The biomimetic catalytic synthesis of acetal compounds using β-cyclodextrin as catalyst☆
Daohong Xia,Shengjuan Jiang,Lantao Li,Yuzhi Xiang,Lijun Zhu
State Key Laboratory of Heavy Oil Processing,College of Chemical Engineering,China University of Petroleum,Qingdao 266580,China
1.Introduction
Acetal compounds can be widely used as flavor and fragrance with a great market potential in food area[1].Moreover,acetals are commonly utilized as the intermediates or final products in fine chemical processes.The acetalation reaction is commonly utilized to protect the carbonyl group in multistep organic synthesis reactions,and the cyclic acetal initiated by radical or light can react with olefin to prepare ester compounds.Conventionally,catalysts in the acetalation reactions are mainly divided into two categories[2-7],one is the protonic acid catalyst(e.g.sulfuric acid,phosphoric acid,citric acid,oxalate,sulfamic acid),and the other is the nonproton solid acid which mainly includes heteropoly acid,niobic acid,FeCl3·6H2O,etc.How ever,these traditional acid catalysts led to the corrosion of equipments,which is uneconomical and not eco-friendly[2,8].In addition,the catalysts cannot be recycled.The increasing attention to the environmental issues has impelled the demand of renew able energy sources.
β-Cyclodextrin(β-CD)has been widely introduced into the biochemical industry[9,10].The particular hollow truncated conical structures of β-CD contribute to its aqueous solubility and the ability to form host-guest inclusion complex with hydrophobic guests under mild conditions[11].The inclusion process is very similar to the mechanism of the enzymatic catalytic reaction in organisms.Thus,β-CD is receiving much attention in the field of biomimetic catalysis which is an environmentally friendly approach mimicking the catalytic microenvironment in natural enzymes with a facile and an efficient catalyst.
Studies have shown that β-CD exhibits high catalytic performance for the reactions of allylation of carbonyl[12,13],synthesis of carbonyl compounds and α-tosylamino ketones[14,15],oxidation of sulfides and alcohols[16,17],and synthesis of thiazoles and aminothiazoles[18,19].However,there are few reportson the a cetalation reactionscatalyzed by β-CD.Compared with acid catalysts,β-CD not only avoids the rigor reaction conditionsand environmental contamination,but also increases the product yields.
In this study,β-CD will be employed to synthesize the acetals under mild conditions,and the plausible catalytic mechanism will be also explored.
2.Experimental
2.1.Equipments and materials
Infrared Spectroscopy(IR)was analyzed by a NEXUS™FT-IR spectrometer.1H NMR spectra were recorded on an Agilent VNMRS 500 MHz spectrometer using TMS as internal reference.Elemental analysis was performed by a Vario ELIIICHNOS instrument.All reagents and solvents were of reagent grade quality obtained from State Chemicals Corp.
2.2.Reaction and synthesis
In this study,β-CD was employed in the synthesis of a series of acetals including benzaldehyde glycol acetal,benzaldehyde-1,2-propylene glycol acetal,benzaldehyde-1,3-propylene glycol acetal,hyacinthin glycol acetal,hyacinthin-1,2-propylene glycol acetal,n-butanal glycol acetal and n-butanal-1,2-propylene glycol acetal.The typical experiment procedure is given below by using the synthesis of benzaldehyde glycol acetal as an example.
2.2.1.Synthesis route
The reaction of be nzaldehyde and glycol is shown in Fig.1.Cyclohexane,as a water-carrying agent for removing water from the reaction system,was added in the reaction mixture to create an azeotrope of cyclohexane and water(the boiling point was 69.8°C,the water content was 8.5%),shifting the acetalization reversible reaction towards the desired direction.
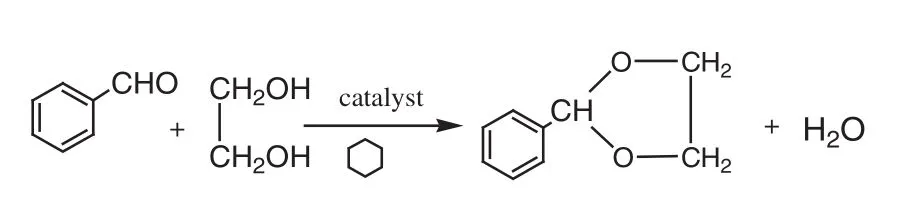
Fig.1.Synthesis route for benzaldehyde glycol acetal.
2.2.2.Procedure
The mixture of benzaldehyde(10.62 g)and cyclohexane(3 ml)was added drop-wise to the mixture of glycol and a small amount of β-CD while stirring.The reaction mixture was continually stirred to dissolve the β-cyclodextrin completely,heated and re fluxed for 2.5 h.Water generated in the process was separated and the organic phase was distilled to remove cyclohexane after the reaction was accomplished.The residue was purified under vacuum distillation.The fraction with a boiling point of 110-112°C at 2.0 k Pa was collected as the pure product.
3.Results and Discussion
3.1.Approaches to the acetal synthesis conditions
3.1.1.Effect of the usage of catalyst
The blank experiment without β-cyclodextrin did not yield any benzaldehyde glycol acetal.Once a certain amount of β cyclodextrin was added,it was found that benzaldehyde glycol acetal was produced,implying the catalytic effect of β-CD even at a very small dosage of 0.2 mmol catalyst·mol-1of benzaldehyde(Fig.2)was excellent.It is interesting to note that an optimal catalyst loading exists.At this optimal loading of 0.4 mmol catalyst·mol-1of benzaldehyde,as high as 81.3%yield of acetal was obtained.Further increase in the catalyst loading led to a decrease in acetal yield.Because the excessive catalyst was precipitated out and the precipitates made the reaction system become to the heterogeneous reaction involving the solid-liquid reaction,which led to the drop of the product yield.
3.1.2.Effect of the reaction time
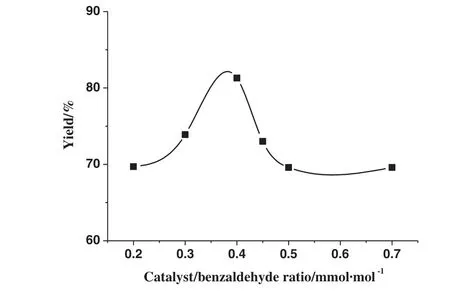
Fig.2.Effect of the molar ratio of catalyst to benzaldehydeon the yield of acetal.The molar ratio of glycol to benzaldehyde was 1.7,the volume of cyclohexane was 3 ml,and the reaction time was 2.5 h.
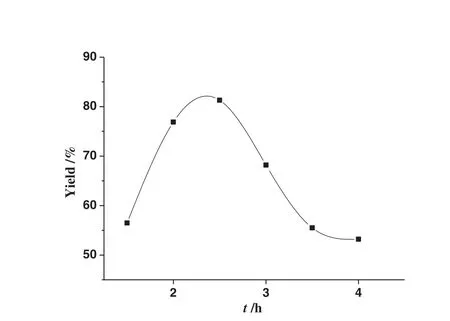
Fig.3.Effect of the reaction time on the yield of acetal.The molar ratio of glycol to benzaldehyde was 1.7,the volume of cyclohexane was 3 ml,and the catalyst loading was 0.4 mmol catalyst·mol-1 of benzaldehyde.
Fig.3 show s the yield of acetal as a function of reaction time.Similar to the effect of catalyst load ing,there is also an op timal reaction time.The yield of acetal significantly increased as the increase of reaction time initially and reached the maximum value of 81.3%at 2.5 h.Afterwards the yield decreased gradually accompanied with the color variety of the mixture from clear to brownish black.The color change implies that the coking of mixture occurred with prolonged heating time,which also led to the drop of the product yield.
3.1.3.Effect of the molar ratio of glycol to benzaldehyde
The level of dissolution of β-CD is closely related to the amount of glycol.As a result,the yield of acetal is expected to be sensitive to the changes in the molar ratio of glycol to benzaldehyde.Fig.4 show s the yield of acetal versus the molar ratio of glycol to benzaldehyde in the reaction mixture.The volume of glycol was in excess of 100%in the experiment,which guaranteed that β-cyclodextrin would dissolve completely.As show n in Fig.4,there was a significant increase from 53.1%to 81.3%in the product yield in the molar ratio range of glycol to benzaldehyde between 1.5 and 1.7,and then the yield displayed a slight drop.As β-CD cannot be dissolved completely in glycol at a lower molar ratio of glycol to benzaldehyde,resulting in the low catalysis to the acetalation reaction.How ever,with a higher molar ratio of glycol to benzaldehyde,the yield decreased gradually ow ing to the excessive glycol which would bring out a portion of acetal w hen they were distilled under vacuum(the boiling points of glycol and acetal were very closely).
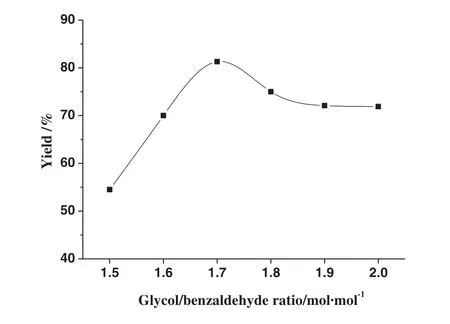
Fig.4.Effect of the molar ratio of glycol to benzaldehydeon the yield of acetal.The volume of cyclohexane was 3 ml;the catalyst loading was 0.4 mmol catalyst·mol-1 of benzaldehyde;and the reaction time was 2.5 h.
3.1.4.Effect of the volume of water-carrying agent
The yield of acetal as a function of the volume of water-carrying agent is show n in Fig.5.In this study,cyclohexane was used as the water-carrying agent to separate out the water formed in the acetalation reaction.In comparison with blank experiment without cyclohexane where the product yield was 38.5%,the yield was improved with more than 80%in the presence of cyclohexane.The maximum yield of acetal reached 81.3%w hen the volume of cyclohexane was 3 ml,while further increase in cyclohexane loading had not show an insignificant effect.

Fig.5.Effect of the volume of water-carryingagent on the yield of acetal.The catalyst loading was 0.4 mmol catalyst·mol-1 of benzaldehyde,the molar ratio of glycol to benzaldehyde was 1.7,and the reaction time was 2.5 h.
3.2.Characterization of product
The elemental analysis gives the atomic composition of C(%),71.44 and H(%),6.57,consistent with the formula of benzaldehyde glycol acetal(C9H10O2).
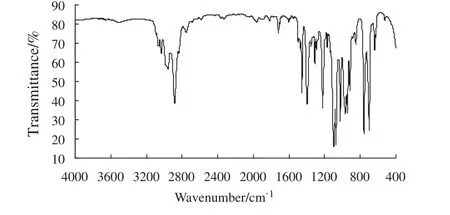
Fig.6.The IR spectra of benzaldehyde glycol acetal.
The IR spectra of benzaldehyde glycol acetal synthesized are show n in Fig.6.The characteristic absorption peak of carbonyl in benzaldehyde at 1706 cm-1and that of-OH in glycol at 3300 cm-1were not observed indicating that reactants were not found in the product.The IR spectra of benzaldehyde glycol acetal show ed that the characteristic bands at 1030 cm-1,1070 cm-1,1100 cm-1and 1220 cm-1might be attributed to the stretching frequency of the C-O group in acetal.The stretching frequency of the C═C group in the aromatic ring contributed to the absorption peak at 1400 cm-1and 1460 cm-1.The peaks at 1460 cm-1,2970 cm-1and 2890 cm-1resulted from the C-H vibration of methylene and the peaks at 760 cm-1,700 cm-1,3040 cm-1and 3070 cm-1resulted from the C-H vibration of the mono-substituted benzene ring in acetal.
The1H NMR spectra of benzaldehyde glycol acetal synthesized are depicted in Fig.7.Resonances belonging to aromatic protons were observed between 7.42 and 7.55 as multiples(m,5H).The peak of the methylene group attaching to the benzene ring at 5.95(s,1H)moved to a low field owing to the linking oxygen atoms.The protons of the methylene group were observed at 4.03(t,2H)and 4.13(t,2H)as two similar multiplets.
3.3.Experimental results for other acetals
The physical state,yield and characterization results for other acetals synthesized are listed in Table 1.
3.4.Possible mechanism for the catalytic synthesis of acetal
In the blank experiment,there was no benzaldehyde glycol acetal produced with the absence of β-CD.How ever,70% acetal yield was obtained even with just a little β-CD.β-CD played a significant role in the synthesis of acetal as a catalyst.Generally speaking,β-CD exhibited preferable catalytic activity for the reaction of aromatic aldehyde than the aliphatic aldehyde.Based on the above experimental data and reports in relevant literatures[9,20-22],a plausible reaction mechanism can be explored as show n in Fig.8.Firstly,benzaldehyde and β-CD were assumed to form the inclusion complex with the hydrophobic portion of benzaldehyde being included in the hydrophobic cavity of β-CD and the aldehyde group stretching out of β-CD.The interaction between the hydrogen of the-OH group in β-CD and oxygen of the C═O group in benzaldehyde contributed to the formation of the intermolecular hydrogen bond O-H…O which activated the C═O group of benzaldehyde molecule.Then,a large amount of benzaldehyde glycol acetal was produced,resulting from the nucleophilic attack of glycol on Cδ+in the C═O double bond.Finally,the catalyst returned to its initial state by releasing the acetal from the cavity of β-CD.
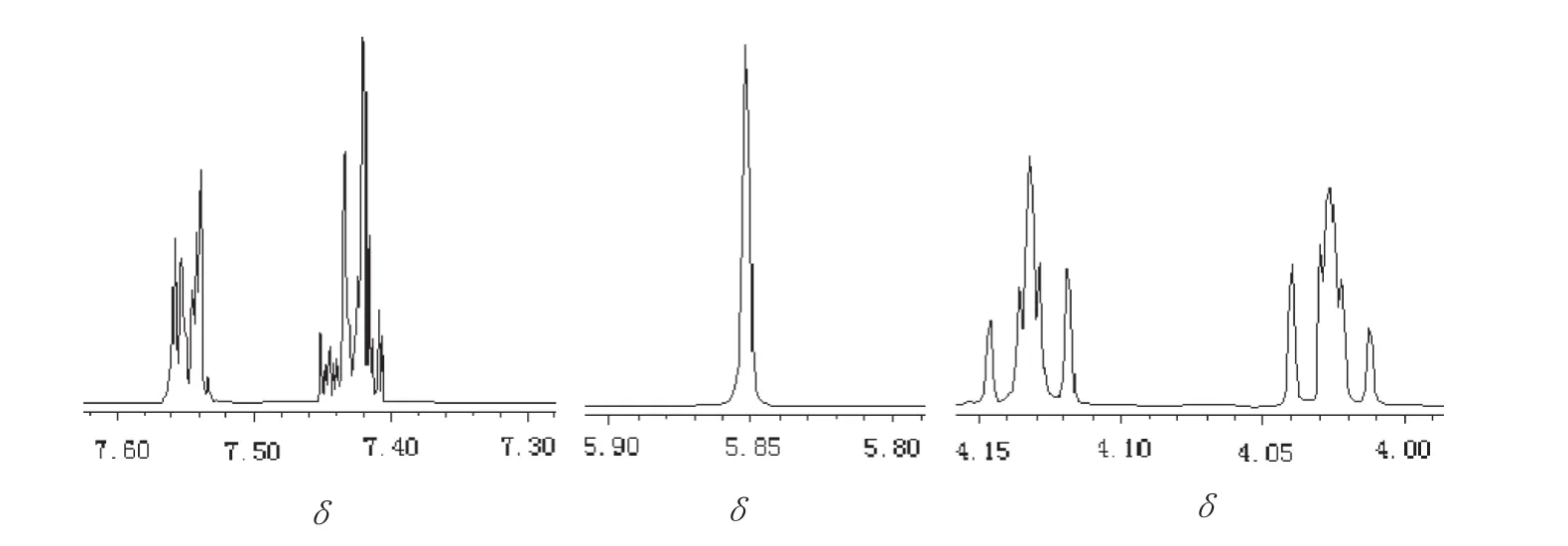
Fig.7.1H NMR spectra of benzaldehyde glycol acetal.

Table 1 Experimental results for other acetals synthesized
4.Conclusions
The biomimetic catalytic synthesis of acetals using β-cyclodextrin as the catalyst was found to be a possible option in the production of acetals.Under the optimum conditions with the catalyst loading of 0.4 mmol catalyst·mol-1of benzaldehyde,the molar ratio of 1.7 of glycol to benzaldehyde,the reaction time of 2.5 h and the cyclohexane volume of 3 ml,81.3%of benzaldehyde glycol acetal was yielded.The structures of acetals were con firmed by IR,1H NMRand elemental analysis.Six of other acetals were also synthesized.Aldehyde andβ-CDwere assumed to form the inclusion complex with the intermolecular hydrogen bond O-H…O,which promoted the acetalation reactions.
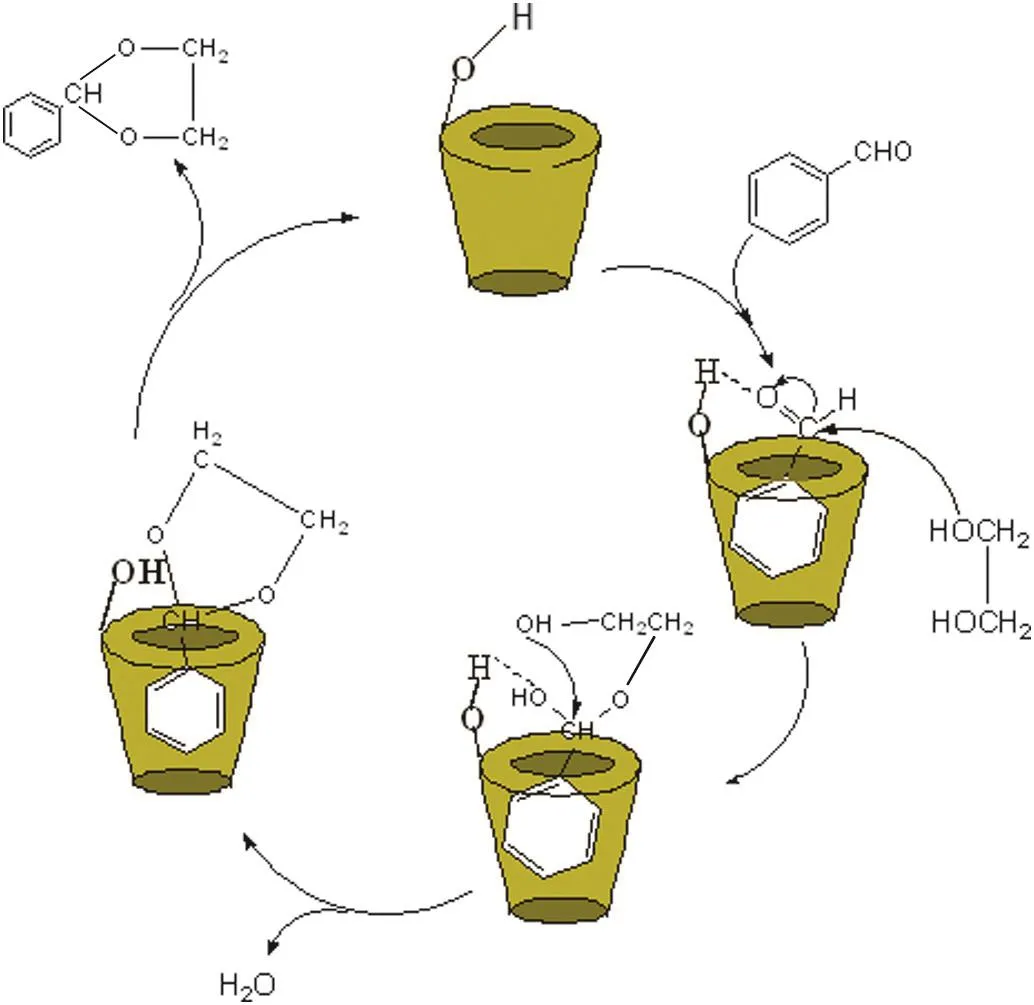
Fig.8.The possible mechanism for the synthesis of acetal catalyzed by β-CD.
[1]I.Agirre,M.B.Güemez,A.Ugarte,J.Requies,V.L.Barrio,J.F.Cambra,P.L.Arias,Kinetics of acetalization of perfumery aldehydes with alkanols over solid acid catalysts,Fuel Process.Technol.116(2013)182-188.
[2]F.Frusteri,L.Spadaro,C.Beatrice,C.Guido,Oxygenated additivesproduction for diesel engine emission improvement,Chem.Eng.J.134(2007)239-245.
[3]K.Stanisław,P.Mateusz,S.Krzyszt of,K.Ewelina,Transition metal compounds and complexes as catalysts in synthesis of acetals and orthoesters:theoretical,mechanistic and practical aspects,Coord.Chem.Rev.256(2012)2057-2095.
[4]M.R.Capeletti,L.Balzano,G.de la Puente,M.Laborde,U.Sedran,Synthesis of acetal(1,1-diethoxyethane)from ethanol and acetaldehyde over acidic catalysts,Appl.Catal.A Gen.198(2000)1-4.
[5]B.Wang,Y.Gu,G.Song,T.Yang,L.Yang,J.Suo,Sulfamic acid as a cost-effective and recyclable catalyst for liquid Beckmann rearrangement,a green process to produce amides from ketoximes without waste,ChemInform 35(2004).
[6]Y.X.Cheng,Y.Y.Zhou,J.D.Zhang,X.D.Fei,M.A.Tengzhou,X.Z.Liang,Synthesis of a novel solid acid with both sulfonic and carbonyl acid groups and its catalytic activities in acetalization,Chin.J.Chem.Eng.22(2014)312-317.
[7]Y.Wang,X.Gong,Z.Wang,L.Dai,SO3H-functionalized ionic liquids as efficient and recyclable catalysts for the synthesis of pentaerythritol diacetals and diketals,J.Mol.Catal.A Chem.322(2010)7-16.
[8]M.R.Capeletti,L.Balzano,G.de la Puente,M.Laborde,U.Sedran,Synthesis of acetal(1,1-diethoxyethane)from ethanol and acetaldehyde over acidic catalysts,Appl.Catal.A Gen.198(2000)L1-L4.
[9]Z.J.Yang,H.Zeng,X.T.Zhou,H.B.Ji,Mechanism into selective oxidation of cinnamaldehyde using β-cyclodextrin polymer as phase-transfer catalyst,Tetrahedron 68(2012)5912-5919.
[10]H.Y.Chen,H.B.Ji,β-Cyclodextrin promoted oxidation of cinnamaldehyde to natural benzaldehyde in water,Chin.J.Chem.Eng.19(2011)972-977.
[11]E.Schneiderman,A.M.Stalcup,Cyclodextrins:A versatile tool in separation science,J.Chromatogr.B 745(2000)83-102.
[12]N.Srilakshmi Krishnaveni,K.Surendra,V.Pavan Kumar,β-Cyclodextrin promoted allylation of aldehydes with allyltributyltin under supramolecular catalysis in water,Tetrahedron Lett.46(2005)4299-4301.
[13]K.Surendra,N.Srilakshmi Krishnaveni,K.Rama Rao,Direct Barbier-type allylation of aromatic acetals and dioxolanes in the presence of β-cyclodextrin in water,Tetrahedron Lett.47(2006)2133-2136.
[14]M.Somi Reddy,M.Narender,K.Rama Rao,A mild and efficient synthesis of α tosylamino ketones from aryl aziridines in the presence of β-cyclodextrin and NBS in water,Tetrahedron Lett.46(2005)1299-1301.
[15]M.Narender,M.Somi Reddy,V.Pavan Kumar,Y.V.D.Nagesw ar,K.Rama Rao,Direct synthesis of carbonyl compounds from THP ethers with IBX in the presence of βcyclodextrin in water,Tetrahedron Lett.46(2005)1971-1973.
[16]K.Surendra,N.S.Krishnaveni,V.Pavan Kumar,R.Sridhar,K.Rama Rao,Selective and efficient oxidation of sulfides to sulfoxides with N-bromosuccinimide in the presence of β-cyclodextrin in water,Tetrahedron Lett.46(2005)4581-4583.
[17]K.Surendra,N.S.Krishnaveni,M.A.Reddy,Y.V.Nagesw ar,K.R.Rao,Mild oxidation of alcohols with o-iodoxybenzoic acid(IBX)in water/acetone mixture in the presence of β-cyclodextrin,J.Org.Chem.68(2003)2058-2059.
[18]H.B.Ji,D.P.Shi,M.Shao,Z.Li,L.F.Wang,Transition metal-free and substrateselective oxidation of alcohols using water as an only solvent in the presence of β-cyclodextrin,Tetrahedron Lett.46(2005)2517-2520.
[19]M.Narender,M.Somi Reddy,R.Sridhar,Y.V.D.Nageswar,K.Rama Rao,Aqueous phase synthesis of thiazoles and aminothiazoles in the presence of β-cyclodextrin,Tetrahedron Lett.46(2005)5953-5955.
[20]H.Y.Chen,H.B.Ji,X.T.Zhou,L.F.Wang,Green synthesis of natural benzaldehyde from cinnamon oil catalyzed by hydroxypropyl-β-cyclodextrin,Tetrahedron 66(2010)9888-9893.
[21]N.Srilakshmi Krishnaveni,K.Surendra,M.Arjun Reddy,Y.V.D.Nageswar,K.Rama Rao,Highly efficient deprotection of aromatic acetals under neutral conditions using β-cyclodextrin in water,J.Org.Chem.68(2003)2018-2019.
[22]H.G.Jiang,Z.J.Yang,X.T.Zhou,Y.X.Fang,H.B.Ji,Immobilization of β-cyclodextrin as insoluble β-cyclodextrin polymer and its catalytic performance,Chin.J.Chem.Eng.20(2012)784-792.
 Chinese Journal of Chemical Engineering2016年1期
Chinese Journal of Chemical Engineering2016年1期
- Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- Process engineering in electrochemical energy devices innovation☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
