Multi-functional forward osmosis draw solutes for seawater desalination☆
Dieling Zhao,Shucheng Chen,Chun Xian Guo,Qipeng Zhao,Xianmao Lu
Department of Chemical&Biomolecular Engineering,National University of Singapore,Singapore 117585,Singapore
1.Introduction
In recent years,fresh water scarcity has become one of the most severe global problems due to the rising population,industrialization,water contamination and climate change[1,2].Therefore,it is of great importance to find other water sources to meet the demand of fresh water.Desalination and water reuse are found to bepromising solutions[3].Therefore,tremendous research effort has been devoted to the development of technologiesto claim fresh water from seawater or wastewater[4-9].Currently,reverse osmosis(RO)has been the most popular method due to its robustness and effectiveness.RO proceeds by applying an external hydraulic pressure to saline water to drive fresh water through a semi-permeable membrane.But the use of a high hydraulic pressure makes such a process quite energy-intensive and the cost is still high for long-term mass water production.
To improve the energy efficiency in desalination and wastewater treatment,forward osmosis(FO)has been given increasing attention in recent years[10,11].It is a naturally occurring process by using a draw agent with sufficiently high osmotic pressure to draw water from saline water,wastewater,or other feed solutions.After the FO process,the draw solute is usually regenerated for subsequent FO cycles.The selection of a suitable draw solute is highly important for an efficient FO process.An ideal draw solute has to meet the following three essential criteria:1)it is able to generate a high osmotic pressure;2)it has considerably low reverse solute flux to reduce replenishment cost;3)and it can be easily and efficiently regenerated with minimized energy consumption.Over the past years,a variety of compounds have been investigated as draw solutes in the FO process,including volatile compounds(e.g.NH3&CO2,SO2)[12-16],inorganic salts(e.g.fertilizer compounds)[17,18],nutrients(e.g.sucrose&fructose)[19-21],organic salts(e.g.polyelectrolyte)[22,23],synthetic nanomaterials(e.g.magnetic nanoparticles)[24,25],and other compounds(Table 1)[26].These draw solutes have been regenerated by hydraulic pres sure assisted process such as nano filtration and ultra filtration,heat-assisted process such as membrane distillation,or the assistance with an external magnetic field.
Despite the repaid progress in the development of FO draws solutes recently,existing draw solutes still have disadvantages such as low osmotic pressure,high reverse solute flux,or complex and energy-intensive regeneration methods.Therefore,it is necessary to explore novel FO draw solutes with high osmotic pressure,minimum reverse solute flux,and facile and energy-efficient regeneration method.Recently,w e investigated a few multi-functional FO draw solutes based on nanoparticles and/or stimuli-responsive compounds.We found that Na+-functionalized carbon quantum dots[27],hydrophilic magnetic nanoparticles functionalized with sodium citrate[28],thermoresponsive copolymer[29],and thermoresponsive magnetic nanoparticles[30]are promising candidates as FO draw solutes.Light responsive nanoparticles[31]and CO2responsive magnetic nanoparticle[32]are also studied as potential FO draw solutes which require further modification to increase the osmotic pressure.

Table 1 Some recently-developed FO draw solutes(DS:draw solution,FS:feed solution,LMH:L·m-2·h-1)
2.Multi-functional Draw Solutes
2.1.Na+-functionalized carbon quantum dots(Na_CQDs)
As a new ly discovered class of nanostructured carbons,carbonaceous quantum dots(CQDs)with size less than 10 nm have attracted increasing attention[33,34].Compared with traditional semiconductor quantum dots,CQDs show advantages of high chemical inertness,bio-compatibility and hydrophilicity[35].In addition,they can be produced inexpensively on large scale based on synthetic approaches such as oxidation of graphite or hydrothermal treatment of biomass[36,37].To date,CQDs have been investigated in various applications including bioimaging,energy conversion/storage,drug delivery and sensing[38,39].We investigated the use of Na+-functionalized carbon quantum dots(Na_CQDs)as a new type of high-performance FO draw solute[27].
Na_CQDs were fabricated as show n in Fig.1(a).Firstly,w e heated citric acid powder in air at 180°C to produce CQDs passivated with carboxyl groups.Then we dispersed the CQDs in water and adjusted the solution p H to 7.0 with NaOH,giving Na+-functionalized CQDs.The Na_CQDs have an average size of 3.5 nm(Fig.1(b)).There exist abundant Na element as w ell as-C═O and-C-O groups for Na_CQDs.Due to the presence of rich ionic species,Na_CQDs disperse w ell in water(0.5 g·ml-1,Fig.1(c))and exhibit high osmolality.At concentrations of 0.4 and 0.5 g·ml-1,the osmolalities reached as high as 3140 and 4350 mOsm·kg-1,corresponding to osmotic pressures of 30.9 and 53.6 atm(1 atm=101325 Pa),respectively.These osmotic pressures are much higher than seawater(~26 atm).When employed as FO draw solution with DI water as the model feed solution,0.4 g·ml-1Na_CQDs solution offered a high FO water flux of 29.8 L·m-2·h-1(LMH)with little change in multiple cycles.This FO water flux exceeds that of previously reported draw solutes such as sugars[11],ethanol[40],hydroacid complexes[26],polymer hydrogels [41],polyelectrolytes [22],and polyelectrolyte-functionalized magnetic nanoparticles[30],and it is among the highest water fluxes reported using the same commercial HTI FO membrane[42].In addition,it show s negligible reverse solute fluxes(<0.05 g·m-2·h-1).Na_CQDs solution(0.4 g·ml-1)was further investigated for seawater desalination and attained high FO water fluxes of 10.4 and 9.6 LMH for the 1st and 5th cycles,respectively(Fig.1(d)).
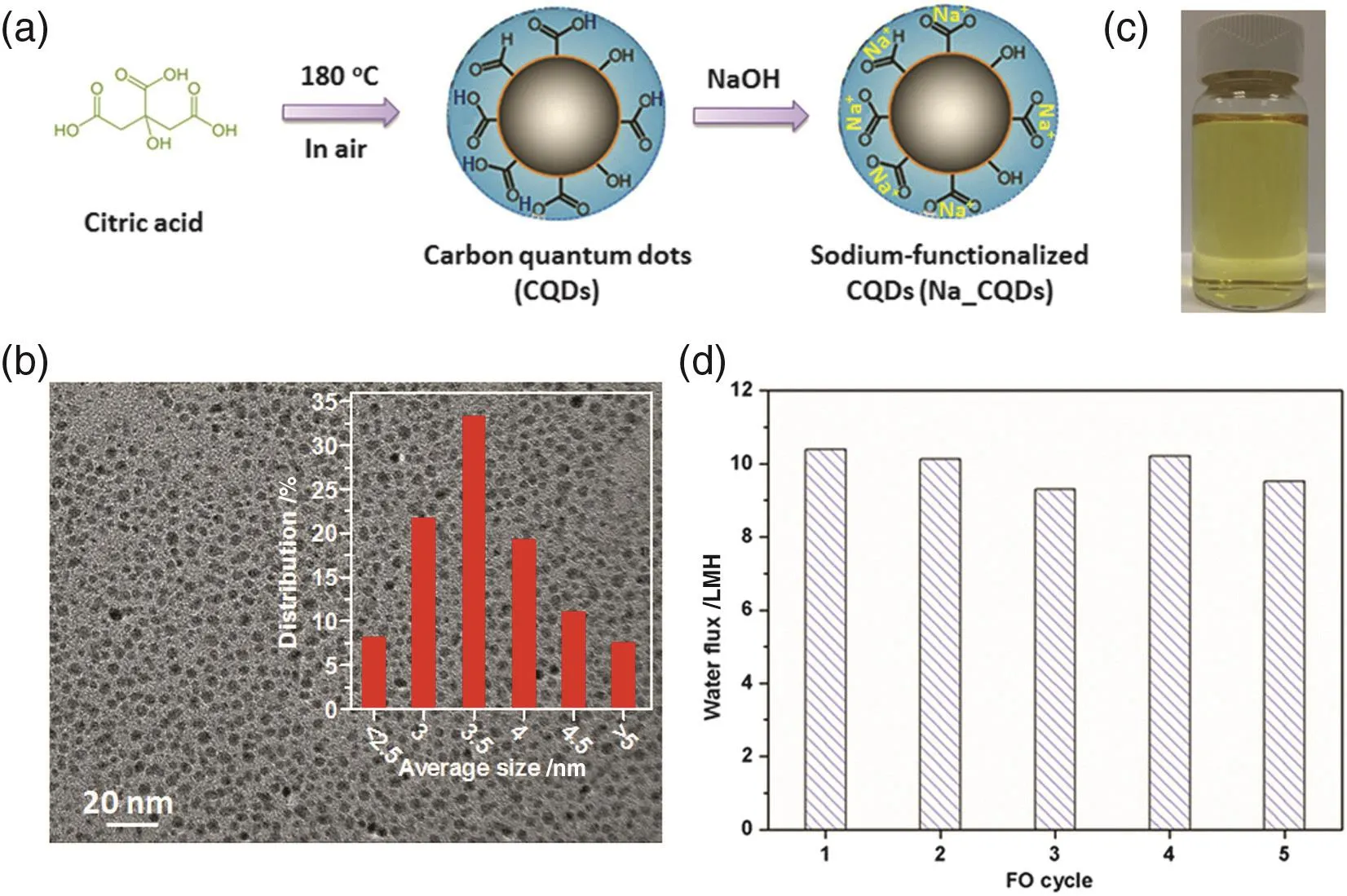
Fig.1.(a)Schematic illustration for the fabrication of Na_CQDs.(b)ATEM image of Na_CQDs.Inset is the size distribution.(c)A photo of Na_CQDs aqueous solution(0.5 g·ml-1).(d)FO water fluxes with 0.4 g·ml-1 Na_CQDs aqueous solution as the draw solution and seawater taken from Singapore coast as the feed solution.The seawater has an osmolality of 880 mOsm·kg-1.Figure reproduced with permission from[27].Copyright 2014 Royal Society of Chemistry.
2.2.Hydrophilic magnetic nanoparticles(MNPs)
In addition to carbon quantum dots,magnetic nanoparticles(MNPs)also represent an important class of draw solutes due to their unique properties.Small magnetic ferrite nanoparticles with size less than 10 nm are superparamagnetic/magnetoresistive and have been used for a wide range of applications such as biomedicine,catalysis and seawater desalination[43-47].Very recently,ferrite magnetic nanoparticles have been explored as magnetically separable draw solutes in FO for seawater desalination and protein enrichment[48,49].To achieve high water flux,nanoparticles with small size and high hydrophilicity are preferred because they have large specific surface area and can offer high osmolality.As a green technique for the fabrication of various nanomaterials,solventless thermolysis offers the advantage of simplicity and minimized use of organic solvents[50].This technique has been used to fabricate magnetite nanoparticles by thermal decomposition of ferrocene in the presence of polyvinyl pyrrolidone[51].How ever,the resultant nanoparticles have poor hydrophilicity and a relatively large size(>25 nm).We employed a one-step solventless thermolysis route to the fabrication of small and hydrophilic magnetic ferrite nanoparticles by simply heating a mixture of metal acetylacetonate and sodium citrate[28].
Wemixed iron(III)acetylacetonate[Fe(acac)3]and sodium citratein solid form via grinding or ball milling.Then,w e heated the mixture at 280°C under air to produce magnetic nanoparticles(shorten as MNPs_SC).Using this method,w e fabricated small magnetic nanoparticles with sizes down to 3.5 nm in large scale(up to~1 kg in one reaction)(Fig.2(a)).The MNPs_SC exhibit a superparamagnetic behavior at room temperature,and have a magnetization of around 10 emu·g-1at an applied field of 15 k Oe.Moreover,without any further ligand exchange,the MNPs_SC show high hydrophilicity.At a concentration of 0.3 g·ml-1,the MNPs_SC solution has a high osmolality of 2290 m Osm·kg-1,much higher than that of protein solutions(<100 m Osm·kg-1).We used MNPs_SC for protein enrichment with an osmotically driven process(Fig.2(b)).The rectangular channel has a size of 8.0 cm×1.0 cm×0.25 cm.Two types of proteins,hemoglobin(Hb)and albumin,were used.The protein concentrations were obtained based the corresponding absorbance intensities.For both proteins,their concentrations gradually increased with enrichment operation time(Fig.2(c)-(d)).The concentration increased from 0.12 to 0.36 g·L-1for Hb after 3.5 h;and from 0.25 to 0.71 g·L-1for albumin after 4 h,demonstrating the efficient protein enrichment.
2.3.Thermoresponsive copolymer

Fig.2.(a)A TEM image of MNPs_SC.Inset is a photo of the as-prepared MNPs_SC.(b)Setup for protein enrichment(co-current cross flow of protein solution and MNPs_SC solution).(c)Protein concentration vs.operation time for hemoglobin(Hb).Inset shows the photos of Hb solutions obtained at different operation times.(d)Protein concentration vs.operation time for albumin.The inset show s corresponding UV-vis spectra of the albumin solution.Figure reproduced with permission from[28].Copyright 2014 Royal Society of Chemistry.
Recently,draw solutes based on thermoresponsive compounds have been intensively studied due to their unique response to temperature[31,49,52].For instance,n-acylated polyethylenimine derivatives are soluble in water below their lower critical solution temperature(LCST),but phase separation occurs when the temperature is increased above the LCST[52].Lee et al.[52]demonstrated temperature-induced reversible water flux between the draw and feed solutions using this type of thermoresponsive draw solute.Stimuli-responsive polymer hydrogels have also been evaluated by Wang et al.[41,53]as FO draw solutes.How ever,it remains a challenge to employ thermoresponsive materials as draw solutes to desalinate seawater because of their relative low osmotic pressure.In our recent study,a thermoresponsive copolymer,poly(sodiumstyrene-4-sulfonate-co-n-isopropylacrylamide)(PSSS-PNIPAM),was used in FO for seawater desalination(Fig.3(a))[29].When PSSS-PNIPAM is dissolved in water to form a draw solution,PSSS generates a high osmotic pressure as astrongpolyelectrolyte.During FO,thepolymer chainscan befully expanded in the solution to provide maximum osmotic pressure.After FO,the polymer solution was re-concentrated by membrane distillation(MD)to produce clean water and to regenerate the draw solution(Fig.3(b)).Because MD was performed at a temperature(50°C)above the LCST of the copolymer,the osmotic pressure of the draw solution decreased significantly dueto theagglomeration of thepolymer chains.Lower osmotic pressure leads to a higher effective water vapor pressure in MD,facilitating the separation of water from the solution.
PSSS-PNIPAM copolymers with different SSS contents were synthesized with a designated feeding ratio(Fig.3(a)).Taking both the osmolality and LCST into consideration,PSSS-PNIPAM with 15 wt%of SSS(15SN)was finally selected as the draw solute in FO for seawater desalination.With simulated seawater(0.6 mol·L-1NaCl solution)as the feed solution,FO water fluxes as high as 3.5 LMH were achieved(Fig.3(c)).The measured reverse solute flux of 15SN in FO tests was 2 g·m-2·h-1(gMH),much lower than 90 gMH obtained with salt water as the draw solution.It was also observed that the osmotic pressure of PSSS-PNIPAM solution dropped significantly at temperatures above its LCST.This thermoresponsive property enhances the regeneration of the draw solution via MDbecause:i)thedecreased osmotic pressure allow s higher water vapor pressure in MD;and ii)PSSS-PNIPAM reduces salt leakage in MD.The MD water flux was 2.5 LMH(Fig.3(c)).
2.4.Thermoresponsive magnetic nanoparticles(MNPs)
Hydrophilic magnetic nanoparticles as emerging draw solutes have been used in the FO process[11,48].Ge and Ling have developed several super hydrophilic magnetic nanoparticles by functionalizing the nanoparticles with variousionic groups[24,25,54,55].The resultant magnetic nanoparticles show good performance as draw solutes in extracting water from feed solution.To further improve the FO performance,higher osmotic pressure generated by the draw solution is desired.Then nanoparticles with smaller size are preferred,since smaller size leads to higher specific area for functionalization.How ever,magnetic nanoparticles with smaller size are difficult to be captured using an external magnetic field.Thermoresponsive polymer PNIPAM has been employed to functionalize magnetic nanoparticles.When heated above its LCST(~32 °C),PNIPAM transits reversibly from hydrophilic to hydrophobic and aggregates in solution[56].Ling has investigated the performance of PNIPAM coated magnetic nanoparticles in FO.Reversible clustering of PNIPAM coated magnetic nanoparticles was observed by altering the temperature.However,the water flux is quite low(<2 LMH)w hen using DI water as the feed solution[49].
To increase the water flux in FO,we recently incorporated polyelectrolyte with thermoresponsive PNIPAM to functionalizemagnetic nanoparticles[30].Specifically,a copolymer,PSSS-PNIPAM,was used to graft onto MNPs via ligand exchange.Theresultant copolymer functionalized MNPs could be dispersed w ell in water and generate high osmolality(Fig.4(a)-(b)).For 5-nm MNPs,the osmolality can reach 2250 m Osm·kg-1at the concentration of 33 w t%.The FO and draw solute regeneration process is show n in Fig.4(c).To regenerate the draw solute,mild heating was applied to the diluted solution after the FO process.When heated above the LCST of the draw solution,14%of the nanoparticles were found to precipitate out.The precipitated nanoparticles were directly recycled back to the draw solution reservoir.The remaining nanoparticles in solution proceeded further to a high gradient magnetic separator(HGMS)for magnetic separation.Since magnetic capturing could not completely remove the nanoparticles,ultra filtration was employed after HGMS to generate product water,whereas the nanoparticles were recycled back.When using simulated seawater(osmolality:1200 m Osm·kg-1)as the feed solution,the FO water flux reached 2.7 LMH(Fig.4(d)).By repeating the FO and regeneration process for multiple cycles,water fluxes above 2 LMH were attained.

Fig.3.(a)Synthesis of PSSS-PNIPAM.(b)Schematic illustration of FO-MD system.(c)FO water fluxes with 15SN solution(33.3 wt%)as the draw solution and simulated sweater(0.6 mol·L-1 NaCl solution)as the feed solution;and MD water fluxes at 50 °C with the diluted 15SN solution as the feed solution.Figure reproduced with permission from[29].Copyright 2014 Elsevier.
2.5.Light-responsive magnetic nanoparticles(MNPs)
Most recently,more efforts have been focused on developing draw solutes that can be regenerated by renew able external stimuli,such as sunlight and CO2.As a renew able and low-cost energy source,sunlight is promisingasan external stimulus to control the dispersity of nanoparticles which have great potential as FO draw solutes.Recently,w e fabricated light-responsive heterodimers composed of a magnetic nanoparticle(Fe3O4)and a plasmonic nanoparticle(Ag)coated with a thermoresponsive polymer PNIPAM[31].Fe3O4nanoparticles were first synthesized in 1-octadecene with iron oleate and oleic acid as the iron source and surfactant,respectively.Then the magnetic nanoparticles were used as seeds to facilitate the grow th of silver nanoparticles to form heterodimers.During this process,PNIPAM was also added to functionalize the silver nanoparticles and provide thermo-sensitivity.When sunlight was directed to the heterodimers,Ag nanoparticles absorbed the energy from sunlight because of theplasmonic effect[8].According to theinterband absorption mechanism,most of the absorbed light energy would be transformed into heat and cause localized heating around the heterodimers.This heat was sufficient to increase the local temperature over the LCST of PNIPAM,leadingto the collapse of the polymer chains and in turn inducing the aggregation of the heterodimers.Once the solar source was removed,the temperature would be brought below the LCST of PNIPAM and the heterodimers could be re-dispersed well again in aqueous solution.A schematic illustration of this process is show n in Fig.5(a).
As seen in Fig.5(b),before solar illumination,the heterodimers had an average size of 10 nm.Under solar illumination,they aggregated and formed clusters with a diameter of around 100 nm(Fig.5(c)).The aggregation-redispersion of these dimers can be consistently achieved under repeated solar illumination cycles(Fig.5(d)).The successful design of such light-responsive heterodimers and their excellent aggregation-reversibility have implied that they are indeed suitable as potential FO draw agents.The formation of large aggregates under sunlight would greatly facilitate the magnetic separation process and reduce the energy consumption.How ever,these heterodimers were only coated with PNIPAM and the expected osmolality would be low.Improvement in osmolality may be achieved by functionalizing the heterodimers with more hydrophilic molecules so that sufficiently high osmotic pressure can be generated for FO application.

Fig.5.(a)Reversible clustering of PNIPAM-capped Ag-Fe3O4 heterodimers via solar illumination.(b)TEM image of dispersed Ag-Fe3O4 heterodimers before solar illumination.(c)TEM image of clustered heterodimers after solar illumination.(d)Average hydrodynamic size of Ag-Fe3O4 heterodimers under repeated solar illumination.Figure reproduced with permission from[31].Copyright 2013 Royal Society of Chemistry.
2.6.CO2-responsive magnetic nanoparticles(MNPs)

Fig.6.(a)Scheme of the reversible aggregation of 1,8-diaminooctane functionalized MNPs induced by purging CO2 or N2 resp ectively.(b)Average hyd rodynamic size of 1,8-diaminooctane functionalized MNPs under repeated CO2 or N2 purging.Figure reproduced with permission from[32].Copyright 2014 Wiley-VCH.
In addition to sunlight,CO2can be advantageous as a stimulus since it can undergo benign reactions with amidines or amines and it can be easily removed by mild heating or/and the charging of inert gas.Moreover,it is safe,cheap and abundant.We recently synthesized CO2-responsive magnetic nanoparticles through a one-pot polyol process using iron acetylacetonate and 1,8-diaminooctane as the iron precursor and surfactant respectively[32].For the two primary amine groups of 1,8-diaminooctane,one can bind to the nanoparticle surface and theother will faceout ward and act asastimulusresponder.Before purging CO2,the hydrophobic alkyl chains of the surfactants cause the nanoparticles to aggregate in the aqueous solution.After charging CO2,the free primary amine group can react with CO2to form alkylammonium ions,which provide good hydrophilicity for the nanoparticles to disperse in water.A schematic illustration of this reversible aggregation process is show n in Fig.6(a).The hydrodynamic sizes of the nanoparticles in repeated CO2/N2purging cycles were recorded and such nanoparticles demonstrated excellent switchability in terms of hydrophilicity with the assistance of gas purging.It is worth noting that after purging N2,the aggregates of the nanoparticles could be as large as 1000 nm and they could be easily separated by an external low-gradient magnetic field.Improvement in osmolality of such CO2-responsive MNPs is underway for further FO testing.
3.Conclusions
Our recent efforts in developing multi-functional draw solutes,including Na+-functionalized carbon quantum dots,hydrophilic magnetic nanoparticles functionalized with sodium citrate,thermoresponsive copolymer,and thermoresponsive magnetic nanoparticles,are summarized.These draw solutes can not only generate high FO water flux,but also reduce reverse solute flux.More importantly,the multifunctional draw solutes may be regenerated with the assistance of waste heat or renewable energy to save energy cost.In addition to seawater desalination,these draw solutes may find application in brackish water and wastewater treatment and protein enrichment.
Abbreviations
gMH unit of reverse solute flux,g·m-2·h-1
LMH unit of water flux,L·m-2·h-1
m Osm·kg-1unit of osmolality,milliosmoles per kilogram of water
[1]M.Elimelech,W.A.Phillip,The future of seawater desalination:Energy,technology,and the environment,Science 333(2011)712-717.
[2]M.A.Shannon,P.W.Bohn,M.Elimelech,J.G.Georgiadis,B.J.Marinas,A.M.Mayes,Science and technology for water purification in thecoming decades,Nature452(2008)301-310.
[3]R.L.McGinnis,M.Elimelech,Global challenges in energy and water supply:The promise of engineered osmosis,Environ.Sci.Technol.42(2008)8625-8629.
[4]G.Chen,Electrochemical technologies in wastewater treatment,Sep.Purif.Technol.38(2004)11-41.
[5]C.Fritzmann,J.Löw enberg,T.Wintgens,T.Melin,State-of-the-art of reverse osmosis desalination,Desalination 216(2007)1-76.
[6]P.R.Gogate,A.B.Pandit,A review of imperative technologies for wastewater treatment II:hybrid methods,Adv.Environ.Res.8(2004)553-597.
[7]A.D.Khawaji,I.K.Kutubkhanah,J.-M.Wie,Advances in seawater desalination technologies,Desalination 221(2008)47-69.
[8]C.Liu,J.Heo,Local heating from silver nanoparticles and its effect on the Er3+upconversion inoxy fluoride glasses,J.Am.Ceram.Soc.93(2010)3349-3353.
[9]T.Matsuura,Progress in membrane science and technology for seawater desalination—A review,Desalination 134(2001)47-54.
[10]T.Y.Cath,A.E.Childress,M.Elimelech,Forward osmosis:principles,applications,and recent developments,J.Membr.Sci.281(2006)70-87.
[11]T.S.Chung,S.Zhang,K.Y.Wang,J.Su,M.M.Ling,Forward osmosis processes:Yesterday,today and tomorrow,Desalination 287(2012)78-81.
[12]A.Achilli,T.Y.Cath,A.E.Childress,Selection of inorganic-based draw solutions for forward osmosis applications,J.Membr.Sci.364(2010)233-241.
[13]K.Gaw el,D.Barriet,M.Sletmoen,B.T.Stokke,Responsive hydrogels for label-free signal transduction w ithin biosensors,Sensors 10(2010)4381-4409.
[14]J.R.McCutcheon,R.L.McGinnis,M.Elimelech,A novel ammonia-carbon dioxide forward(direct)osmosis desalination process,Desalination 174(2005)1-11.
[15]J.R.Mc Cutcheon,R.L.McGinnis,M.Elimelech,Desalination by ammonia-carbon dioxide forward osmosis:influence of draw and feed solution concentrations on process performance,J.Membr.Sci.278(2006)114-123.
[16]R.L.McGinnis,M.Elimelech,Energy requirements of ammonia-carbon dioxide forward osmosis desalination,Desalination 207(2007)370-382.
[17]S.Phuntsho,H.Shon,S.Hong,S.Lee,S.Vigneswaran,J.Kandasamy,Fertiliser drawn forward osmosis desalination:the concept,performance and limitations for fertigation,Rev.Environ.Sci.Biotechnol.11(2012)147-168.
[18]S.Phuntsho,H.K.Shon,S.Hong,S.Lee,S.Vigneswaran,A novel low energy fertilizer driven forward osmosis desalination for direct fertigation:evaluating the performance of fertilizer draw solutions,J.Membr.Sci.375(2011)172-181.
[19]J.Yaeli,Method and apparatus for processing liquid solutions of suspensions particularly useful in the desalination of saline water,Google Pat.(1992).
[20]J.O.Kessler,C.D.Moody,Drinking water from sea water by forward osmosis,Desalination 18(1976)297-306.
[21]K.Stache,Apparatus for transforming sea water,brackish water,polluted water or the like into a nutritious drink by means of osmosis.Google Pat.(1989).
[22]Q.Ge,J.Su,G.L.Amy,T.-S.Chung,Exploration of polyelectrolytes as draw solutes in forward osmosis processes,Water Res.46(2012)1318-1326.
[23]Q.Ge,P.Wang,C.Wan,T.-S.Chung,Polyelectrolyte-promoted forward osmosismembrane distillation(FO-MD)hybrid process for dye wastewater treatment,Environ.Sci.Technol.46(2012)6236-6243.
[24]Q.Ge,J.Su,T.-S.Chung,G.Amy,Hydrophilic superparamagnetic nanoparticles:synthesis,characterization,and performance in forward osmosis processes,Ind.Eng.Chem.Res.50(2010)382-388.
[25]M.M.Ling,K.Y.Wang,T.-S.Chung,Highly water-soluble magnetic nanoparticles as novel draw solutes in forward osmosis for water reuse,Ind.Eng.Chem.Res.49(2010)5869-5876.
[26]Q.Ge,T.-S.Chung,Hydroacid complexes:A new class of draw solutes to promote forward osmosis(fo)processes,Chem.Commun.49(2013)8471-8473.
[27]C.X.Guo,D.Zhao,Q.Zhao,P.Wang,X.Lu,Na+-functionalized carbon quantum dots:a new draw solute in forward osmosis for seawater desalination,Chem.Commun.50(2014)7318-7321.
[28]C.X.Guo,S.Huang,X.Lu,Asolventlessthermolysisroute to large-scale production of ultra-small hydrophilic and biocompatible magnetic ferrite nanocrystals and their application for efficient protein enrichment,Green Chem.16(2014)2571-2579.
[29]D.Zhao,P.Wang,Q.Zhao,N.Chen,X.Lu,Thermoresponsive copolymer-based draw solution for seawater desalination in a combined process of forward osmosis and membrane distillation,Desalination 348(2014)26-32.
[30]Q.Zhao,N.Chen,D.Zhao,X.Lu,Thermoresponsive magnetic nanoparticles for seawater desalination,ACS Appl.Mater.Interfaces 5(2013)11453-11461.
[31]H.Han,J.Y.Lee,X.Lu,Thermoresponsive nanoparticles+plasmonic nanoparticles=photoresponsive heterodimers:Facile synthesis and sunlightinduced reversible clustering,Chem.Commun.49(2013)6122-6124.
[32]S.Chen,C.X.Guo,Q.Zhao,X.Lu,One-pot synthesisof CO2-responsive magnetic nanoparticles with switchable hydrophilicity,Chem.Eur.J.43(2014)14057-14062.
[33]S.N.Baker,G.A.Baker,Luminescent carbon nanodots:Emergent nanolights,Angew.Chem.Int.Ed.49(2010)6726-6744.
[34]L.Zhou,Y.Lin,Z.Huang,J.Ren,X.Qu,Carbon nanodots as fluorescence probes for rapid,sensitive,and label-free detection of Hg2+and biothiols in complex matrices,Chem.Commun.48(2012)1147-1149.
[35]S.Qu,X.Wang,Q.Lu,X.Liu,L.Wang,A biocompatible fluorescent ink based on water soluble luminescent carbon nanodots,Angew.Chem.Int.Ed.51(2012)12215-12218.
[36]C.X.Guo,J.Xie,B.Wang,X.Zheng,H.B.Yang,C.M.Li,A new class of fluorescent-dots:long luminescent lifetime bio-dots self-assembled from DNA at low temperatures,Sci.Rep.3(2013).
[37]L.Lin,S.Zhang,Creating high yield water soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes,Chem.Commun.48(2012)10177-10179.
[38]Y.Dong,H.Pang,H.B.Yang,C.Guo,J.Shao,Y.Chi,C.M.Li,T.Yu,Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitationindependent emission,Angew.Chem.Int.Ed.52(2013)7800-7804.
[39]C.X.Guo,Y.Dong,H.B.Yang,C.M.Li,Grap hene quantum dots as a gr eensensitizer to functionalize Zn O nanow ire arrays on F-doped Sn O2glass for enhanced photoelectrochemical water splitting,Adv.Energy Mater.3(2013)997-1003.
[40]P.McCormick,J.Pellegrino,F.Mantovani,G.Sarti,Water,salt,and ethanol diffusion through membranes for water recovery by forw ard(direct)osmosis processes,J.Membr.Sci.325(2008)467-478.
[41]D.Li,X.Zhang,J.Yao,G.P.Simon,H.Wang,Stimuli-responsive polymer hydrogels as a new class of draw agent for forw ard osmosis desalination,Chem.Commun.47(2011)1710-1712.
[42]K.Chon,J.Cho,H.K.Shon,Fouling characteristics of a membrane bioreactor and nano filtration hybrid system for municipal wastewater reclamation,Bioresour.Technol.130(2013)239-247.
[43]N.A.Frey,S.Peng,K.Cheng,S.Sun,Magnetic nanoparticles:synthesis,functionalization,and applications in bioimaging and magnetic energy storage,Chem.Soc.Rev.38(2009)2532-2542.
[44]R.Hao,R.Xing,Z.Xu,Y.Hou,S.Gao,S.Sun,Synthesis,functionalization,and biomedical applications of multifunctional magnetic nanoparticles,Adv.Mater.22(2010)2729-2742.
[45]Z.Li,S.X.Wang,Q.Sun,H.L.Zhao,H.Lei,M.B.Lan,Z.X.Cheng,X.L.Wang,S.X.Dou,G.Q.Lu,Ultrasmall manganese ferrite nanoparticles as positive contrast agent for magnetic resonance imaging,Adv.Healthc.Mater.2(2013)958-964.
[46]A.Pourjavadi,S.H.Hosseini,F.Matloubi Moghaddam,B.Koushki Foroushani,C.Bennett,Tungstate based poly(ionic liquid)entrapped magnetic nanoparticles:a robust oxidation catalyst,Green Chem.15(2013)2913-2919.
[47]S.Sun,H.Zeng,Size-controlled synthesis of magnetite nanoparticles,J.Am.Chem.Soc.124(2002)8204-8205.
[48]Q.Ge,M.Ling,T.-S.Chung,Draw solutions for forward osmosis processes:developments,challenges,and prospects for the future,J.Membr.Sci.442(2013)225-237.
[49]M.M.Ling,T.S.Chung,X.Lu,Facile synthesis of thermosensitive magnetic nanoparticles as “smart”draw solutes in forw ard osmosis,Chem.Commun.47(2011)10788-10790.
[50]T.H.Larsen,M.Sigman,A.Ghezelbash,R.C.Doty,B.A.Korgel,Solventless synthesis of copper sulfide nanorods by thermolysis of a single source thiolate-derived precursor,J.Am.Chem.Soc.125(2003)5638-5639.
[51]D.Amara,J.Grinblat,S.Margel,Solventless thermal decomposition of ferrocene as a new approach for one-step synthesis of magnetite nanocubes and nanospheres,J.Mater.Chem.22(2012)2188-2195.
[52]M.Noh,Y.Mok,S.Lee,H.Kim,S.H.Lee,G.W.Jin,J.H.Seo,H.Koo,T.H.Park,Y.Lee,Novel lower critical solution temperature phase transition materials effectively control osmosis by mild temperature changes,Chem.Commun.48(2012)3845-3847.
[53]A.Razmjou,G.P.Simon,H.Wang,Effect of particle size on the performance of forward osmosis desalination by stimuli-responsive polymer hydrogels as a draw agent,Chem.Eng.J.215-216(2013)913-920.
[54]M.M.Ling,T.S.Chung,Novel dual-stage FO system for sustainable protein enrichment using nanoparticles as intermediate draw solutes,J.Membr.Sci.372(2011)201-209.
[55]M.M.Ling,T.S.Chung,Surface-dissociated nanoparticle draw solutions in forward osmosis and the regeneration in an integrated electric field and nano filtration system,Ind.Eng.Chem.Res.51(2012)15463-15471.
[56]H.G.Schild,Poly(N-isopropylacrylamide):Experiment,theory and application,Prog.Polym.Sci.17(1992)163-249.
 Chinese Journal of Chemical Engineering2016年1期
Chinese Journal of Chemical Engineering2016年1期
- Chinese Journal of Chemical Engineering的其它文章
- Economic analysis in product design—A case study of a TCM dietary supplement
- Effect of ionic liquids on stability of O/W miniemulsion for application of low emission coating products☆
- Characterization and adsorption behaviors of a novel synthesized mesoporous silica coated carbon composite☆
- Experiment and simulation of foaming injection molding of polypropylene/nano-calcium carbonate composites by supercritical carbon dioxide☆
- Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation☆
- A computational analysis of the impact of mass transport and shear on three-dimensional stem cell cultures in perfused micro-bioreactors
