Effect of ionic liquids on stability of O/W miniemulsion for application of low emission coating products☆
Yiyang Kong,Binjie Hu,Yanqing Guo,Yifan Wu
Department of Chemical and Environmental Engineering,University of Nottingham Ningbo China,Ningbo 315100,China
1.Introduction
As legislations against volatile organic components(VOCs)become more stringent,there has been a trend towards waterborne coating.Among waterborne coatings,latex coating,prepared by mixing latex with fillers,pigments,and additives,has attracted increasing attention[1].It is widely applied in architectural field,including interior coating,exterior coating,and wood paint.However,VOC emission from coalescing agent is a main challenge of latex coating.Coalescing agent can be classified as a plasticizer that reduces the glass transition temperature(Tg)of polymer.It functions as enhancing the deformation and coalescence of particles,which is essential for forming a continuous film.But after film formation coalescing agent diffuses and evaporates as VOCs.Some of them,for example,the w ell-know n Texanol,even release a weak and unpleasant odor persisting for days[2].
It isnotable that some of the room temperature ionic liquids(RTILs),kinds of non-volatile organic salts,are proven to be effective plasticizers to reduce Tgof polymers[3,4].Due to plasticizer effect and nonvolatility,RTILs may act the same as coalescing agents,but eliminate the emission of VOCs.After film formation,RTILs remain in the film as polymer/RTILs composite,and provide the film with some special functions like thermal stability,conductivity,and antifouling property[5-9].It can be seen that a certain RTIL may have the potential to be a coalescing agent with multi-function and minimum VOC emission,which coincides with the development tendency of additives for latex coating.
The method of adding RTILs into latex coating is worth considering.To improve water resistance of film,RTILs must be hydrophobic;and hydrophobic RTILs cannot be dispersed in water phase directly.Encapsulation of RTILs inside polymer particles by miniemulsion polymerization is a good choice.In miniemulsion polymerization,polymerization carries out primarily according to droplet nucleation mechanism,w here monomers are polymerized directly inside droplets[10].Because the final particles originate from droplets,hydrophobic compounds presented in droplets would be gradually encapsulated by polymers formed till the end of polymerization.Some hydrophobic compounds like Miglyol 812,castor oil,and n-heptane[11-13],have been successfully encapsulated by this method.This indicates the potential to encapsulate RTILs by miniemulsion polymerization.
To prepare homogeneous latex containing RTILs,the stability of miniemulsion should be concerned.Such stability includes the storage period before polymerization.This would further affect the stability of miniemulsion during polymerization[10].During storage,the instability of miniemulsion can be classified into tw o types:reversible and irreversible change[14].For reversible change,droplets first flocculate,follow ed by creaming or sedimentation.Irreversible change includes coalescence and Ostwald ripening,thusleading to the formation of larger droplets.Ostwald ripening is a phenomenon that describes the diffusion of monomer from small droplet into large droplet caused by the higher interfacial energy of small droplet than that of large one.Coalescence is the process of formation of larger droplet caused by the collision of smaller droplets.The unstable phenomenon of miniemulsion observed during polymerization has some similarity to the one observed during storage.Oil phase may separate from miniemulsion at the early stage of polymerization.Miniemulsion could become viscous follow ed by the coagulation of particles which would separate from miniemulsion at the end of polymerization.Sometimes products are stable and homogeneous based on direct observation,however,under the observation via microscope,the particles could be bridged together[15].
So far,no report has been published on relations between RTILs and stability of miniemulsion during storage as w ell as during the polymerization process.Our work will focus on the investigation on the effect of RTILs on stability of miniemulsion before and during polymerization.1-Octyl-3-methyl-imidazolium hexafluoro phosphate(C8mim PF6)is chosen as the target RTIL.Reasons have been explained elsew here[16].
2.Materials and Methods
2.1.Chemicals
Methyl methacrylate(MMA,CP)was purchased from Sinopharm Chemical Reagent Co.,Ltd and puri fied by vacuum distillation to remove inhibitor.L-Ascorbic acid(Vc,AR),hydrogen peroxide(H2O2,AR),and sodium dodecyl sulfonate(SDSO,CP)were purchased from Sinopharm Chemical Reagent Co.,Ltd.Hexadecane(HD,98%)was purchased from Aladdin Industrial Inc.1-Octyl-3-methylimidazolium hexa fluorophosphate(C8mim PF6,99%)wassupplied by Shanghai Cheng Jie Chemical Co.,Ltd.Ultra-pure water with a resistivity of 18.2 MΩ·cm-1was used in all experiments.
2.2.Preparation of miniemulsion
Oil in water miniemulsion(O/W)was prepared with a volume ratio of oil to water phase equal to 3 to 7.The oil phase contained 5 w t%HD,the remaining 95 w t%containing C8mim PF6and MMA.Both C8mimPF6and HD were in finitely miscible in MMA.The water phase contained 30 mmol∙L-1SDSO.After HD and C8mim PF6were fully dissolved into MMA,the oil phase was added into water phase in a 100 ml plastic container and kept at 40°C.Ultrasound probe was then placed 1 cm below the surface of the mixture in the middle of the container.The output power of the sonicator(Xinzhi Scientz II)was set as 95 W.The miniemulsion was formed by homogenizing the mixture for 6 min in a state of 1 s on and 1 s off.
2.3.Miniemulsion polymerization
The polymerization was carried out in a 250 ml four-necked flask equipped with a stirrer,a re flux condenser,a thermometer,and a nitrogen inlet.The flask was immersed in a water bath for controlling reaction temperature.150 ml miniemulsion was loaded into the flask,and stored under stirring and nitrogen bubbling for 1.5 h to remove oxygen.Then set the temperature of water bath to the reaction temperature.After the desired reaction temperature,40°C,was reached,adding initiator solution,H2O2+Vc,into water phase to start polymerization reaction.The initiator solution was prepared by dissolving agiven amount of Vc in 5 ml ultra-pure water,then adding H2O2in to Vc solution.The molar ratio of H2O2to Vc equal to 1 to 1.3.In this w ork,1 mol%of H2O2based in MMA is used.
2.4.Characterization
2.4.1.Stability of miniemulsion
Miniemulsion was visually observed during storage and polymerization to evaluate if any creaming,sedimentation,phase separation,and coagulation occurred.
2.4.2.Droplet size measurement
The droplet/particle sizes and distributions of miniemulsion at different storage/reaction times were measured using the laser diffraction method(Mastersizer 3000,Malvern Inc).This technique has been w idely accepted as a tool to evaluate droplet size[17-19].Before the size measurement,the sample was diluted with water solution to meet the requirement of obscuration.The diluted water solution was saturated with SDSO and monomers to prevent coalescence during sample measurement[20].All measurements were repeated three times.
2.4.3.Interfacial tension measurement
The interfacial tension between oil phase and water phase was measured using the Du Noüy ring method(BZY-2tensiometer,Hengping Instruments).Since above a certain concentration,the surfactant(SDSO)cannot be dissolved in water phase at the storage temperature,20°C,the measuring temperature was set at 40°C.Measurements were repeated three times.
2.4.4.Zeta potential measurement
The Zeta potentials of the miniemulsion were measured using trace laser Doppler electrophoresis method(Zetasizer Nano ZS,Malvern Inc)at 20°C.Measurements were carried out by injecting the miniemulsion sample into high concentration sample cell and it was repeated three times.
2.4.5.Determination of conversion
The conversion of monomer was determined gravimetrically.The product was sampled at regular intervals during reaction,and stored in refrigerator immediately after being taken out to terminate the reaction.After evaporating monomer under ventilated condition and storing in an oven at 120°C for 4 h,polymer,C8mim PF6,and SDSO remained in the solid product.Because the content of C8mim PF6and SDSO can be calculated according to raw material ratio,the yield of polymer can be obtained by subtracting the content of C8mim PF6and SDSO from the solid product.
3.Results and Discussion
In this section,effect of C8mim PF6concentration on stability of miniemulsion during storage and polymerization,was systematically investigated.
3.1.Effect of C8mimPF6 concentration on stability of miniemulsion during storage
In order to evaluate the role of C8mim PF6on stability of miniemulsion during storage,miniemulsions containing different concentrations of C8mim PF6ranging from 0 w t%,1 w t%,5 w t%,10 w t%,20 w t%to 30 w t%based on oil phase were prepared,and were stored at 20°C for 288 h.Results were listed in Table 1.
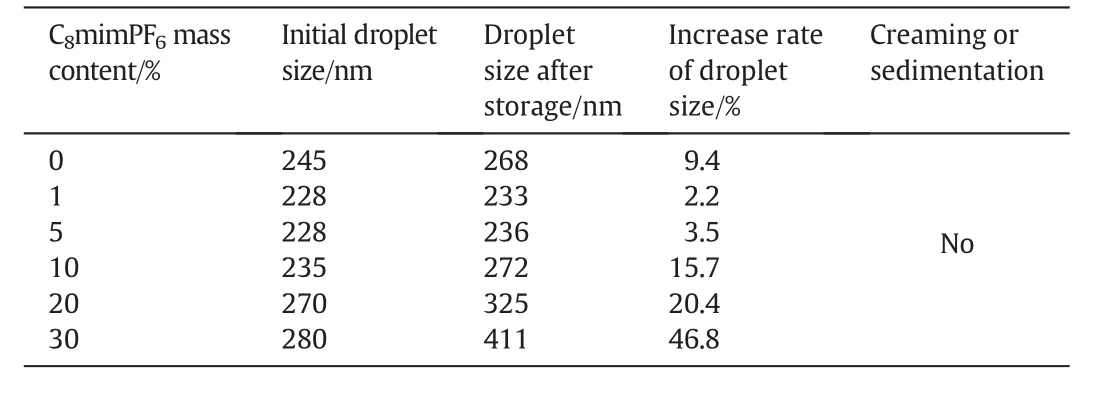
Table 1 Droplet sizes of miniemulsions containing different concentrations of C8mimPF6 before and after 288 h storage
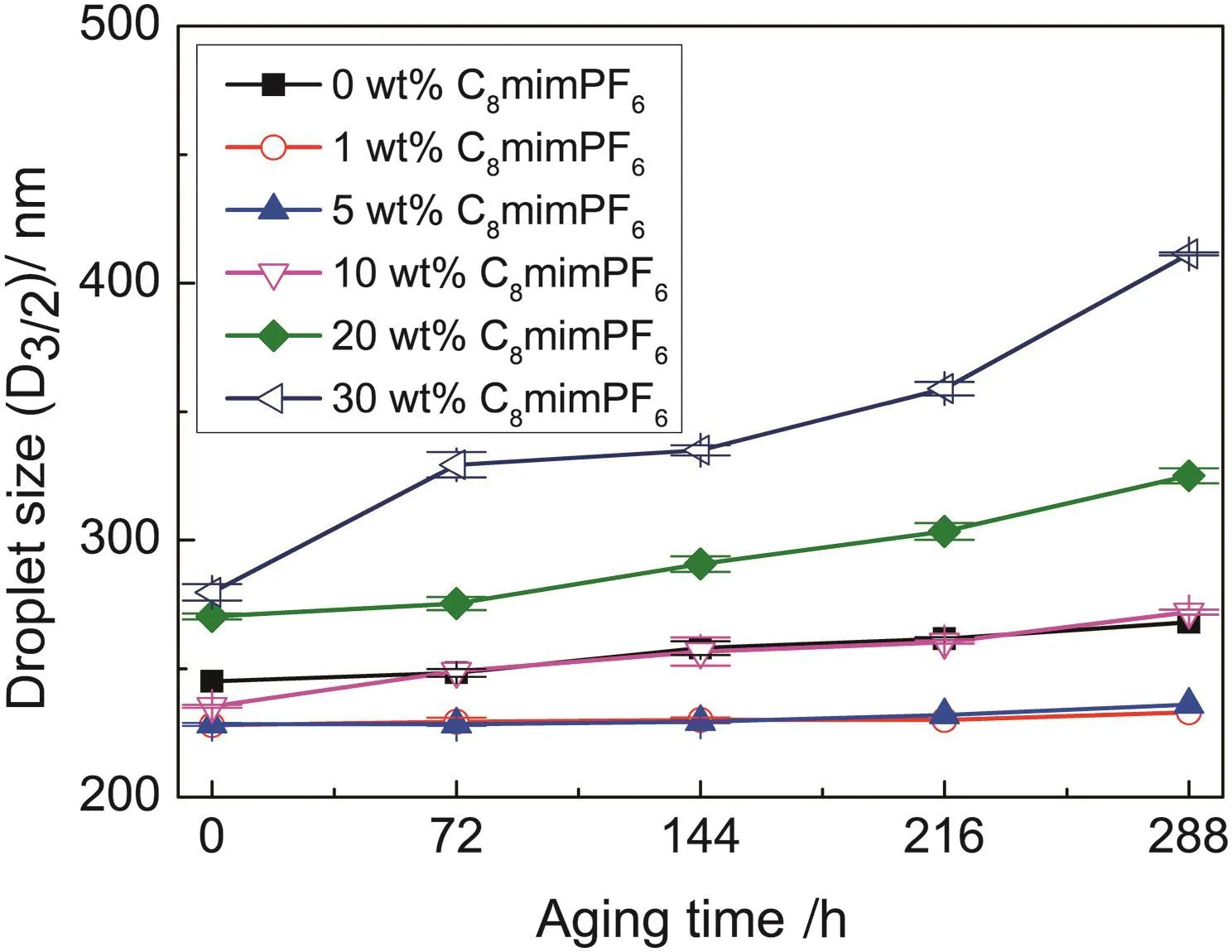
Fig.1.Variation of droplet sizes as a function of time for miniemulsions containing different C8mimPF6 concentrations at 20°C.
It was observed that all miniemulsions were visually stable,without creaming or sedimentation within 288 h storage.Creaming or sedimentation is normally induced by gravity force.Accordingto Stoke'slaw,migration rate of droplets induced by gravity force is in proportion to the square of droplet diameter.For miniemulsion with nanoscale droplet sizes,the migration rate of droplets could be less apparent,hence inhibiting creaming or sedimentation[21].
Even though miniemulsions were visually stable,after 288 h storage,droplet sizes(show n in Table 1)were found to be increased,especially at concentration of C8mim PF6above 10 w t%.The rate of increase on droplet size became more pronounced up to 46.8%compared to the low er one 2.2%obtained at 1 w t%of C8mim PF6.Such an increase in droplet sizes might be attributed to the effect of coalescence and Ostw ald ripening,which could lead to the instability of miniemulsion.It is interesting to note,such instability of miniemulsion seemed independent of initialdroplet size.Table1 showed that the initialdroplet size for miniemulsion at 10 w t%C8mim PF6was 235 nm,which was smaller than the one obtained at 0 w t%C8mim PF6,245 nm.The rate of increase in droplet size for miniemulsion at 10 w t%C8mim PF6was faster,272 nm,while for miniemulsion at 0 w t%C8mim PF6,the droplet size after storage was 268 nm.Such unusual change on droplet size in the presence of C8mim PF6might be due to the interaction between C8mim PF6and SDSO at droplet interface.The underneath mechanism were explained in the following section.To have a full picture on stability of droplet during 288 h storage time,droplet sizes of miniemulsions containing different concentrations of C8mim PF6were continually measured at 72,144,216 and 288 h.Results were show n in Fig.1.
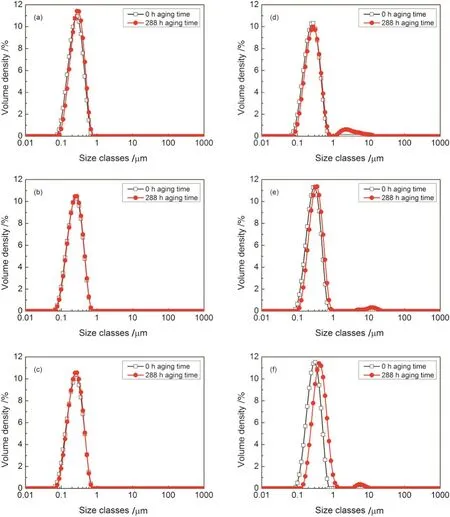
Fig.2.Droplet size distribution at 0 h and 288 h aging time for miniemulsions containing different concentrations of C8mimPF6.(a)0 wt%;(b)1 w t%;(c)5 w t%;(d)10 w t%;(e)20 w t%;(f)30 w t%.
It was found that the increase of droplets for miniemulsions containing 10 wt%,20 wt%,and 30 w t%C8mim PF6were much rapid compared with those containing 0 w t%,1 w t%,and 5 w t%C8mim PF6.This suggested that above certain concentration of C8mim PF6,e.g.at 10 w t%,more C8mim PF6in oil phase had a negative effect on the stability of miniemulsion.
To have a better understanding of miniemulsions at initial stage and after 288 h storage,droplet size distributions at 0 h and 288 h storage for miniemulsions containing 0 w t%,1 w t%,5 w t%,10 w t%,20 w t%,and 30 w t%of C8mim PF6are illustrated(Fig.2).It was found,for miniemulsion w ithout C8mim PF6,the droplet distribution peak moved towards larger size slightly.No obvious changes on distributions could be detected for miniemulsions with concentration of C8mim PF6at 1 w t%and 5 w t%after 288 h storage.How ever,further increase the concentration of C8mim PF6,droplet distribution exhibited bimodal distributions with larger size appearing after 288 h storage.The results were consistent with the mean droplet size measured at the same concentration range of C8mim PF6show n in Fig.1.Clearly,above a critical concentration,e.g.at 10 w t%,further increase in C8mim PF6concentration accelerated the movement of droplets tow ards larger size,indicating the instability of miniemulsion.
The instability of miniemulsion at higher concentration of C8mimPF6might be due to two factors:coalescence and Ostwald ripening.Based on our previous study,Ostwald ripening could be well inhibited in the presence of co-stabilizer,HD[16].Thus the increase of droplet size observed in Fig.1 might be attributed to the coalescence effect.Coalescence could take place into tw o processes:collision of droplets and drainage of the liquid between the droplets to form one large droplet[10].Collision was mainly due to Brownian motion and van der Waals force[21].If droplets were stabilized by ionic surfactants,the induced electrostatic repulsion due to oriented adsorption of the ionic surfactants at oil/water interface could prevent the collision of droplets[22].Thus the coalescence rate during storage of miniemulsions was strongly dependent on interfacial activity between water and oil phases[23].
The interfacial activity of solution could be characterized by measuring interfacial tensions between water phase and oil phase containing different concentrations of C8mim PF6.Table 2 show ed that the interfacial tension decreased signi ficantly from 3.94 m N∙m-1to 0.31 m N∙m-1with the concentration of C8mim PF6increased from 0 w t%to 1 w t%.Such a reduction on interfacial tension con firmed the adsorption of surfactant on the surface of droplet mentioned above,and hence preventing droplet from coalescence.This partially explained why miniemulsion with 1 w t%concentration of C8mim PF6had better stability during storage than 0 w t%miniemulsion as Fig.1 show ed.How ever,further increase in the concentration of C8mim PF6,i.e.above 10 w t%,interfacial tension became independent of change of the concentration of C8mim PF6though at the same range of concentration of C8mim PF6,the increase of droplet sizebecamemore pronounced indicating the instability of droplets.This suggested that interfacial tension was no longer a suitable property for characterizing the interfacial activity in the miniemulsion system w e studied.Consider the conductivity of C8mim PF6,surface charge could be a more sensible parameter for evaluating interfacial activity of the liquid system we studied.
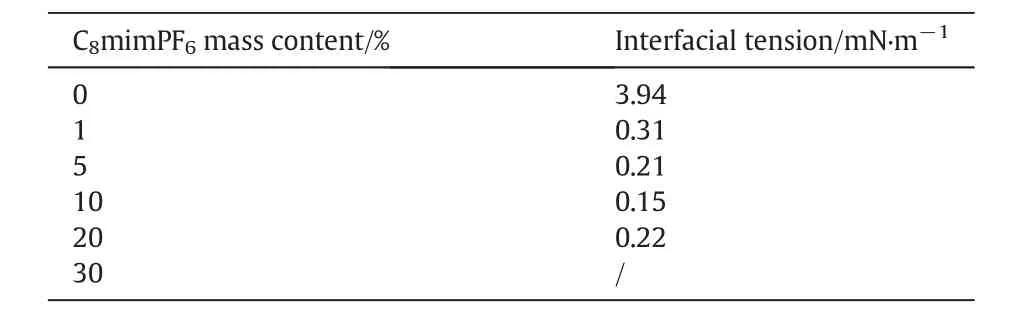
Table 2 Interfacial tension between water phase and oil phase containing different concentrations of C8mimPF6
The surface charge could be evaluated using zeta potential,which is a measure for the degree of electrostatic repulsion between adjacent droplets/particles.Droplets in a dispersion system could be relatively stable if the absolute value of zeta potential is higher than 30 m V.Table 3 illustrated zeta potential values for miniemulsions containing different concentrations of C8mim PF6.Negative value suggested that droplets were negatively charged.It was found that for miniemulsions containing C8mim PF6at 0 w t%,1 w t%,5 w t%and 10 w t%,the absolute zeta potential values were larger than 30 m V,indicating the stronger electric repulsions between droplets,and hence can effectively prevent coalescence of droplets.For miniemulsions containing C8mim PF6above 10 wt%,i.e.at 20 wt%and 30 wt%,smaller absolute zeta potential values,i.e.13.8 m V and 7.8 m V(less than 30 m V)were detected,indicating that the droplets in this region were unstable.Such observations a greed with the results of the droplet stability show n in Fig.1.This further suggested that at higher concentration of C8mim PF6,(above 10 w t%)surface charge of droplets was the dominating factor for droplet stability in the dispersion.
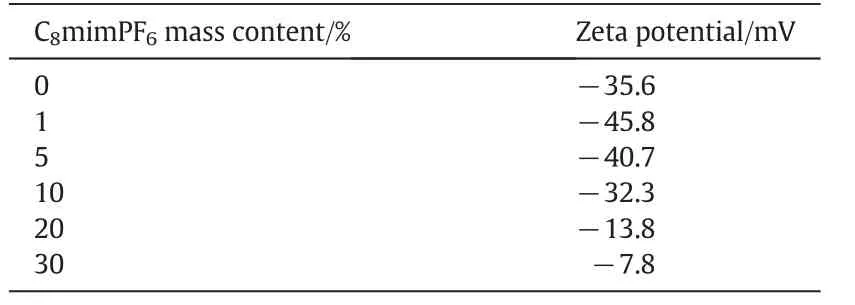
Table 3 Zeta potential of miniemulsions containing different concentrations of C8mimPF6
3.2.Effect of C8mimPF6 concentration on stability of miniemulsion during polymerization
To have a better understanding on the relation between the initial droplet size obtained before polymerization and the one after polymerization,i.e.latex,which is the key stage to form coating product,a series of investigations on the effect of C8mim PF6concentration on miniemulsion droplets during polymerization were conducted.The miniemulsions containing different concentrations of C8mim PF6ranging from 0 w t%,1 w t%,5 w t%,10 w t%,20 w t%to 30 w t%based on oil phase were prepared because H2O2+Vc was hydrophilic,and could be added just before the polymerization reaction to start.We could compare the droplet size before and after reaction directly.H2O2+Vc was chosen as the initiator,with fixed concentration of H2O2at 1.0 mol%based on MMA.The reaction temperature was fixed at 40°C,which allowed the polymerization reaction giving maximum yield whilst keeping miniemulsion droplets to remain stable.Evaluation for miniemulsions containing different concentrations of C8mim PF6after polymerization with H2O2+Vc as an initiator were listed in Table 4.
Table 4 showed that monomersin all miniemulsions were completely converted into polymers,indicating that H2O2+Vc was an efficient initiator for this system.All miniemulsions were visually stable with no phase separation,sedimentation or coagulation beingobserved during polymerization.Droplet sizes at different reaction times were also measured.
Fig.3 show ed the variation of droplet sizesasafunction of C8mimPF6concentration initiated by H2O2+Vc at different reaction times.It is interesting to note that immediately after reaction started,i.e.10 min,all droplets became smaller.This might be attributed to homogeneous or micellar nucleation[10,24,25].For homogeneous nucleation,primary particles generate in the aqueous phase by the chain growth reaction of radicals with monomers dissolved in water.They were stabilized by the surfactant dissolved in water or released from droplet surface.As to micellar nucleation,polymerization would be carried out in micellar and form primary particles,and these smaller primary particles coulddominate the mean droplet size,which were subjected to a reduction compared to the initial mean droplet size.This trend was also reported in similar system initiated by Vc+H2O2[26],and miniemulsion polymerization of styrene initiated by sodium persulfate[25].The results suggested that homogeneousor micellar nucleation could be a dominating factor to determine droplet size throughout the entire reaction,90 min.In addition,it was noted that no further decrease in droplet sizes as reaction continues,e.g.,50 min and 90 min,which suggested that such micellar nucleation only occurred at initial reaction period.By comparing the relation between droplet size and the concentration of C8mim PF6before reaction(0 min)and after reaction at different reaction times(10 min,50 min,and 90 min),the trend in both case were very similar.
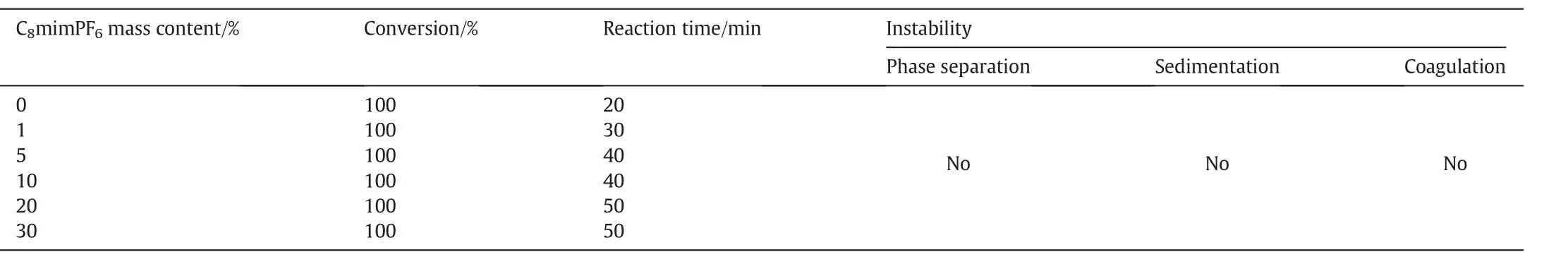
Table 4 Conditions of miniemulsions containing different concentrations of C8mim PF6 after polymerization
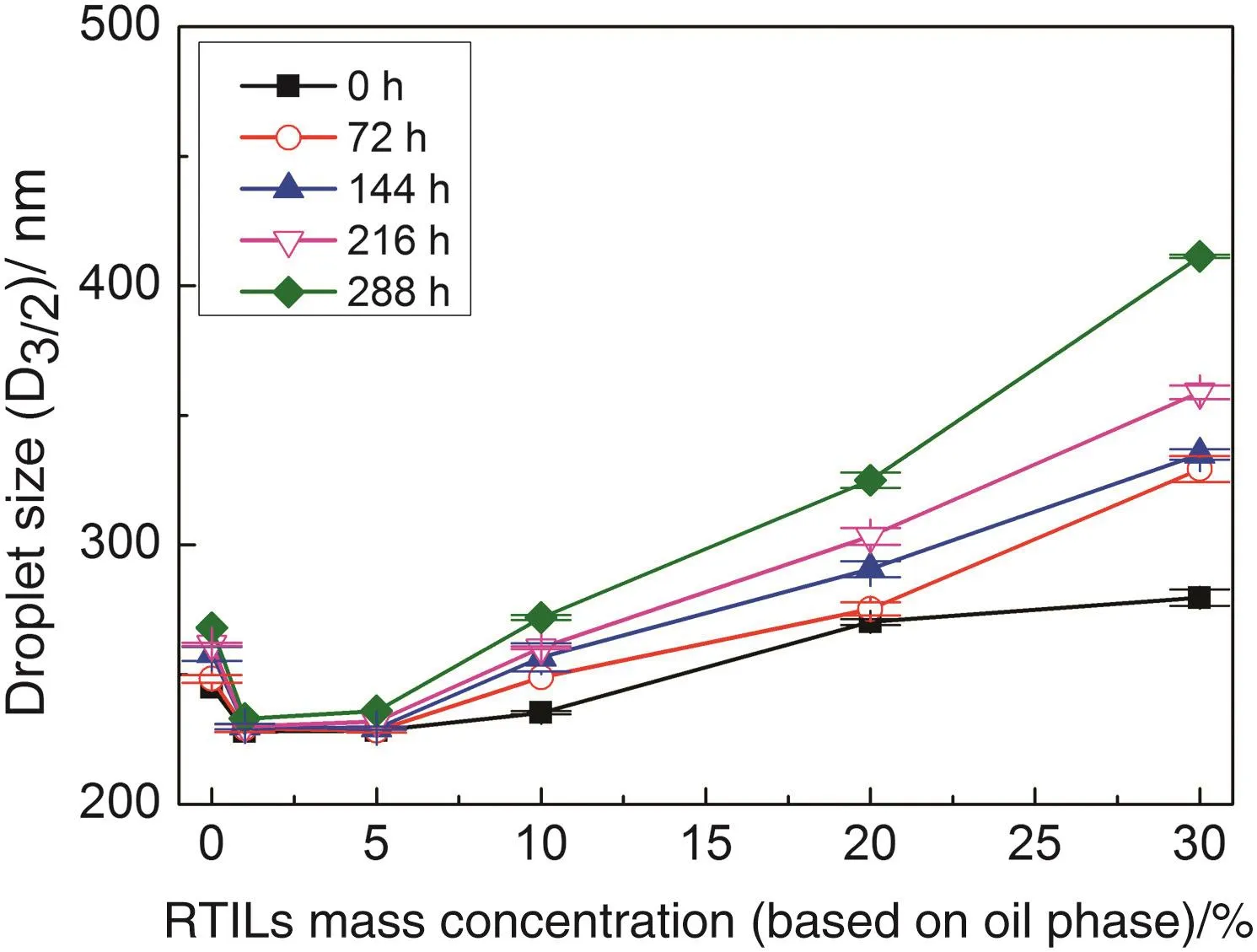
Fig.4.Variation of droplet sizes as a function of C8mimPF6 concentration for miniemulsions at different storage times.
To have a full picture about controlling factors on droplet size before and after reaction,the droplet sizes as a function of concentration of C8mim PF6for miniemulsions at different storage times were plotted in Fig.4.It was found that all droplets increased with the increase of storage time,and the rate of increase in droplet size for miniemulsions containing 10 w t%,20 w t%and 30 w t%C8mim PF6were much faster.This indicated that the dominating factor for stability of droplet size before the reaction was controlled by coalescence as described in Section 3.1,though the overall trend for variation of droplet size with concentration of C8mim PF6were still similar.
4.Conclusions
A new latex containing C8mim PF6,a kind of RTILs,had been developed for low VOC latex coating.This was achieved by encapsulating C8mim PF6inside particles via miniemulsion polymerization which was initiated by H2O2+Vc at 40°C.The concentration of C8mim PF6played a key role on stability of miniemulsion during storage and polymerization.Regarding storage stability,there was a critical concentration for C8mim PF6,i.e.10 w t%C8mim PF6.Below such value,the miniemulsions were relatively stable during storage.Above 10 wt%,adding more C8mim PF6to the oil phase might weaken the droplet stability significantly.The instability of droplet size at higher concentration of C8mim PF6was caused by a decrease in the absolute zeta potential value thus the electrostatic repulsion between droplets were not strong enough to prevent droplets from coalescence.As to polymerization process,the reaction mechanism appeared to be more important in determining the droplet size.A reduction on droplet size in all ranges of concentration of C8mim PF6was observed during polymerization reaction.Such droplet size reductions were probably caused by homogeneous or micellar nucleation mechanism,which eventually dominated the droplet size throughout the reaction.Both during storage of miniemulsion and after polymerization reaction,the trend on relation between droplet size and concentration of C8mim PF6remained unchanged.
[1]J.V.Barbosa,E.Veludo,J.Moniz,A.Mendes,F.D.Magalhães,M.M.Bastos,Low VOC self-crosslinking waterborne acrylic coatings incorporating fatty acid derivatives,Prog.Org.Coat.76(11)(2013)1691-1696.
[2]Y.-M.Chang,W.-H.Hu,W.-B.Fang,S.-S.Chen,C.-T.Chang,H.-W.Ching,A study on dynamic volatile organic compound emission characterization of water-based paints,J.Air Waste Manage.Assoc.61(1)(2011)35-45.
[3]M.P.Scott,C.S.Brazel,M.G.Benton,J.W.Mays,J.D.Holbrey,R.D.Rogers,Application of ionic liquids as plasticizers for poly(methyl methacrylate),Chem.Commun.(2002)1370-1371.
[4]M.M.Mok,X.Liu,Z.Bai,Y.Lei,T.P.Lodge,Effect of concentration on the glass transition and viscoelastic properties of poly(methyl methacrylate)/ionic liquid solutions,Macromolecules 44(4)(2011)1016-1025.
[5]M.P.Scott,M.Rahman,C.S.Brazel,Application of ionic liquids as low-volatility plasticizers for PMMA,Eur.Polym.J.39(10)(2003)1947-1953.
[6]A.Noda,M.Watanabe,Highly conductive polymer electrolytes prepared by in situ polymerization of vinyl monomers in room temperature molten salts,Electrochim.Acta 45(2000)1265-1270.
[7]K.Matsumoto,T.Endo,Con finement of ionic liquid by networked polymers based on multifunctional epoxy resins,Macromolecules 41(2008)6981-6986.
[8]M.A.B.H.Susan,T.Kaneko,A.Noda,M.Watanabe,Ion gels prepared by in situ radical polymerization of vinyl monomers in an ionic liquid and their characterization as polymer electrolytes,J.Am.Chem.Soc.127(2005)4976-4983.
[9]Q.Ye,T.Gao,F.Wan,B.Yu,X.Pei,F.Zhou,Q.Xue,Grafting poly(ionic liquid)brushes for anti-bacterial and anti-biofouling applications,J.Mater.Chem.22(26)(2012)13123.
[10]J.M.Asua,Miniemulsion polymerization,Prog.Polym.Sci.27(7)(2002)1283-1346.
[11]A.P.Romio,C.Sayer,P.H.H.Araújo,M.Al-Haydari,L.Wu,S.R.P.da Rocha,Nanocapsules by miniemulsion polymerization with biodegradable surfactant and hydrophobe,Macromol.Chem.Phys.210(9)(2009)747-751.
[12]F.R.Steinmacher,N.Bernardy,J.B.Moretto,E.I.Barcelos,P.H.H.Araújo,C.Sayer,Kinetics of MMA and VAc miniemulsion polymerizations using miglyol and castor oil as hydrophobe and liquid core,Chem.Eng.Technol.33(11)(2010)1877-1887.
[13]C.A.Capeletto,C.Sayer,P.H.H.de Araújo,Styrene miniemulsion polymerization:Incorporation of N-alkanes,Macromol.Symp.319(1)(2012)54-63.
[14]B.Abismaïl,J.P.Canselier,A.M.Wilhelm,H.Delmas,C.Gourdon,Emulsi fication by ultrasound:Drop size distribution and stability,Ultrason.Sonochem.6(1999)75-83.
[15]A.Musyanovych,R.Rossmanith,C.Tontsch,K.Landfester,Effect of hydrophilic comonomer and surfactant type on the colloidal stability and size distribution of carboxyl-and amino-functionalized polystyrene particles prepared by miniemulsion polymerization,Langmuir 23(2007)5367-5376.
[16]Y.Kong,B.Hu,K.-L.Choy,X.Li,G.Chen,Study of miniemulsion formulation containing 1-octyl-3-methylimidazolium hexa fluorophosphate for its application in lowemitting coating products,Soft Matter 11(7)(2015)1293-1302.
[17]Q.Zhang,Y.Shi,X.Zhan,F.Chen,In situ miniemulsion polymerization for waterborne polyurethanes:Kinetics and modeling of interfacial hydrolysis of isocyanate,Colloids Surf.A Physicochem.Eng.Asp.393(2012)17-26.
[18]M.Winkelmann,H.P.Schuchmann,Precipitation of metal oxide nanoparticles using a miniemulsion technique,Particuology 9(5)(2011)502-505.
[19]Y.Wang,Y.Zhang,T.Xia,W.Zhao,W.Yang,Effects of fabricated technology on particle size distribution and thermal properties of stearic-eicosanoic acid/polymethylmethacrylate nanocapsules,Sol.Energy Mater.Sol.Cells 120(2014)481-490.
[20]Y.Meliana,L.Suprianti,Y.C.Huang,C.T.Lin,C.-S.Chern,Effect of mixed costabilizers on Ostwald ripening of monomer miniemulsions,Colloids Surf.A Physicochem.Eng.Asp.389(1-3)(2011)76-81.
[21]U.Bazylińska,J.Kulbacka,K.A.Wilk,Dicephalic ionic surfactants in fabrication of biocompatible nanoemulsions:Factors in fluencing droplet size and stability,Colloids Surf.A Physicochem.Eng.Asp.460(2014)312-320.
[22]T.L.Doane,C.-H.Chuang,R.J.Hill,C.Burda,Nanoparticle ζ-potentials,Acc.Chem.Res.45(2011)317-326.
[23]J.C.Berg,An introduction to interfaces and colloids:The bridge to nanoscience,World Scienti fic Publishing Co.Pte.Ltd,5 Toh Tuck Link,Singapore,2010.
[24]F.J.Schork,Y.Luo,W.Smulders,J.P.Russum,A.Butté,K.Fontenot,Miniemulsion polymerization,Adv.Polym.Sci.175(2005)129-255.
[25]C.-S.Chern,Y.-C.Liou,Kinetics of styreneminiemulsion polymerization stabilized by nonionic surfactant/alkyl methacrylate,Polymer 40(13)(1999)3763-3772.
[26]A.P.Romio,N.Bernardy,E.Lemos Senna,P.H.H.Araújo,C.Sayer,Polymeric nanocapsules via miniemulsion polymerization using redox initiation,Mater.Sci.Eng.C 29(2)(2009)514-518.
 Chinese Journal of Chemical Engineering2016年1期
Chinese Journal of Chemical Engineering2016年1期
- Chinese Journal of Chemical Engineering的其它文章
- Economic analysis in product design—A case study of a TCM dietary supplement
- Characterization and adsorption behaviors of a novel synthesized mesoporous silica coated carbon composite☆
- Experiment and simulation of foaming injection molding of polypropylene/nano-calcium carbonate composites by supercritical carbon dioxide☆
- Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation☆
- A computational analysis of the impact of mass transport and shear on three-dimensional stem cell cultures in perfused micro-bioreactors
- Production and characterization of exopolysaccharides in mycelial culture of Cordyceps sinensis fungus Cs-HK1 with different carbon sources☆
