氨基酸席夫碱氧钒(IV)配合物的合成、晶体结构及其抗肿瘤活性
曹亚萍,易芩兰,刘洪梅,李海霞,左建丽,袁泽利
(遵义医学院 药学院,贵州 遵义 563099)
基础医学研究
氨基酸席夫碱氧钒(IV)配合物的合成、晶体结构及其抗肿瘤活性
曹亚萍,易芩兰,刘洪梅,李海霞,左建丽,袁泽利
(遵义医学院 药学院,贵州 遵义563099)
[摘要]目的 开发具有新生物活性的非铂系前药。方法 采用一锅合成法合成3个新的氨基酸席夫碱和1,10-邻菲罗啉(IV)配合物[VO(o-van-Naph-L-Ana)(Phen)](o-van-Naph-L-Ana为邻香草醛与 3-(1-萘基)-L-丙氨酸缩合成的席夫碱,Phen为1,10-邻菲罗啉)(1)、[VO(Hynaph-L-Tyr)(Phen)] (Hynaph-L-Tyr为2-羟基-1-萘甲醛与L-色氨酸缩合成的席夫碱, Phen=1,10-邻菲罗啉)(2)和[VO(o-van-L-Trp)(Phen)](o-van-L-Trp为邻香草醛与L-色氨酸缩合成的席夫碱, Phen=1,10-邻菲罗啉)(3),利用高分辨质谱、FT-IR谱及摩尔电导进行了表征研究,并通过X 射线单晶衍射测定了其晶体结构。用MTT法测定目标配合物1、2和3对A549(人肺腺癌细胞)和 HepG2(人源肝癌细胞)的体外抗肿瘤活性。结果 成功合成了3个新的钒氧氨基酸席夫碱配合物。3个目标配合物的体外抗肿瘤实验结果表明:配合物2和3对两种受试细胞株均表现出一定的细胞毒性,2和3对A549的IC50分别为58.88和53.08 μmol/L,对HepG2的IC50分别为:83.95和44.74 μmol/L。结论 探寻了一种具有反应条件温和、后处理简单的一锅合成方法。合成得到的3个目标配合物中,化合物2和3对A549和HepG2细胞具有中等活性。
[关键词]氨基酸;席夫碱;氧钒(IV)配合物;晶体结构;抗肿瘤活性
In the past few decades, transition metal complexes have been extensively studied as anticancer agents following the discovery ofcis-platin as an effective chemotherapeutic drug[1-3]. Vanadium is an nessential trace metal in life processes, and it occupies an important position inmetallo -pharmaceuticals due to its pharmacological functions. Meanwhile, in many transition metal materials, oxovanadium salts are less expensive than K2PtCl4, RuCl3, or VCl3materials and make for more suitable vanadium-based drug research and development for inexpensive drugs. On the other hand, current research suggests that transformed cells maintain an abnormal redox homeostasis that helps them to obtain optimal genetic instability, which is conducive to cancer progression[4-5]. Vanadium’s intrinsic redox activity can generate reactive oxygen species (ROS). This enhanced level of ROS can disorganize the redox homeostasis in neoplastic cells and impart redox stress to the cell apoptosis either by cleaving DNA or by promoting mitochondrial membrane permeabilisation[6]. Moreover, a growing body of evidence reveals that vanadium complexes can act as potent anticancer agents[7-8]. Although inorganic vanadium salts have relatively high toxicity and poor biological activity, some research has shown that the complexation of vanadium with organic ligands minimizes adverse effects and usually aggrandizes the important benefits. Therefore, it is important to synthesize novel oxovanadium complexes to investigate their medical application.
Amino acids and their derivatives are very important in molecular biology because of their roles in biochemical reactions[1,9]. So, efforts have been made to synthesize and characterize amino acid Schiff base complexes with transition metals[10-11]. Recently, our research group, and others, reported some vanadium complexes with anticancer activities[6,9]. In this work, three novel amino acid Schiff base oxidovanadium (IV) complexes have been synthesized and characterized (Fig 1) to evaluate their pharmacological properties. Moreover, their anticancer activities have been investigated against A-549 and HeGp2 by the MTT assay.
1Materials and methods
1.1MaterialsAll chemicals and reagents obtained from commercial sources were AR grade and used without further purification. H RMS(ESI-MS) were recorded using a time-of-flight Micromass LCT PremierXE spectrometer. FT-IR spectra were recorded, using KBr pellets, with a Vary FT-IR 1000 spectrophotometer in the range 400-4 000 cm-1. Molar conductivity measurements were done using a QCJD-2010 (China) conductivity meter.
1.2Methods
1.2.1Synthesis of [VO(o-van-Naph-L-Ana)(Phen)](1), [VO(Hynaph-L-Trp)(Phen)](2) and [VOo-van-L-Trp)(Phen)](3)The complexe 1 was prepared by a one-pot synthetic procedure in which a mixture of 3-(1-Naphthyl)-L-alanine (0.215 g, 1.0 mmol) and KOH (0.06 g, 1.0 mmol) in 15 ml 50% hot methanol were added to a 5 ml methanol solution ofo-vanillin (0.15 g, 1.0 mmol) and the mixture allowed to reflux for 2 h, followed by addition of 5 ml aqueous solution of vanadyl sulfate (0.16 g, 1.0 mmol). The solution was then heated to reflux for another 1 h, followed by the addition of 1,10-phenanthroline (0.20 g, 1.0 mmol) taken in 5 ml of methanol. The resulting solution after further refluxing for 1 h gave a red precipitate. The solid was isolated, washed with a small amount of water, methanol and diethyl ether, and dried. The complexes 2 and 3 were prepared according to the similar routes of 1. Single crystals of 1, 2 and 3 suitable for X-ray diffraction were obtained over one weekviaslow evaporation of the MeOH -CH2Cl2(v:v=1∶1) solution at room temperature. The data of 1, yield: 68.6%. H RMS (ESI -MS) calculated for C33H25N3O5VNa: 617.1132 (M++ Na), foundm/z: 617.109 4 (M++ Na);FT-IR(4 000-400 cm-1): 3 445, 2 965, 2 925, 2 854, 1 643, 1 592, 1 518, 1 499, 1 425, 1 390, 957, 850, 727, 604, 441; Λm(CH3OH:DMF) = 1∶1-v/v, 1.0 × 10-5mol/l, Ω-1·mol-1·cm2): 6.47. The data of 2, yield: 73.8%. H RMS (ESI -MS) calculated for C34H24N4O4VNa: 627.1168 (M++ Na), foundm/z:627.136 5 (M++ Na). FT-IR(4 000-400 cm-1): 3 456, 3 443,1 648,1 617, 1 549,1 515, 1 468, 1 400, 962, 861, 747, 729, 626, 429; Λm(CH3OH:DMF) = 1∶1 -v/v, 1.0 × 10-5mol/l, Ω-1·mol-1·cm2): 8.37. The data of 3, yield: 75.4%. H RMS (ESI-MS) calculated for C31H24N4O5Na: 606.108 4 (M++ Na), foundm/z: 606.107 5 (M++ Na), FT-IR(4 000-400 cm-1):3 456, 3 443, 1 648, 1 617, 1 549, 1 515, 1 468, 1 400, 962, 861, 747, 729, 626, 429; Λm(CH3OH:DMF) = 1∶1-v/v, 1.0 × 10-5mol/l, Ω-1·mol-1·cm2): 5.96.
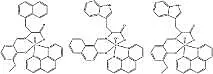
Fig 1 The molecular structure of complexes 1, 2 and 3
1.2.2Crystal structure determinationSingle-crystal XRD data were collected using a Rigaku diffractometer with a mercury charge-coupled device area detector (Mo Kα;λ= 0.710 73 Å) at room temperature. Empirical absorption corrections were applied to the data using the Crystal Clear program. The structure was solved by the direct method and refined by the full-matrix least-squares method onF2using the SHELXTL-97 program. Metal atoms were located fromE-maps and other non-hydrogen atoms were located in successive difference Fourier syntheses. All non-hydrogen atoms were refined anisotropically. The organic hydrogen atoms were positioned geometrically, and those in water molecules were located using the difference Fourier method and refined freely. The C atoms in the pyridine ring were disordered equally over two positions. PLATON/SQUEEZE was used to remove the heavily disordered water molecules. Crystallographic data and other pertinent information for 1-3 are summarized in Table 1. Selected bond distances and angles are listed in Table 2.
1.2.3Invitroanticancer activitiesA549 cells (human lung carcinoma cell line) and HepG2 cells (human hepatoma cell line) were purchase from ATCC. A549 cells were grown in DMEM/HIGH GLUCOSE(1X) (Dulbecco’s modified eagle’s medium) and HepG2 cells were maintained in Modified Roswell Park Memorial Institute 1640 (RPMI-1640) which were supplemented with 10% fetal bovine serum, and 1% penicillin streptomycin solution in a humidified atmosphere of 5% CO2, 95% air at 37 ℃. The passage number range for both cell lines was maintained between 10 and 20. The cells were cultured in 25 cm2cell culture flasks. For experimental purposes, A549 and HepG2 cells were cultured in 96-well plates respectively (5×104cells/ml, 100 ml/well). Cells were allowed to attach for 24 h before treatment with title complexes 1-3. The stock solution of complexes 1-3 was dissolved in dimethyl sulfoxide (DMSO) and filtered with Minisart Filters (0.45 μm), then the solution was diluted with serum free medium to different concentration(0 to 120 μmol/l). Cell monolayers were washed with PBS and then addition the complexes 1-3 within a range of concentrations from 0 to 120 μmol/l for 24 h. The concentration range for the compounds 1-3 and the exposure times have been selected based on preliminary studies performed in our laboratory.
A549 cells were maintained in Dulbecco’s modified eagle’s medium DMEM/HIGH GLUCOSE(1X) medium and HepG2 cells were maintained in Modified Roswell Park Memorial Institute 1640 (RPMI-1640) medium which were supplemented with 10% fetal bovine serum and culture medium, and were kept at 37 ℃ in a humidified atmosphere containing 5% CO2.
The MTT assay is based on the protocol described for the first time by Mossmann.The assay was optimized for the cell lines used in this experiment. Briefly, at the end of the incubation time, cells were incubated for 4 h with 0.5 mg/ml of MTT, dissolved in serum free medium (DMEM or RPMI -1640 for A459 and HepG2 cells respectively). Washing with PBS (100 μl) was followed by the addition of DMSO (200 μl), gentle shaking for 10 min and absorbance was recorded at 490nm using the Multiskan Spectrun. The cell viability ratio was calculated by the following formula: inhibitory ratio (%) = 1-(ODtreated-ODblack)/(ODcontrol-ODblack)×100%.
Cytotoxicity was expressed as the concentration of the complex inhibiting cell growth by 50% (IC50value see Table 3).
2Results
2.1Characterisation and spectroscopic propertiesThe H RMS (ESI-MS) mass spectra of complexes 1-3 exhibit a parent ion peak atm/z617.109 4 for 1, 627.136 5 for 2 and 606.107 5 for 3.
FT-IR spectra of 1-3 exhibit typical bands at 957 cm-1for 1, 962 cm-1for 2 and 962 cm-1for 3, assigned to aυ(V=O)stretching vibration, which is typical for oxovanadium complexes[9]. The very sharp absorptions at 1 657 to 1 636 cm-1are characteristic vibrations of the imine group,υ(C=N), in the complexes[12-13]. The weak bands at 850 and 728 cm-1are attributed to the ring stretching frequencies [υ(C=C)andυ(C=N)] of 1,10-phenanthroline[14]. Two moderate absorptions at 1 617 cm-1and 1 399 cm-1can be assigned to the asymmetric and symmetric stretching vibrations of theυ(CO2-)group[9], respectively. The frequency separation (Δυ) is greater than 200 cm-1, suggesting unidentate bonding for the carboxyl group[9]. The weak peaks in the low wave number region 400-650 cm-1may be attributed toυ(V-O)andυ(V-N)bonds in the complexes[15-17].
The three complexes have small molar conductivity values in 50% CH3OH-DMF at 25 ℃, indicating that they are non-electrolytes.
2.2Description of crystal structureSingle-crystal XRD analysis showed that complex 1 crystallizes in the monoclinic space groupC2. The asymmetric unit consists of one VO(II) moiety, one 1,10-phenanthroline ligand, one amino acid Schiff base ligand and one guest MeOH molecule. The V(IV) ions are six-coordinated with a distorted octahedral geometry, by two N atoms from one phen ligand, one O atom from the VO(II) moiety, two O atoms and another N atom from the tridentate Schiff base ligand (Fig 2). Bond lengths are V(1)-N(1) 2.352(8) Å and V(1)-N(2) 2.140(7) Å, with the amine nitrogen involved in a bond length of V(1)-N(3) 2.061(7) Å, and two V-O distances involving the Schiff bases ofca. 2.0 Å. The V(1)=O(1) and V(2)=O(6) bond distance are 1.599(6) Å and 1.611(8) Å, which is typical for oxovanadium complexes (Table 2)[9].
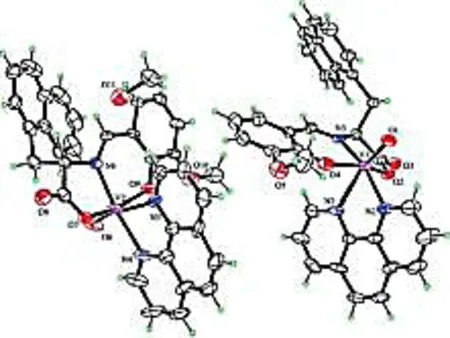
Fig 2 Molecular structure of the complexe 1 with thermal ellipsoids at 30% probability
Complex 2 crystallizes in the monoclinic space groupP-1. The asymmetric unit consists of one VO(II) moiety, one 1,10-phenanthroline ligand, one amino acid Schiff base ligand and one guest MeOH molecule. In the structure of 2, the V(IV) ions are six-coordinated with a distorted octahedral geometry, by two N atoms from one phen ligand, one O atom from the VO(II) moiety, two O atoms and another N atom from the tridentate Schiff base ligand (Fig 3). The structure contains bond lengths V(1)-N(1) 2.028(17) Å and V(1)-N(2) 2.151(17) Å, V(1)-N(3) 2.356(19) Å. The V(1)=O(4) bond distance is 1.590 7(17) Å, which also is typical for oxovanadium complexes (Table 2)[9].
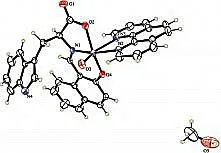
Fig 3 Molecular structure of the complexe 2 with thermal ellipsoids at 30% probability
Complex 3 crystallizes in the chiral space groupP21. The asymmetric unit consists of one VO(II) moiety, one 1,10-phenanthroline ligand and one Schiff base ligand. The V(IV) ions are six-coordinated with a distorted octahedral geometry, by two N atoms from one phen ligand, one O atom from the VO(II) moiety, two O atoms and another N atom from the tridentate Schiff base ligand (Fig 4). Bond lengths involving V(1) to the carboxylic oxygen atom O(2), imines nitrogen atom N(2) and the phenolate O(3) atom from the Schiff base ligand are 1.975(9),2.020(15) and 1.946(9) Å, respectively.
The bond distances V(1)-N(3) and V(1)-N(4) from 1,10-phenanthroline ligand are 2.403(11) Å and 2.142(13) Å, respectively. The V(1)=O(4), V(2)=O(8) and V(3)=O(13) bond distance are 1.598(11), Å 1.598(10) Å and 1.599(10) Å, which is typical for oxovanadium complexes, respectively (Table 2)[9].
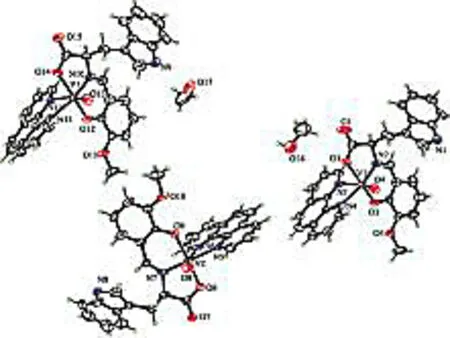
Fig 4 Molecular structure of the complexe 3 with thermal ellipsoids at 30% probability
Tab 1Pertinent crystal data and structure refinement results for complexes 1,2 and 3a
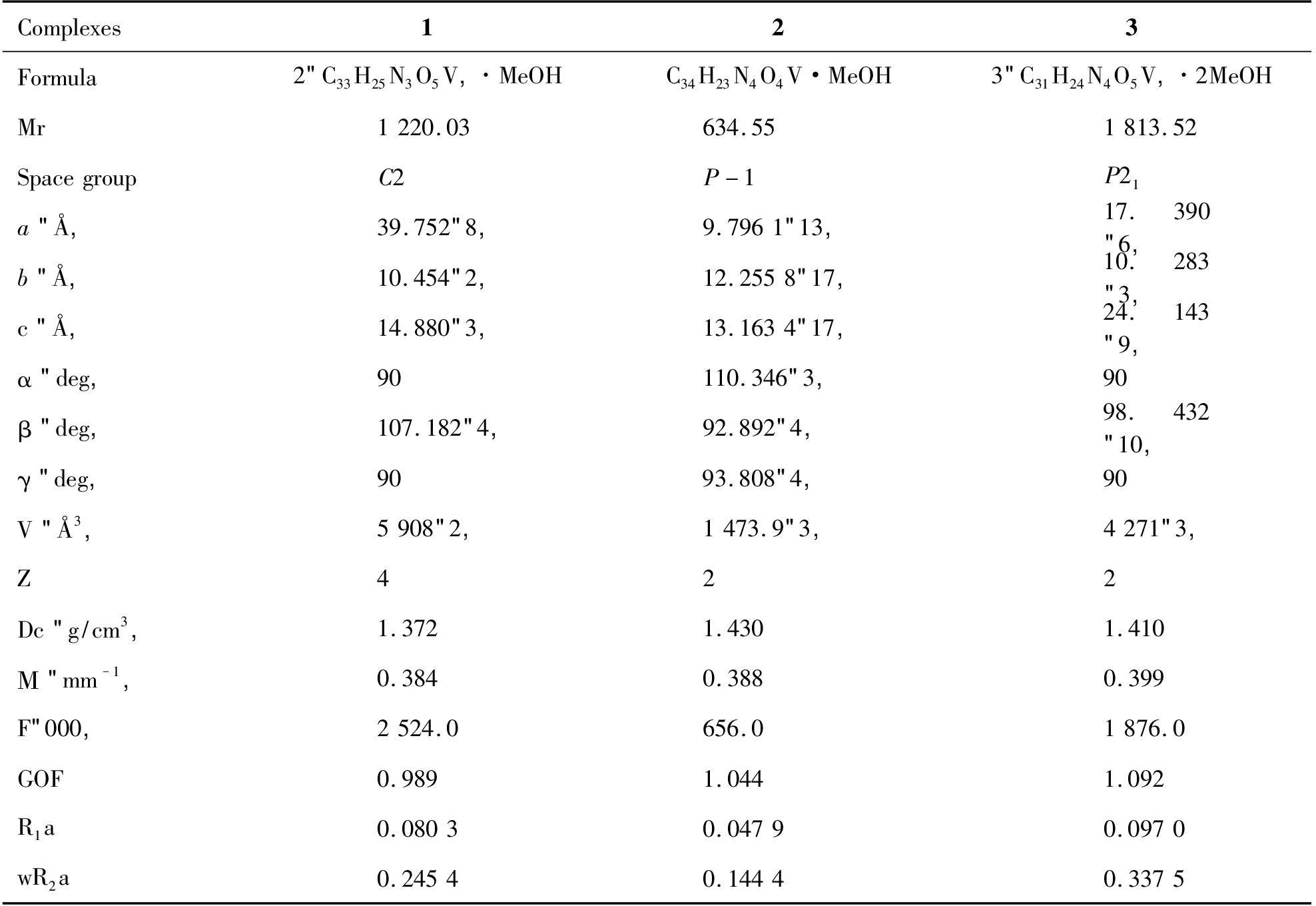
ThebonddistancesV(1)-N(3)andV(1)-N(4)from1,10-phenanthrolineligandare2.403(11)Åand2.142(13)Å,respectively.TheV(1)=O(4),V(2)=O(8)andV(3)=O(13)bonddistanceare1.598(11),Å1.598(10)Åand1.599(10)Å,whichistypicalforoxovanadiumcomplexes,respec-tively(Table2)[9].Complexes123Formula2(C33H25N3O5V)·MeOHC34H23N4O4V·MeOH3(C31H24N4O5V)·2MeOHMr1220.03634.551813.52SpacegroupC2P-1P21a(Å)39.752(8)9.7961(13)17.390(6)b(Å)10.454(2)12.2558(17)10.283(3)c(Å)14.880(3)13.1634(17)24.143(9)α(deg)90110.346(3)90β(deg)107.182(4)92.892(4)98.432(10)γ(deg)9093.808(4)90V(Å3)5908(2)1473.9(3)4271(3)Z422Dc(g/cm3)1.3721.4301.410Μ(mm-1)0.3840.3880.399F(000)2524.0656.01876.0GOF0.9891.0441.092R1a0.08030.04790.0970wR2a0.24540.14440.3375遵义医学院学报39卷
aR1= ∑ (||Fo| - |Fc||)/∑ |Fo|,wR= {∑w[(Fo2- Fc2)2]/∑w[(Fo2)2]}1/2.
Tab 2Selected bond lengths (Å) and bond angles (°) for complexes 1, 2 and 3 continues
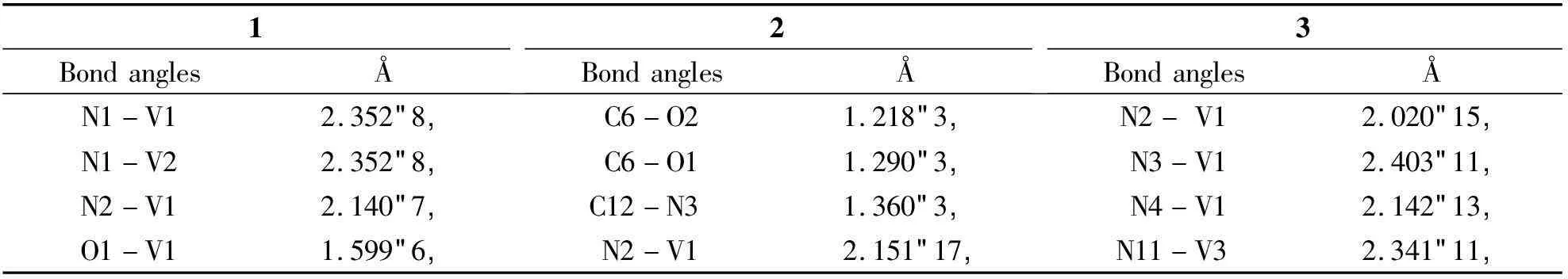
1BondanglesÅ2BondanglesÅ3BondanglesÅN1-V12.352(8)C6-O21.218(3)N2-V12.020(15)N1-V22.352(8)C6-O11.290(3)N3-V12.403(11)N2-V12.140(7)C12-N31.360(3)N4-V12.142(13)O1-V11.599(6)N2-V12.151(17)N11-V32.341(11)
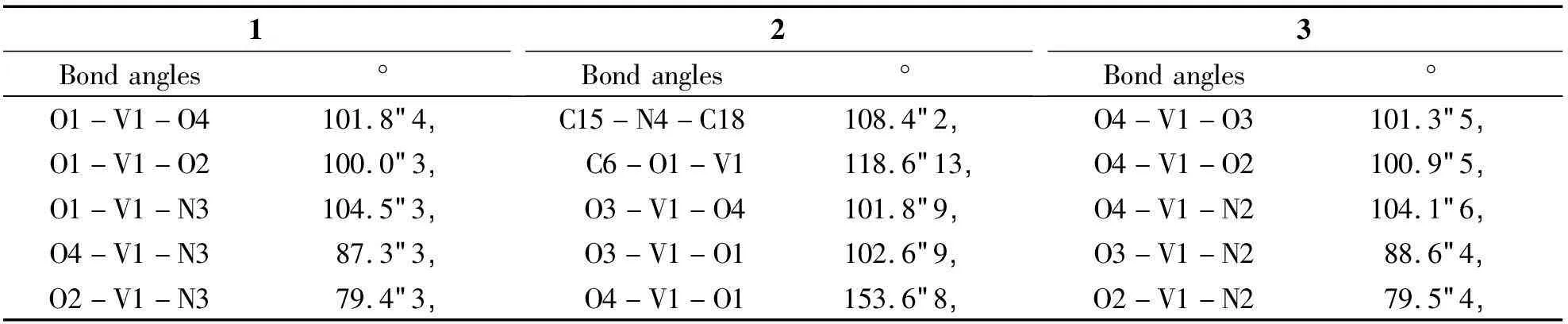
1Bondangles°2Bondangles°3Bondangles°O1-V1-O4101.8(4)C15-N4-C18108.4(2)O4-V1-O3101.3(5)O1-V1-O2100.0(3)C6-O1-V1118.6(13)O4-V1-O2100.9(5)O1-V1-N3104.5(3)O3-V1-O4101.8(9)O4-V1-N2104.1(6)O4-V1-N387.3(3)O3-V1-O1102.6(9)O3-V1-N288.6(4)O2-V1-N379.4(3)O4-V1-O1153.6(8)O2-V1-N279.5(4)plexesweredeterminedusingXRDtoshowthattheV(IV)atomsinallthreecomplexesaresix-coordina-tedinadistortedoctahedralenvironment.tivity[J].Polyhedron,2015,93:99-105.[7]BalajiB,SomyajitK,BanikB,etal.PhotoactivatedDNAcleavageandanti-canceractivityofoxovanadium(IV)complexesofcurcumin[J].InorgChimActa,·721·
2.3Invitroanticancer activitiesThe anticancer properties of 1-3 and ofcis-Pt against A-549 and HeGp2 were tested by the MTT assay[9]. The cytotoxicity was expressed as the concentration of the complex inhibiting cell growth by 50% (Table 3). Results showed that complexes 2 and 3 exhibited certain antitumor activities.The IC50values of the synthetic test complexes 2 and 3 are 58.88 and 53.08 μmol/L to A549, 83.95 and 44.74 μmol/L to HepG2, respectively.
Tab 3In vitro antitumor activities of complexes 1,2 and 3

ComplexesIC50(μmol/L)A549HeGP21>100>100258.8883.95353.0844.74cis-Pt2.991.66
3Discussion
Complexes 1-3 have been prepared in high yield from a one-pot synthetic procedure in which vanadyl sulfate is reacted with dianionic α-amino acid Schiff base ligands and 1,10-phenanthroline in aqueous methanol. And their stuctures have been characterized by H RMS(ESI-MS), IR spectra and molar conductance.The crystal structures of the complexes were determined using XRD to show that the V(IV) atoms in all three complexes are six-coordinated in a distorted octahedral environment.
Frominvitroexperiments carried out, results show that 2 and 3 have moderate anticancer activity towards A549 cells (human lung carcinoma cell line) and HepG2 cells (human hepatoma cell line).
[References]
[1] Dong J F, Jing B Q, Li L Z. Synthesis and crystal structure of an oxovanadium(IV) complex containingL-phenylalanine Schiff base and 1,10-phenanthroline[J]. Advanc Mater Research, 2014, 1033-1034: 588-591.
[2] Sinha A, Banerjee K, Banerjee A, et al. Synthesis, characterization and biological evaluation of a novel vanadium complex as a possible anticancer agent[J]. J Organomet Chem, 2014, 772-773: 34-41.
[3] Wu Q, Yuan Z L, Xu Y F, et al. Synthesis of new bis-β-diketonato complexes of titanium(IV) containing S-heterocyclic [J]. Journal of Zunyi Medical University,2010, 33(5): 426-430.
[4] Ku W J, Suzuki T, SugiuraY, et al. Effective DNA cleavage by bleomycin-vanadium(IV) complex plus hydrogen-peroxide[J]. Biochem Biophys Res Commun,1985, 129(2): 368-374.
[5] Bishayee A, Waghray A, Patel M A, et al. Synthesis and characterization of some oxovana -dium complexes[J]. Cancer Lett, 2010, 294(19): 1-12.
[6] Ebrahimipour S Y, Sheikhshoaie I, Kautz A C, et al. Mono- and dioxido-vanadium(V) complexes of a tridentate ONO Schiff base ligand: Synthesis, spectral characterization, X-ray crystal structure, and anticancer activity[J]. Polyhedron, 2015, 93: 99-105.
[7] Balaji B, Somyajit K, Banik B, et al. Photoactivated DNA cleavage and anti-cancer activity of oxovanadium(IV) complexes of curcumin[J]. Inorg Chim Acta, 2013, 400(5): 142-150.
[8] Sasmal K P, Patra K A, Nethaji M, et al. DNA cleavage by new Oxovanadium(IV) complexes of N-salicylidener-amino acids and phenanthroline bases in the photo-dynamic therapy window[J]. Inorg Chem, 2007, 46(26):11112-11121.
[9] Cao Y P, Yi C L, Liu H M, et al. Synthesisand anticancer activitiesof novel oxovanadium (Ⅳ) complexes[VO(dtbsal-met) (phen) and VO(naph-met) (phen)] [J]. Chinese Journal of Synthetic Chemistry, 2015, 23(6): 1124-1129.
[10] Prasad P, K. Sasmal P, Majumdar R, et al. Photocytotoxicity and near-IR light DNA cleavage activity of oxovanadium(IV) Schiff base complexes having phenanthroline bases[J]. Inorg Chim Acta, 2010, 363(12):2743-2751.
[11] Patra A K, Bhowmick T, Ramakumar S, et al. DNA cleavage in red light promoted by copper(II) complexes of α-amino acids and photoactive phenanthroline bases[J]. Dalton Trans, 2009, 48(48):6966-6976.
[12] Yuan Z L, Wu Q, Yang X B, et al. Synthesis and antibacterial activity of novel Schiff base macrocyclic compounds cotaining triazole[J]. Chin J Org Chem, 2011, 31(10): 1698-1702.
[13] Yuan Z L, Yu G Q, Luo Y L, et al. Efficient synthesis of involving nitrogen-oxygen donor macrocyclic compounds by microwave-assisted witting reaction[J]. Journal of Zunyi Medical University, 2014, 37(4): 366-368.
[14] Sasmal P K, Ritankar M, Dighe R R, et al. Photocytotoxicity and DNA cleavage activity of L-arg and L-lys Schiff base oxovanadium(IV) complexes having phenanthroline bases[J]. Dalton Trans, 2010, 39(30), 7104-7113.
[15] Yuan Z L, Shen X M, Huang J D. Syntheses, crystal structures and antimicrobialactivities of Cu(II), Ru(II), and Pt(II) compounds with an anthracene-containing tripodal ligand[J]. Rsc Advances, 2015, 5(14): 10521-10528.
[16] Yuan Z L, Wang L, Shen X M, et al. Copper(II) and platinum(II) compounds with pyrene-appended dipicolylamine ligand: syntheses, crystal structures and biological evaluation[J]. J Incl Phenom Macrocycl Chem, 2015, 82(1-2): 135-143.
[17] Cao Y P, Yi C L, Liu H M, et al. Synthesis, and interaction with DNA of a new oxovanadium (IV) complex containingL-Methionine Schiff base and 1,10-phenanthroline[J]. Journal of Zunyi Medical University, 2015, 38(6): 584-590.
[收稿2016-01-03;修回2016-03-15]
(编辑:王静)
Study on the synthesis, crystal structure andinvitroanticancer activities of three novel oxovanadium (IV) complexes with ligands of amino acid schiff base and 1,10-phenanthroline
CaoYaping,YiCenlan,LiuHongmei,LiHaixia,ZuoJianli,YuanZeli
(School of Pharmacy, Zunyi Medical University, Zunyi Guizhou 563099, China)
[Abstract]Objective To discover the novel bioactivities of non-platinum metallic prodrug.Methods Three novel new oxovanadium (IV) complex, [VO(o-van-Naph-L-Ana)(Phen)] (1) (o-van-Naph -L-Ana = Schiff base derived from o-vanillin and 3-(1-Naphthyl)-L-alanine, phen = 1,10-phenanthroline), [VO(Hynaph-L-Tyr)(Phen)] (2) (Hynaph-L-Tyr = Schiff base derived from 2-hydroxy-1-naphthaldehyde and L-tryptophan, phen = 1,10-phenanthroline) and [VO o-van -L-Trp)(Phen)] (3) (o-van-L-Trp = Schiff base derived from o-vanillin and L-tryptophan, phen = 1,10-phen -anthroline), were synthesized by one-pot, and characterized by H RMS, FT-IR spectra, molar conductance and single-crystal XRD. The in vitro anticancer activities of 1, 2 and 3 against A-549 and HeGp2 were tested by MTT assay.Results Three new amino acid Schiff base oxovanadium (IV) complexes 1-3 were synthesized. These results suggested that the complexes 2 and 3 exhibited certain antitumor activities. The IC50values of the synthetic test complexes 2 and 3 were 58.88 and 53.08 μmol/l in A549, 83.95 and 44.74 μmol/l in HepG2, respectively.Conclusion An one-pot synthetic protocol with mild reaction conditions and convenient purification was developed. The complexes 2 and 3 have moderate anticancer activities towards A549 cells (human lung carcinoma cell line) and HepG2 cells (human hepatoma cell line).
[Key words]amino acid; schiff base; oxovanadium (IV) complex; crystal structure; anticancer activity
[中图法分类号]R914.5
[文献标志码]A
[文章编号]1000-2715(2016)02-0122-07
[通信作者]袁泽利,男,博士,教授,硕士生导师,研究方向:药物设计合成及性能研究,E-mail:zlyuan2002@126.com。
[基金项目]国家自然科学基金资助项目(NO:81360471);贵州省国际合作项目(NO:[2012]7036);贵州省科技创新人才团队项目(NO:2014GZ 71255);国家大学生创新项目、贵州省大学生创新项目(NO:201510661007);遵义医学院大学生创新项目(NO:[2014]5809)。

