Effects of variations in culture media and hormonal treatments upon callus induction potential in endosperm explant of Barringtonia racemosa L.
Nurul Izzati Osman, Norrizah Jaafar Sidik, Asmah AwalFaculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, MalaysiaFaculty of Plantation and Agrotechnology, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
Effects of variations in culture media and hormonal treatments upon callus induction potential in endosperm explant of Barringtonia racemosa L.
Nurul Izzati Osman1*, Norrizah Jaafar Sidik1, Asmah Awal21Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
2Faculty of Plantation and Agrotechnology, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
Original article http://dx.doi.org/10.1016/j.apjtb.2015.10.007
Tel: +60 17 3681685
E-mail: nurulizzati70@gmail.com
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
Foundation project: Supported by Ministry of Education under Higher Education Department through Fundamental Research Grant Scheme (FRGS) with the grant number of FRGS/1/2012/STW/N03/UITM/02/01.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 24 Aug 2015
Received in revised form 2 Sep 2015 Accepted 20 Oct 2015
Available online 10 Dec 2015
Keywords:
Callus induction
Barringtonia racemosa
Plant growth regulators
2,4-Dichlorophenoxyacetic acid
Kinetin
Lloyd and McCown's woody plant medium
Murashige and Skoog's medium
ABSTRACT
Objective: To induce callus from the medicinally valuable species, Barringtonia racemosa L. (B. racemosa) whereby the formation of callus is essential for micropropagation studies and in vitro plant secondary metabolites production.
Methods: The callus induction potential in B. racemosa was assessed from endosperm explant cultured on different culture media and plant hormonal treatments. Lloyd and McCown's woody plant medium and Murashige and Skoog's medium were used in the study as culture media. On the other hand, various concentrations and combinations of 2,4-dichlorophenoxyacetic acid (1.0–2.0 mg/L) and kinetin (0.5–2.5 mg/L) had been incorporated in the culture media to exert the effects of auxin and cytokinin on callus induction.
Results: From the present study, it was found that the profuse [(1.681±0.770) g fresh weight, (0.239±0.239) g dry weight] and friable callus formation was optimally produced with desirable morphology and considerable percentage of callus induction (56.70%) in endosperm explants cultured on 1.0 mg/L 2,4-dichlorophenoxyacetic acid and 1.5 mg/L kinetin in Murashige and Skoog's medium.
Conclusions: A reliable protocol for inducing callus formation of profuse and friable morphology in endosperm explant of B. racemosa had therefore been successfully established.
1. Introduction
Barringtonia racemosa L. (B. racemosa) is a type of medicinal plant species native to East Africa, Pacific Islands and Southeast Asia including Malaysia. Known as a type of mangrove plants, B. racemosa grows well in wet and watery areas such as along fresh water swamps, riverbanks and lakes with the height of approximately 4–8 m but can grow up to 15 m [1–3]. This species gained less attention due to lack of effort in promoting their development and commercialization. Furthermore, in certain regions of the world for instance in Singapore, the species is considered endangered and identified to be at the verge of extinction.
Despite being disregarded especially in modern societies, this species however is a rich source of phytomedicines and has long been used by the elderly as a type of traditional remedies, being included in the dishes whereby the shoots and fruits are usually eaten raw. The medicinal values of the B. racemosa have been acknowledged to be among the herbs of choice in various tribes around the world. Furthermore, the pharmacological properties of the species had been scientifically proven and recorded in a number of studies [4]. B. racemosa have been documented in Ayurvedic literature in which the fruits of B. racemosa are prescribed for the treatment of pain, inflammation and rheumatic conditions [5]. The leaves are traditionally used for treating high blood pressure [3], ulcer, cancer [6] and the pounded leaves, roots and barks are used to reduce itchinessand chicken pox[7]. Due to the medicinally valuable properties of the species, the effort to micropropagate this species through plant tissue culture techniques is seen to be useful to ensure its continuous supply.
The induction of callus serves as a basis in plant biotechnology studies in which the development of various plant regeneration studies and somatic embryogenesis may be initiated from callus [8,9]. In addition, the formation of friable callus is essential to initiate and establish a homogeneous cell suspension cultures for plant bioactive compound studies [10]. In order to get the optimum callus induction with the desirable morphogenic response, various callus induction factors may be applied. Variations in external factors such as types of basal culture media, plant growth regulators, sucrose compositions, pH of the culture medium and incubation temperatures are among the factors which may influence the induction potential and morphology of the callus induced [10]. The present study described the works that had been conducted covering the aspect of callus induction potentials under different exogenous factors which were culture media and plant growth regulators supplementation. The effects of those factors had been studied to fulfill the aim of obtaining callus with profuse amount of production and having greatest score of callus weight with friable morphology.
2. Materials and methods
2.1. Surface sterilization of explants
Matured fruits of B. racemosa were obtained from Nasuha Herbal Farm, Johor, Malaysia. The fruits were initially washed under running tap water. They were then carefully dissected and the endosperms were taken out from the fruits (Figure 1). Afterwards, surface sterilization procedures for endosperms were carried out by soaking the explants in filtered distilled water followed by 70% (v/v) ethanol for 3 min in a sterile beaker. After that, the explants were soaked in 5.25% sodium hypochlorite solution (Cocorex®) added with two drops of Tween 80 (Sigma, USA) for 30 min. The explants were then thoroughly rinsed with sterile distilled water to remove any traces of remaining detergents.
2.2. Media preparation
Different concentrations and combinations of 2,4-dichlorophenoxyacetic acid (2,4-D) (1.0–2.0 mg/L) and kinetin (0.5–2.5 mg/L) were used in the study. Two types of culture media used were Murashige and Skoog's (MS) medium and Lloyd and McCown's woody plant medium (WPM). The prepared media were consisted of 30 g/L sucrose (R&M Chemicals,UK)and7g/Lgelriteagar(Duchefa,Netherland)andthepH was adjusted to be within 5.6–5.8 by using 1 mol/L HCl and NaOH after the addition of plant hormones. The media were autoclavedat121°Cunderthepressureof1.06kg/cm2for15min.
2.3. In vitro callus induction
The sterilized endosperm were further aseptically cut into small pieces approximately 0.5 cm2and cultured onto callus induction media. Twenty replications of inoculated explants had been prepared for each treatment and the experiments were repeated thrice. The cultures were incubated at (25±2)°C under dark condition and subcultured onto fresh media after four weeks of culture. The observation was done on weekly basis. At the end of six weeks, the formed calli were scraped and isolated from the explants. The data for callus induction were recorded in which the morphology, fresh and dry weights (g) of callus in each treatment were taken.
2.4. Data collection and statistical analysis
The study was conducted according to Completely Randomized Design (CRD). Each week, the percentage of callus induction and callus morphologies from each treatment and media combination were observed. After six weeks of culture period, the percentage of callus induction, weights of calli (fresh weight and dry weight) and the morphology of calli formed were all documented. The percentage of callus induction in each treatment was calculated by the following formula:

The optimum treatment was determined by considering the highestcallusinductionpercentage,freshanddryweightofcallus (g) with the optimum intended morphologies of profuse and completely friable calli. The data were presented as mean±SD. The means comparison and statistical analysis were done using SPSS 17.0 software whereby the effects of hormonal treatments and culture medium types on callus induction were analyzed utilizing paired samples t-test at 95% confidence interval. The value of P<0.05 was considered as statistically significant.
3. Results
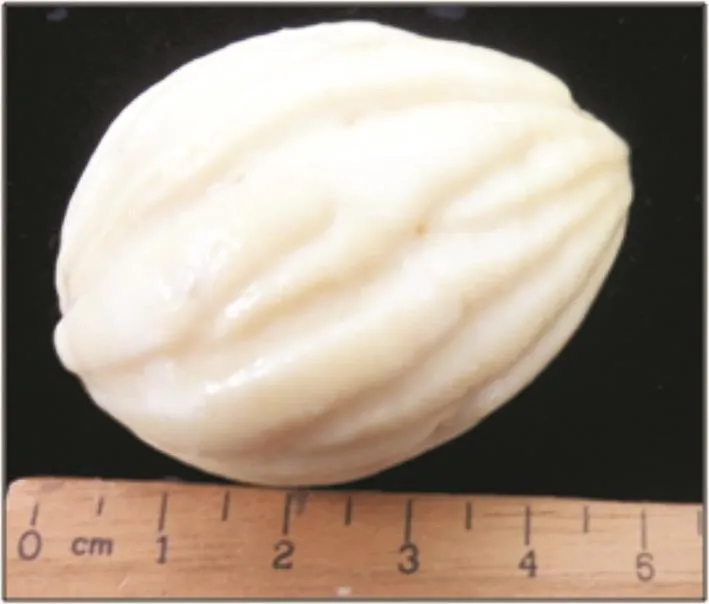
Figure 1. Endosperm of B. racemosa which had been taken out from the matured fruit.
After six weeks of incubation, the progress of callus induction in B. racemosa was observed and recorded. Generally, the calli formed were all yellowish. The highest callus formation with 56.7% callus induction percentage was found in the explants cultured on MS medium supplemented with 1.0 mg/L 2,4-D and 1.5 mg/L kinetin (Table 1). The calli produced from such treatment were found to be completely friable so as to fulfill the desirable morphology (Figure 2) and recorded the greatest fresh [(1.681±0.768) g] and dry weight [(0.239±0.239) g]. After being subcultured (after four weeks of culture period), the calli were proliferated and enlarged (Figure 3). In the present study, most of the calli formed were found to be compact,nodular and brittle (Figure 4). And the findings were prominent in those cultured on WPM medium whereby only one treatment produced friable calli (Table 2). On the contrary, four out of five treatments which produced friable calli in this study were derived from those cultured on MS medium. In terms of percentage of callus induction, the scores were ranging from 1.7% to 56.7% except those cultured on the media treated with 2.0 mg/ L 2,4-D and 1.5 mg/L kinetin on MS medium which produced no callus at all.
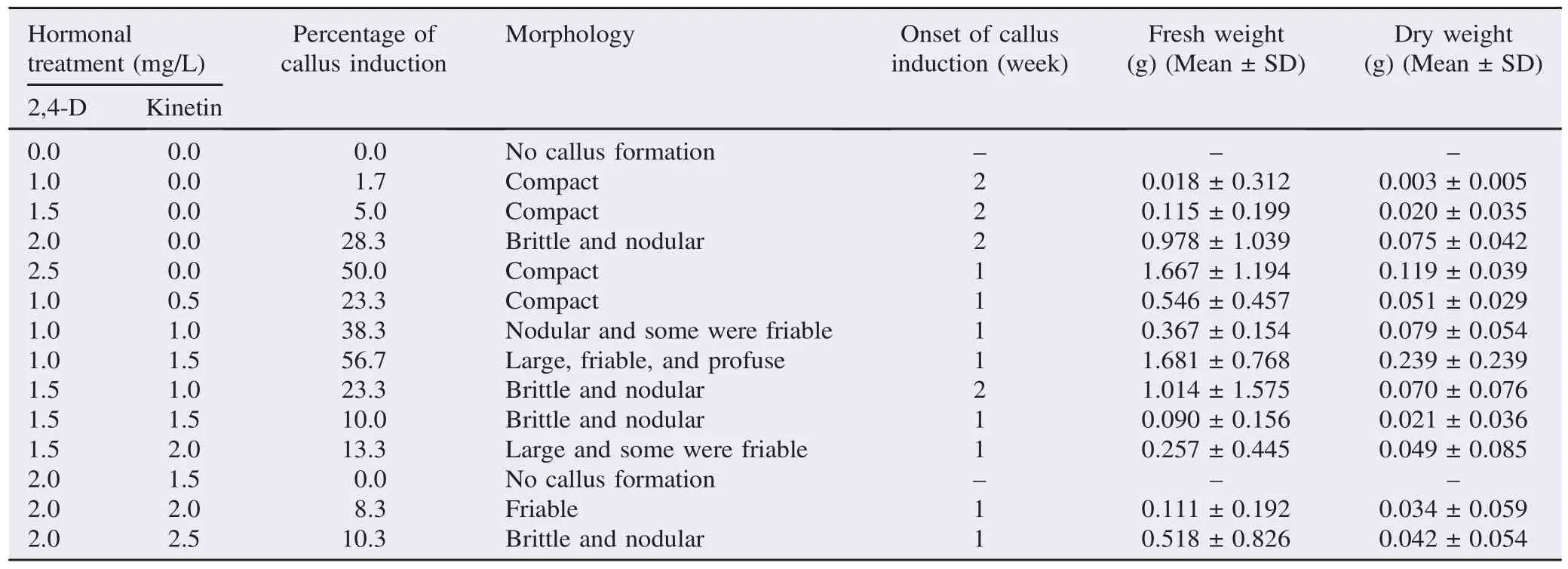
Table 1Callus induction in B. racemosa from endosperm explant cultured on MS medium treated with various concentrations and combinations of 2,4-D and kinetin hormones after six weeks of culture at (25±2)°C under dark condition.
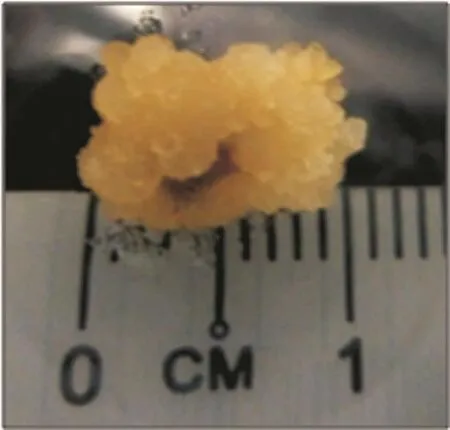
Figure 2. Formation of callus from endosperm explant cultured on optimum treatment [1.0 mg/L 2,4-D and 1.5 mg/L kinetin on MS medium after five weeks of culture at (25±2)°C under dark condition].The calli formed were yellowish, friable and covered almost the entire explants.
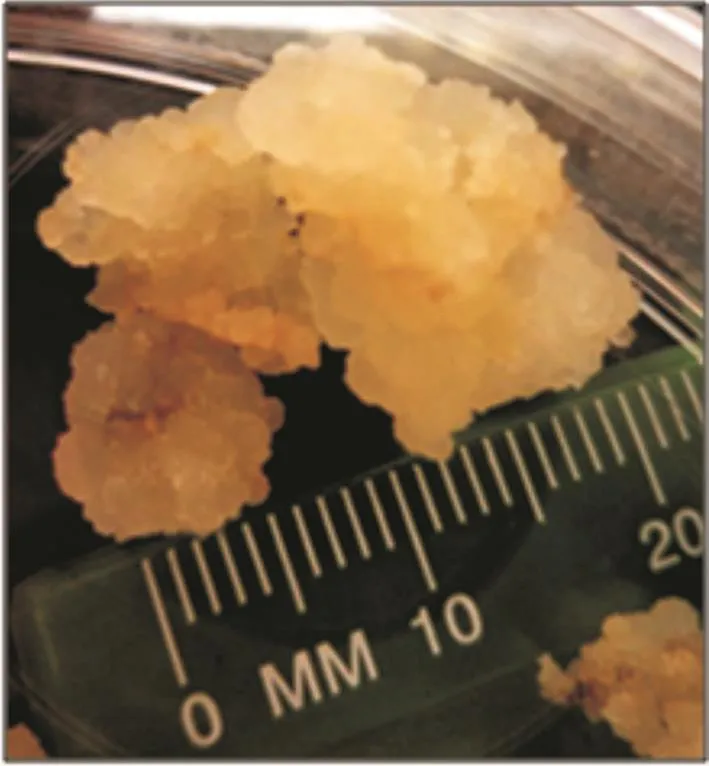
Figure 3. The proliferation and enlargement of friable calli formed from optimum treatment (1.0 mg/L 2,4-D and 1.5 mg/L kinetin in MS medium) after being isolated and subcultured onto fresh medium after six weeks of culture.
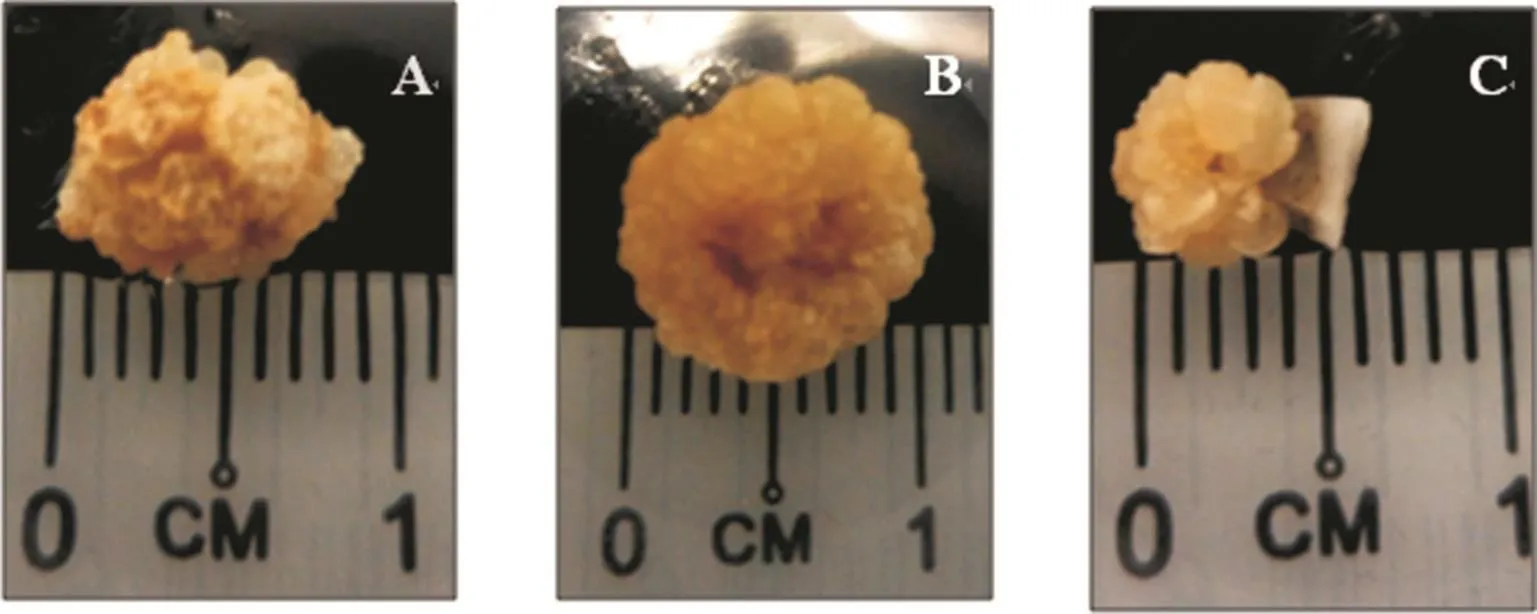
Figure 4. Examples of callus with compact (A and B) and brittle and nodular (C) morphologies.
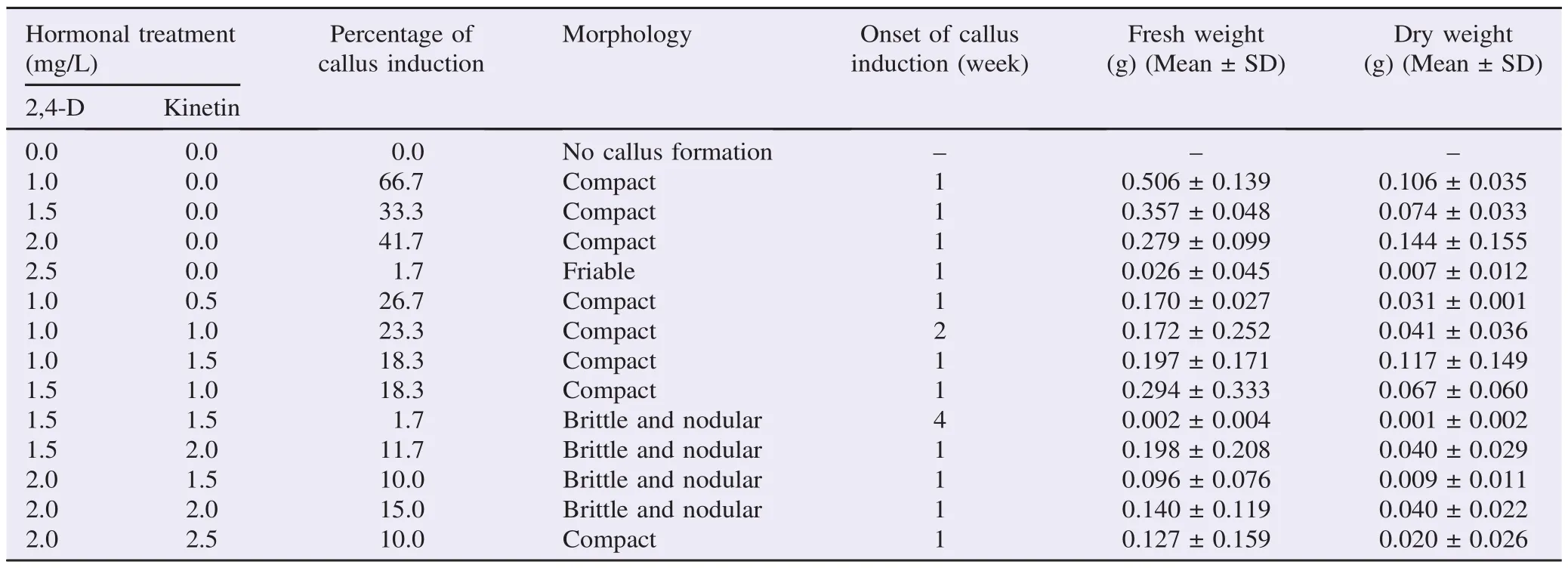
Table 2Callus induction in B. racemosa from endosperm explant cultured on WPM medium treated with various concentrations and combinations of 2,4-D and kinetin hormones after six weeks of culture at (25±2)°C under dark condition.
4. Discussion
4.1. Effects of hormonal treatments on callus induction
Plant growth regulators are synthetic molecules used in plants and supplemented at a relatively low concentrations to work as signaling compounds for plant growth and development [11]. They can be classified into different types according to their molecular structures and physiological functions. The most extensively used and studied class of plant growth regulators in plant tissue cultures are auxin and cytokinin [8]. 2,4-D which is known as a type of auxin has been acknowledged to effectively induce callus formation in many plant species.
Theresultfromthisstudyrevealedthatthepresenceof2,4-Din the culture media was essentially required to induce callus formation in this species even though the cytokinin was absent. The effectiveness of 2,4-D in inducing the formation of callus is attributed to its main characteristic which can stimulate cell divisionofplanttissuesandstrongly suppressorganogenesis.It is also considered to be the most potent among any other commonly usedauxins[10].Nevertheless,inthecurrentstudy,thepresenceof 2,4-D alone at the concentration of 1.0 mg/L–2.0 mg/L in MS mediumwasfoundtodelaytheformationofcalliinwhichthecalli onlystarted to form duringthe secondweek of culture.Therefore, theadditionofkinetinisrequiredtoexertadditionalphysiological effect. The optimum treatment for callus induction in this study was identified in MS medium supplemented with 1.0 mg/L 2,4-D and 1.5 mg/L kinetin. The findings revealed that the supplementation of kinetin at an optimum concentration and combination with 2,4-D is required to produce calli with the desirable morphology.Thehormonalcombinationof2,4-Dandkinetinwas previously found to be effective in producing optimum callus induction in Aquilaria malaccensis Lam, in which 70%–73% of callus induction was recorded[12].
From the preliminary study conducted previously, the supplementation of singly present kinetin in the culture media did not promote callus induction and was not useful in promoting the formation of profuse calli (data not shown). However, the results from this study had shown that the addition of kinetin in the culture media in combination with 2,4-D was fruitful in producing callus. This is in contrast to Rashid et al. in which they found that the addition of kinetin affected the callus formation negatively in Triticum aestivum [13]. By looking at the trend of callus formation in this study, an increasing induction percentage was noted associated with an increase in kinetin concentration supplemented together with 2,4-D hormone at the concentration of 1.0 mg/L and 1.5 mg/L. In addition, the positive effect was also noted especially in the media with 1.0 mg/L 2,4-D whereby the morphogenic responses were identified to be improved as the friability was enhanced with an increase in the concentration of kinetin (Table 1). According to the statistical analysis conducted, hormonal combinations do have significant effect towards the formation of callus in this study whereby the value of P is<0.05.
4.2. Effects of culture media on callus induction
The nutrition components in each medium do affect the callus induction potential hence the morphology of calli formed. Obviously observed in the present study, the friability status of the calli formed was completely different in which all the explants cultured on WPM medium produced compact and nodular calli except those cultured on 2.5 mg/L 2,4-D while some treatments used MS medium produced friable calli. Apart from that, it was clearly observed that the calli isolated from WPM culture media were smaller and less profuse than those cultured on MS media (Table 2). Similarly, considering the effects of using WPM medium, a study on plant regeneration in bay leaf tree (Cinnamomum tamala) by Sharma and Nautiyal which used WPM as their sole culture medium reported that almost all explants and plant growth regulator treatments produced compact callus in their study [14].
The impact of culture media towards callus formation and consistency was also reported previously by Avil´es et al. following their studies in Juglans regia L. in which they testified that calli produced from the explants cultured on MS medium were more friable and had less browning effects [15]. On theother hand, those cultured on WPM medium were more likely to appear nodular. Nevertheless, no generalization could be made in terms of plant response towards culture media factor since morphogenic capacity of the induced calli may vary depending on the species [15]. In contrast with the study by Behbahani et al. [16], the greatest callus induction percentage (90%) in B. racemosa from leaf explants incubated at 25°C under dark condition was obtained from the treatment of 2.0 mg/L 2,4-D in WPM medium and they found that the scores were relatively lower in MS medium (13%–48%). Therefore, it has been verified that the contents of the nutritive media influence the morphogenic responses of the species being cultured and the explants used as well.
In terms of time taken for the callus to be induced, generally WPM medium exerted faster induction response in which 11 out of 13 treatments induced the formation of callus as early as during first week of culture (Table 2). In contrast to MS medium, the treatment consisted of 2,4-D hormone alone at the concentration of 1.0–2.0 mg/L resulted in a delayed response in the onset of callus formation (Table 1). The superiority of WPM medium in producing earlier callus induction response was previously reported by Behbahani et al. as well wherein it took three weeks for the callus to be produced in leaf explants of B. racemosa as compared to other media which required five weeks [16]. On the contrary, Avil´es et al. reported that callogenesis in Juglans regia L. (walnut) started a week earlier in MS medium than in WPM medium [15]. Upon statistical analysis, in terms of callus induction percentage, the effect of different culture media was found to be statistically insignificant with the value of P>0.05. Nevertheless, the difference in culture media exerted significant effects towards callus morphology and fresh weight (P<0.05).
More further studies are potentially conducted to examine the impacts of medium components towards callus morphogenic responses since there are some great differences in the content of macronutrients and micronutrients available in different basal culture media. For instance, there are a great amount of potassium nitrate (1900 mg/L) found in MS medium while the component is absent in WPM medium. In addition to that, there are nutrients whichareavailableatarelativelyhigheramountthanthoseinWPM medium and vice versa. Ammonium nitrate and calcium chloride forinstancearefoundmuchhigherin MSmedium(1650mg/Land 440 mg/L respectively) than in WPM medium (400 mg/L and 96mg/Lrespectively).Similarly,therearesomenutrientswhichare onlyfoundin WPMmediumwhiletheyarelackingin MSmedium such as calcium nitrate, potassium sulfate and mangan sulfate[17]. The differences in the compositions of such nutrients are likely to be the factor which influence the responses of explants of each plant species towards the formation of callus.
Theeffectsofplantgrowthregulatorsandculturemediaonthe induction and formation of calli from endosperm of B. racemosa had been elucidated from this study. The findings gathered are useful for production of calli, which is required for plant regeneration studies, somatic embryogenesis or it may function as a starting point for establishing cell suspension cultures, plant bioreactor and bioactive compounds studies in the species.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to express gratitude to Ministry of Education under Higher Education Department for the funding of the project through Fundamental Research Grant Scheme (FRGS) with the grant number of FRGS/1/2012/STW/N03/ UITM/02/01 facilitated by Research Management Centre, Universiti Teknologi MARA.
References
[1] Deraniyagala SA, Ratnasooriya WD, Goonasekara CL. Antinociceptive effect and toxicological study of the aqueous bark extract of Barringtonia racemosa on rats. J Ethnopharmacol 2003; 86(1): 21-6.
[2] Kong KW, Mat-Junit S, Aminuddin N, Ismail A, Abdul-Aziz A. Antioxidant activities and polyphenolics from the shoots of Barringtonia racemosa (L.) spreng in a polar to apolar medium system. Food Chem 2012; 134(1): 324-32.
[3] Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S. Agroforestree database: a tree reference and selection guide version 4.0. Nairobi: World Agroforestry Centre; 2009. [Online] Available from: http://worldagroforestry.org/treedb2/AFTPDFS/Rauvolfia_ vomitoria.PDF [Accessed on 5th January, 2014]
[4] Osman NI, Sidik NJ, Awal A. Pharmacological activities of Barringtonia racemosa L. (Putat), a tropical medicinal plant species. J Pharm Sci Res 2015; 7(4): 185-8.
[5] Patil KR, Patil CR, Jadhav RB, Mahajan VK, Patil PR, Gaikwad PS. Anti-arthritic activity of bartogenic acid isolated from fruits of Barringtonia racemosa Roxb. (Lecythidaceae). Evid Based Complement Altern Med 2011; 2011: 785245.
[6] Thomas TJ, Panikkar B, Subramoniam A, Nair MK, Panikkar KR. Antitumour property and toxicity of Barringtonia racemosa Roxb. seed extract in mice. J Ethnopharmacol 2002; 82(2–3): 223-7.
[7] Ong HC, Nordiana M. Malay ethno-medico botany in Machang, Kelantan, Malaysia. Fitoterapia 1999; 70(5): 502-13.
[8] Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell 2013; 25(9): 3159-73.
[9] Osman NI, Awal A, Sidik NJ, Abdullah S. Callus induction and somatic embryogenesis from leaf and nodal explants of Lycium barbarum L. (Goji). Biotechnology 2013; 12(1): 36-45.
[10] Staba EJ. Plant tissue culture as a source of biochemicals. Florida: CRC Press; 1980.
[11] Sauer M, Robert S, Klein-Vehn J. Auxin: simply complicated. J Exp Bot 2013; 64(9): 2565-77.
[12] Saikia M, Shrivastava K, Singh SS. Effect of culture media and growth hormones on callus induction in Aquilaria malaccensis Lam., a medicinally and commercially important tree species of North East India. Asian J Biol Sci 2013; 6(2): 96-105.
[13] Rashid U, Ali S, Ali GM, Ayub N, Masood MS. Establishment of an efficient callus induction and plant regeneration system in Pakistani wheat (Triticum aestivum) cultivars. Electron J Biotechnol 2009; 12(3): 4-5.
[14] Sharma G, Nautiyal AR. Influence of explants type and plant growth regulators on in vitro multiple shoots regeneration of a laurel from Himalaya. Nat Sci 2009; 7(9): 1-7.
[15] Avil´es F, Rios D, Gonz´alez R, S´anchez-Olate M. Effect of culture medium in callogenesis from adult walnut leaves (Juglans regia L.). Chil J Agric Res 2009; 69(3): 460-7.
[16] Behbahani M, Shanehsazzadeh M, Hessami MJ. Optimization of callus and cell suspension cultures of Barringtonia racmosa (Lecythidaceae family) for lycopene production. Sci Agric 2011; 68(1): 69-76.
[17] Saad AIM, Elshahed AM. Plant tissue culture media. In: Leva A, Rinaldi LMR, editors. Recent advances in plant in vitro culture. Winchester: InTech; 2012.
*Corresponding author:Nurul Izzati Osman, Faculty of Applied Sciences Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia.
 Asian Pacific Journal of Tropical Biomedicine2016年2期
Asian Pacific Journal of Tropical Biomedicine2016年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Prevalence of refractive errors among primary school children in a tropical area, Southeastern Iran
- Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
