R20.997).The sensitivities for electrode3 are 3.4 times and 3.6 times higher than those of non-porous Bi electrode1 for Pb(II)and Cd(II).As was indicated in Section 3.1,because of low current efficiency at high current density,electrode3 has low Bi mass but high sensitivity,implying high surface area.The electrode3 offers detection limits of 0.1 μg·L-1for Pb(II)and 0.4 μg·L-1for Cd(II),whereas the electrode1 is found to be 0.4 μg·L-1for Pb(II)and 2.1 μg·L-1for Cd(II)on the basis of the responses for blank solutions following 200 s accumulation(S/N ratio is 3).Obviously,dendritic Bi film electrode with porous structure improves significantly electroanalytical performance.It is expected that lower detection limits for both ions can be achieved by increasing deposition time.
3.5.The repeatability of the Bi electrode3

Fig.2.Square wave stripping voltammograms for successive addition of Pb(II)and Cd(II)from 0 to 50 μg·L-1 for different electrodes:(a)electrode1,(b)electrode2 and(c)electrode3.Supporting electrolyte:0.1 mol·L-1 sodium acetate/acetic acid buffer(pH 4.5),deposition potential:-1.0 V,deposition time:200 s.
To evaluate the repeatability of the developed electrode3, five repetitive measurements of a solution containing 40 μg·L-1Pb(II)and Cd(II)in 0.1 mol·L-1sodium acetate/acetic acid buffer(pH 4.5)were carried out.As illustrated in Fig.5,the stripping voltammograms recorded for electrode3 exhibit a well-defined and undistorted stripping peaks for Pb(II)and Cd(II)and the peak potentials are fixed at-0.825 V and-0.574 V.The relative standard deviations(RSDs)of the results for Pb(II)and Cd(II)are 0.12%and 0.14%,respectively.The measurement reproducibility at different electrodes was also studied.Three electrodes were prepared from the same batch and they were evaluated by performing the determination of 40 μg·L-1Pb(II)and Cd(II).The RSD for the response among electrodes is 3.7%and 4.1%for Pb(II)and Cd(II),respectively.Additionally,under the same conditions,electrode3 was reused for 10 consecutive measurements with signal minimal degradation,which displays a good stability and durability of the Bi film electrode3.

Fig.3.Effect of deposition time on the stripping current signals in a solution containing 40 μg·L-1 Pb(II)and Cd(II).
3.6.The application to real samples
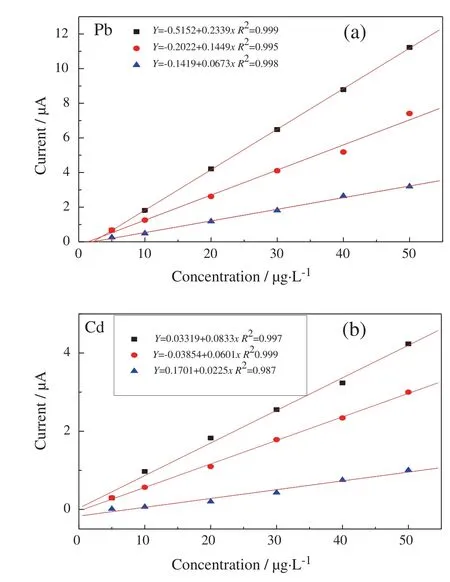
Fig.4.Calibration curves of determination of Pb(II)(a)and Cd(II)(b)using three different Bi film electrodes.
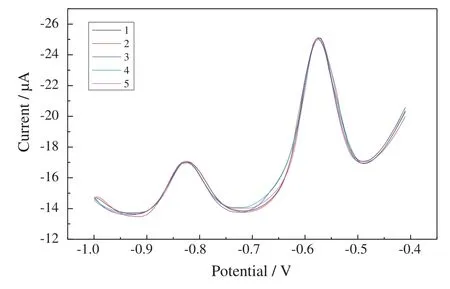
Fig.5.The repeatability of determination of Pb(II)and Cd(II)using the porous Bi electrode3.
To illustrate the application potential of the dendritic Bi film electrode with porous structure in practical analysis,the electrode3 was employed for simultaneous analysis of Pb(II)and Cd(II)in industrial wastewater sample from Guye District,Tangshan,Hebei Province.The water sample filtered with a filter paper was added to 0.1 mol·L-1sodium acetate/acetic acid buffer with pH=4.5.The standard addition method was used to determine the concentrations of Pb(II)and Cd(II)with 10 μg·L-1steps in the concentration range of 10-50 μg·L-1[27].Fig.6 showed the voltammograms recorded during the analysis of the water sample.According to stripping peak currents recorded for Pb(II)and Cd(II)before and after the additions of their standard solutions to the sample,the concentration of Pb(II)and Cd(II)in sample are obtained to be 5.34 μg·L-1and 4.26 μg·L-1,which are comparable with the results obtained by atomic absorption spectroscopy(USA,Buck 210 VGP)based on standard addition method(5.13 μg·L-1and 4.38μg·L-1for Pb(II)and Cd(II)),indicating a acceptable agreement between both techniques.The results should suggest that the Bi film electrode prepared by hydrogen bubble dynamic templates has a great application potential for analysis of trace Pb(II)and Cd(II)in real samples.

Fig.6.The voltammograms of determination of Pb(II)and Cd(II)in water sample by standard addition method.
4.Conclusions
The dendritic Bi film electrodes with porous structure in this work were deposited by constant current electrolysis at a high current density based on hydrogen bubble dynamic templates.The electrodes show highly reproducible and well-defined stripping signals in the square wave stripping analysis of Pb(II)and Cd(II).The sensitivities of the dendritic Bi film electrode with porous structure are 3.4 and 3.6 times higher than those of non-porous Bi film electrode for Pb(II)and Cd(II).And the detection limits obtained on the aforementioned Bi film electrode are 0.1 μg·L-1for Pb(II)and 0.4 μg·L-1for Cd(II).However,the interference from other metal ions needs to be investigated.
[1]A.Economou,P.R.Fielden,Selective determination of Ni(II)and Co(II)by flow injection analysis and adsorptive cathodic stripping voltammetry on a wall jet mercury if lm electrode,Talanta46(1998)1137-1146.
[2]W.Zhang,Z.Y.Liu,S.Y.Zhu,J.Chen,G.B.Xu,Electrochemical stripping analysis of cadmium on tantalum electrode,Electrochem.Commun.123(2010)1291-1293.
[3]S.B.Hocevar,B.Ogorevc,J.Wang,B.Pihlar,A study on operational parameters for advanced use of bismuth film electrode in anodic stripping voltammetry,Electroanalysis4(2002)1707-1712.
[4]J.Wang,J.M.Lu,S.B.Hocevar,P.A.M.Farias,Bismuth-coated carbon electrodes for anodic stripping voltammetry,Anal.Chem.72(2000)3218-3222.
[5]K.Vytras,I.Svancara,R.Metelka,A novelty in potentiometric stripping analysis:Total replacement of mercury by bismuth,Electroanalysis14(2002)1359-1364.
[6]S.Legeai,S.Bois,O.Vittori,A copper bismuth film electrode for adsorptive cathodic stripping analysis of trace nickel using square wave voltammetry,J.Electroanal.Chem.591(2010)93-98.
[7]C.Chen,X.H.Niu,Y.Chai,H.L.Zhao,M.B.Lan,Bismuth-based porous screen-printed carbon electrode with enhanced sensitivity for trace heavy metal detection by stripping voltammetry,Sensors Actuators B Chem.178(2013)339-342.
[8]A.Królicka,R.Pauliukaite,I.Svancara,R.Metelka,A.Bobrowski,E.Norkus,K.Kalcher,K.Vytřas,Bismuth- film-plated carbon paste electrodes,Electrochem.Commun.4(2002)193-196.
[9]A.Królicka,A.Bobrowski,K.Kalcher,J.Mocak,I.Svancara,K.Vytras,Study on catalytic adsorptive stripping voltammetry of trace cobalt at bismuth film electrodes,Electroanalysis15(2003)1859-1863.
[10]I.Svancara,L.Baldrianova,M.Vlcek,R.Metelka,K.Vytras,A role of the plating regime in the deposition of bismuth films onto a carbon paste electrode,Electroanalysis17(2005)120-126.
[11]L.Baldrianová,P.Agrafiotou,I.Svancara,K.Vytras,S.Sotiropoulos,The determination of cysteine at Bi-powder carbon paste electrodes by cathodic stripping voltammetry,Electrochem.Commun.10(2008)918-921.
[12]M.Stoces,I.Svancara,Bismuth trifluoride-modified carbon paste electrode for electro-stripping analysis of heavy metals,Int.J.Electrochem.Sci.8(2013)5657-5671.
[13]C.Kokkinos,A.Economou,Stripping analysis at bismuth-based electrodes,Curr.Anal.Chem.4(2008)183-190.
[14]G.Kefala,A.Economou,M.Sofoniou,Determination of trace aluminium by adsorptive stripping voltammetry on a preplated bismuth- film electrode in the presence of cupferron,Talanta8(2006)1013-1019.
[15]G.Grinciene,A.Selskiene,R.Verbickas,E.Norkus,R.Pauliukaite,Peculiarities of electrochemical bismuth film formation in the presence of bromide and heavy metal ions,Electroanalysis21(2009)1743-1749.
[16]A.Królicka,R.Pauliukaite,I.Svancara,R.Metelka,A.Bobrowski,E.Norkus,K.Kalcher,K.Vytras,Bismuth- film-plated carbon paste electrodes,Electrochem.Commun.4(2002)193-196.
[17]S.B.Hocevar,I.Svancara,K.Vytras,B.Ogorevc,Novel electrode for electrochemical stripping analysis based on carbon paste modified with bismuth powder,Electrochim.Acta51(2005)706-710.
[18]J.Wang,Stripping analysis at bismuth electrodes:A review,Electroanalysis17(2005)1341-1346.
[19]I.Svancara,L.Baldrianová,E.Tesarová,S.B.Hocevar,S.A.A.Elsuccary,A.Economou,S.Sotiropoulos,B.Ogorevc,K.Vytras,Recent advances in anodic stripping voltammetry with bismuth-modified carbon paste electrodes,Electroanalysis18(2006)177-185.
[20]A.Walcarius,A.Kuhn,Ordered porous thin films in electrochemical analysis,Anal.Chem.27(2008)593-603.
[21]J.Saturno,D.Varela,H.Carrero,L.Fernandez,Electroanalytical detection of Pb,Cd and traces of Cr at micro/nano-structured bismuth film electrodes,Sensors Actuators B Chem.1(2011)92-96.
[22]V.Urbanová,M.Bartoš,K.Vytras,A.Kuhn,Porous bismuth film electrodes for signal increase in anodic stripping voltammetry,Electroanalysis22(2010)1524-1530.
[23]S.Cherevko,X.L.Xing,C.H.Chung,Electro-deposition of three-dimensional porous silver foams,Electrochem.Commun.12(2010)467-470.
[24]A.Ott,L.A.Jones,K.S.Bhargava,Direct electro-deposition of porous platinum honeycomb structures,Electrochem.Commun.13(2011)1248-1251.
[25]M.D.Morales,M.R.P.Marin,L.C.Blazquez,E.P.Gil,Performance of a bismuth bulk rotating disk electrode for heavy metal analysis:determination of lead in environmental samples,Electroanalysis24(2012)1170-1177.
[26]Y.Liang,P.Wang,H.B.Dai,Hydrogen bubbles dynamic template preparation of a porous Fe-Co-B/Ni foam catalyst for hydrogen generation from hydrolysis of alkaline sodium borohydride solution,J.Alloys Compd.491(2010)359-365.
[27]N.Serrano,J.M.Díaz-Cruz,C.Ariño,M.Esteban,Stripping analysis of heavy metals in tap water using the bismuth film electrode,Anal.Bioanal.Chem.396(2010)1365-1369.
 Chinese Journal of Chemical Engineering2016年3期
Chinese Journal of Chemical Engineering2016年3期
- Chinese Journal of Chemical Engineering的其它文章
- Influence of synthesis parameters on the properties of LiFePO4/C cathode material☆
- Controlled release and enhanced antibacterial activity of salicylic acid by hydrogen bonding with chitosan☆
- The structure,tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics☆
- Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion☆
- Performance evaluation of a modified step-feed anaerobic/anoxic/oxic process for organic and nutrient removal
- Biodiesel synthesis via metal oxides and metal chlorides catalysis from marine alga Melanothamnus afaqhusainii
