Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
Nastaran Mohseni, Fatemeh Salehi Sarvestani, Mehdi Shafiee Ardestani, Fatemeh Kazemi-Lomedasht,Masoud Ghorbani,*Biotechnology Research Center, Biotechnology Department, Venom and Biotherapeutics Molecules Laboratory, PasteurInstitute of Iran, Tehran, IranDepartment of Biology, Science and Research Branch, Islamic Azad University, Tehran, IranDepartment of Radiopharmacy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, IranResearch and Development Department, Pasteur Institute of Iran, Tehran, IranDepartment of Virology, University of Ottawa, Ottawa, Canada
Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
Nastaran Mohseni1, Fatemeh Salehi Sarvestani2, Mehdi Shafiee Ardestani3, Fatemeh Kazemi-Lomedasht1,
Masoud Ghorbani4,5*1Biotechnology Research Center, Biotechnology Department, Venom and Biotherapeutics Molecules Laboratory, Pasteur
Institute of Iran, Tehran, Iran
2Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
3Department of Radiopharmacy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
4Research and Development Department, Pasteur Institute of Iran, Tehran, Iran
5Department of Virology, University of Ottawa, Ottawa, Canada
Original article http://dx.doi.org/10.1016/j.apjtb.2015.10.014
Tel: +98 9102104228
E-mail: Mghorbani2000@yahoo.com
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
Foundation Project: Supported by a grant from Iran National Science Foundation under the grant number 91002993.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 24 Jun 2015
Received in revised form 6 Aug, 2nd revised form 10 Aug, 3rd revised form 17 Aug 2015
Accepted 5 Oct 2015
Available online 19 Dec 2015
Keywords:
Gold nanorods
Breast cancer cell line Interferon gamma
Near-infrared laser
ABSTRACT
Objective: To develop a gold nanoparticles complex conjugated with interferon-gamma (IFN-γ) and methionine along with application of hyperthermia using near-infrared laser beams for the treatment of cancer cells.
Methods: Gold nanorods (10 nm) were conjugated with IFN-γ and methionine using carbodiimide family and characterized after purification by dialysis bags. Breast cancer cells were cultured and incubated with gold nanorods at different concentrations followed by irradiation with near-infrared laser beam. Samples were then evaluated for their viability in order to determine the effect of treatment and variables by MTT assy.
Results: Zetasizer results confirmed the conjugation of gold nanorods with methionine and IFN-γ. The median percentage of cell viability in 0.30 μg/mL concentration of gold nanorods was 82%. The cell viability reached to 85% at the same concentration of gold nanorods, which existed in the assayed complex. The results of MTT assay showed that the 0.60 μg/mL concentration of gold nanoparticles complex was toxic on tumor cells (P<0.05). After exposure to hyperthermia, the viability of cells at 6 min decreased to 77% in 0.30 μg/mL concentration of gold nanorods complex.
Conclusions: The size and concentration of gold nanorods was not cytotoxic. However, their presence during irradiation near-infrared laser increased the number of dead cells during the treatment of cells.
1. Introduction
Cancer is a leading cause of disease worldwide[1]. Cancer is a potentially fatal disease caused mainly by environmental factors that mutate genes encoding critical cell regulatory proteins. The resultant aberrant cell behavior leads to expansive masses of abnormal cells that destroy surrounding normal tissue that can spread to vital organs resulting in disseminated disease, commonly a harbinger of imminent patient death [2]. An estimated 12.7 million new cancer cases occurred in 2008. Lung, female breast, colorectal and stomach cancers accounted for 40% of all cases diagnosed worldwide. In men, lung cancer was the most common cancer (16.5% of all new cases in men). Breast cancer was by far the most common cancer diagnosed in women (23% of all new cases in women)[1].
Current cancer therapy usually involves intrusive processes including application of catheters to allow chemotherapy, initial chemotherapy to shrink any cancer present, surgery to then remove the tumor(s) if possible, followed by more chemotherapy and radiation. The purpose of the chemotherapy andradiation is to kill the tumor cells as these cells are more susceptible to the actions of these drugs and methods because of their growth at a much faster rate than healthy cells, at least in adults. Current research areas include the development of carriers to allow alternative dosing routes, new therapeutic targets such as blood vessels fueling tumor growth and targeted therapeutics that are more specific in their activity. Clinical trials have shown that patients are open to new therapeutic options and the goal of these new chemotherapeutics is to increase survival time and the quality of life for cancer patients. In all cases, the effectiveness of the treatment is directly related to the treatment's ability to target and to kill the cancer cells while affecting as few healthy cells as possible[3]. Today, nanotechnology can help the diagnosis and treatment of cancer cells by detecting and destroying them in nanometer dimensions [4].
The use of nanoparticles as a drug for the treatment of malignant cells does not have any adverse effect on healthy cells and tissues[5]. Among the applications of nanoparticles, treatment of malignant tumors by thermotherapy is more common. Gold nanostructures have been extensively studied in a variety of applications due to their interesting electrical, conductive and optical properties. Among these nanostructures, spherical gold nanoparticles during the last 20 years have been used to research photothermal cancer therapies. The other types of gold nanoparticles can be noted gold nanorods[6].
By deformation of gold nanospheres to cylindrical nanotubes, theycanrecognizeanddestroythemalignanttumorsinthedeeper parts of the skin condition that is observed in breast cancer by selectively applying a beam of laser with half of the power and it was used previously without destroying healthy cells. When gold was attached to the cancer cells, it would reflect light and distinguish cancer cells from healthy cells easily. Gold nanoparticles can also absorb the laser beam very easily and thus malignantcellscoatedwiththisparticlewillbedestroyedquickly. By deformation of the gold nanoparticles from sphere shape to rod-shape, we can use optical spectra at lower frequency. In other words, near-infrared light can be used instead of visible light and it is commonly used for gold nanospheres[7,8].
Hyperthermia or thermotherapy is a type of cancer treatment in which body tissue is exposed to temperatures above 45°C, usually with minimal injury to normal tissue [9]. This treatment is almost always used with other forms of cancer therapy, such as radiation therapy and chemotherapy[9,10]. Hyperthermia may make some cancer cells more sensitive to radiation or harm other cancer cells that radiation cannot damage. Most normal tissues are not damaged during hyperthermia if the temperature remains under 45°C[9,11,12].
In this method of treatment, different ways are being used to create heat including the use of laser irradiation. In the meantime, near-infrared laser has more applications. In this study, we attempted to evaluate the treatment of human breast cancer cell line (MCF-7) with conjugated gold nanorods. In this study, the cancer cells death rate was compared with the survival rate of normal healthy cells in the presence of hyperthermia induced by near-infrared laser beam in the presence of gold nanoparticles.
2. Materials and methods
2.1. The proliferation of cell line and culture conditions
MCF-7 cells were cultured in a 25 T-flask in medium containing Dulbecco's modified Eagle's medium, 10% fetal calf serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C with 5% CO2, 95% air and complete humidity. Once reached~90% confluency, cells were detached using 0.05% trypsin/ethylene diamine tetraacetic acid and counted using trypan blueon ahemocytometer slide under an inverted microscope.
2.2. Conjugation of gold nanorods with interferon-gamma (IFN-γ)
For conjugation of gold nanorods and IFN-γ, diethylene triamine pentacetic acid was used as a linker. To perform the reaction, 0.1 mg diethylene triamine pentacetic acid was initially mixed with 0.5 mL sterile poly butylenes succinate (PBS) and the content was added to 0.5 mL PBS containing 50 μg IFN-γ and vortexed for 10 min at room temperature. The mixture was then kept in the refrigerator to rest for 24 h. This rest can help to stabilize the complex. On the next day, 3 mg 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide was mixed with 1.5 mg methionine in 0.2 mL PBS. The content was then added to 0.5 mL PBS containing 10 μg nanorods and mixed with 2 mL syringe. The complex of nanorods, methionine and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide was then added to our complex of diethylene triamine pentacetic acid and IFN-γ from the previous day and mixed by vortexing for 20 min at room temperature. The final product was purified using a dialysis bag (Sigma, Canada) with a cut off of 1 kDa. The final complex was kept at 4°C until further use. The structure of conjugate was then confirmed by UV spectroscopy. The size and charge of gold nanorods were measured before and after conjugation by a zetasizer machine (Zetasizer Nano S90, Malvern Instruments Ltd., UK). The size and the charge of the complex were expected to be increased after conjugation.
Cells were treated with various concentrations of unconjugated (gold nanorods) and conjugated gold nanorods (the mixture of gold nanorods, IFN-γ and methionine).
2.3. Test groups
To evaluate the effects of conjugated gold nanorods on MCF-7 cell line, cells were incubated at a concentration of 2×104cell/well on 96 well plates[13]. Cells on each plate were divided into 3 groups and repeated 6 times per group.
Group 1 contained six different concentrations of conjugated complex including 0.08, 0.16, 0.30, 0.60, 1.20 and 2.50 μg/mL gold nanorods. Group 2 contained six different concentrations including 0.3, 0.6, 1.2, 2.5, 5.0, and 10.0 μg/mL of unconjugated gold nanorods. Group 3 contained four different concentrations of IFN-γ at 12.5, 25.0, 50.0, and 100.0 μg/mL. Briefly, the culture media was removed, 50 μL of each concentration was added to each well and cell was incubated for 24 h followed by MTT assay to measure the viability of cells.
2.4. Hyperthermia test
At first, the cells were exposed to near-infrared laser light at a wavelength of 980 nm (980 nm Infrared Laser Diode Module, China)foraperiodof1–6min.Allcellswereexpectedtosurviveat this stage of experiment. Twenty-four hours after treating cells with conjugated complex or gold nanorods alone, the medium of all plates including the treated and untreated cells was replaced withfreshmediumandthecellswereexposedtonear-infraredlaser light for a period of 1–6 min. Each test was repeated three times.
2.5. MTT assay for evaluating cell viability
Cell survival rate and cell proliferation were evaluated based on the ability of mitochondria to convert MTT to formazan crystalswithapalebluecolor.Briefly,10μLof MTTplus100μL of RPMI 1640 were added to each well, except the cell-free blank wells.Cellswereincubatedfor4hat37°Cwith5%CO2,95%air andcompletehumidity.After 4hofincubation,the MTTsolution was removed and replaced with 50 μL of dimethylsulfoxide and the plates were further incubated for 20 min at 37°C, and the optical density of the wells was determined using a Bio-Rad plate reader machine at a test wavelength of 570 nm and a reference wavelength of 630 nm[14].
2.6. Statistical analysis
To determine the effect of treatment in different groups, all data were collected and analyzed using a t-test of SPSS 17.0 software and the charts were drew in Excel 2007.
3. Results
3.1. Confirmation of the complex structure
3.1.1. UV spectrophotometry
To confirm the structure of conjugated and unconjugated gold nanorods, a UV spectrophotometer was used which indicated a peak absorbance at 520 nm wavelength corresponding to gold nanorods alone (Figure 1, red peak). IFN-γ, however, created peak absorption at 240 nm wavelength (Figure 1, blue peak). After conjugation of gold nanorods with IFN-γ, two different peaks were appeared at 240 nm and 520 nm wavelength respectively, confirming the attachment of IFN-γ to gold nanoparticles (Figure 1, green peak).
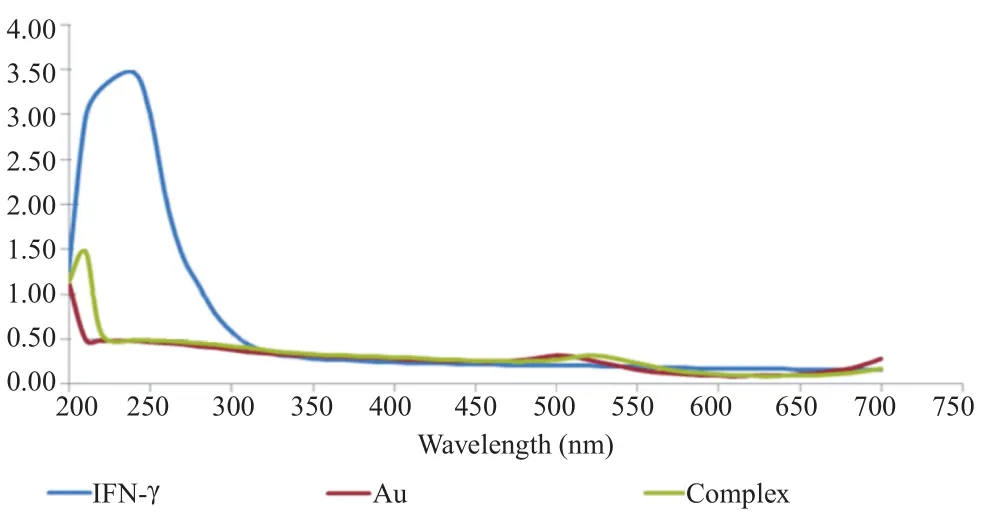
Figure 1. UV spectrophotometry of the conjugated and unconjugated gold nanorods.
3.1.2. Measurement of size and charge of the complex by a Zetasizer machine
IFN-γ was negatively charged due to its carboxylic group that was detected using a Zetasizer machine (Figure 2A). After conjugation of IFN-γ with gold nanorods, we expected an exchange of electrons with the positively charged gold nanorods that were detected with the Zetasizer machine (Figure 2B). In the mean time, the size of the complex including gold nanorods and IFN-γ was increased to 42.9 nm whereas the size of gold nanorods alone was 10 nm confirming the attachment of the protein to gold nanorods (Figure 2C). As it was expected, the size and charge of complex was increased after conjugation.
Figure 2. Measurement of the complex's size and charge by Zetasizer machine.
3.2. MTT assay on kidney adenocarcinoma of hamster (BSR) cell
The MTT was performed to evaluate the toxicity effect of pure gold nanorods on normal BSR cells. Data were obtained at a wavelength of 570 nm with a reference wavelength of 630 nm and evaluated using SPSS software. Cells were first treated with different concentrations of gold nanorods for 24 h. MTT assay results revealed that gold nanorods were significantly toxic to the cells at the concentration of 2.50 μg/mL and higher. Therefore, all none toxic concentrations lower than 2.50 μg/mL were used for the rest of the experiments. The complex was prepared based on different concentrations of gold nanorods and 5.00 μg/mL IFN-γ. Only at the highest concentration of 2.50 μg/mL, the complex showed toxicity to normal cells (Figure 3).
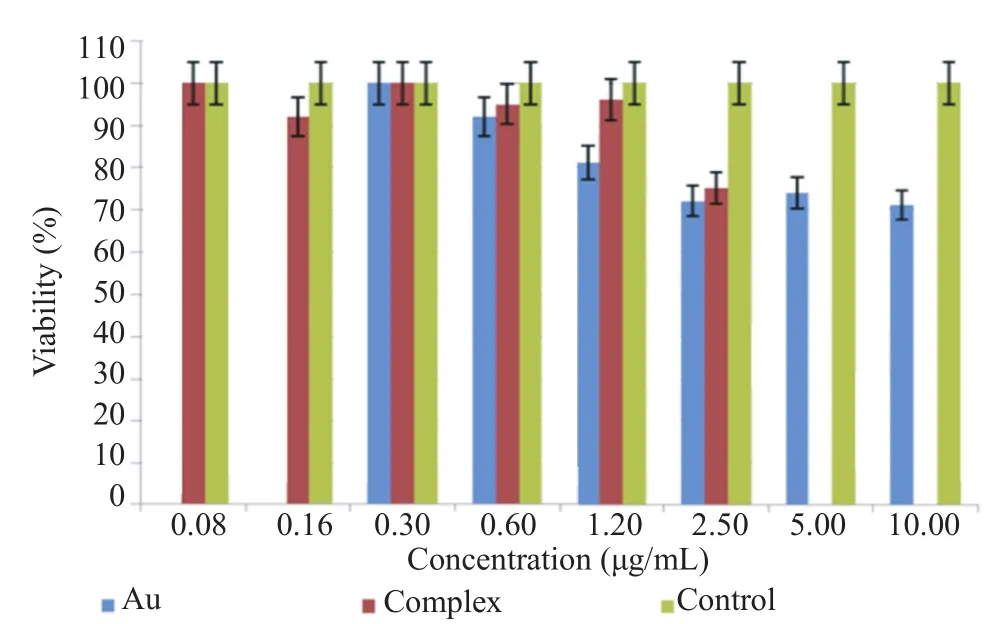
Figure 3. MTT assay evaluations on viability BSR cell line.
3.3. Effects of conjugated and unconjugated gold nanorods on MCF-7 cancer cells
As compared to normal BSR cells, unconjugated gold nanorods demonstrated similar effects at the concentration of 10.00, 5.00 and 2.50 μg/mL, and two lower concentrations (1.20 and 0.60 μg/mL) had toxicity effect on MCF-7 cells and only 0.30 μg/mL concentration which was its lowest had no toxicity effect on MCF-7 cells. Interestingly, nontoxic concentrations of unconjugated gold nanorods were significantly toxic to MCF-7 cells when conjugated with IFN-γ at 0.60 and 1.20 and 2.50 μg/mL. The concentration of 0.30 μg/mL had no toxicity effect on MCF-7 cells (Figure 4).
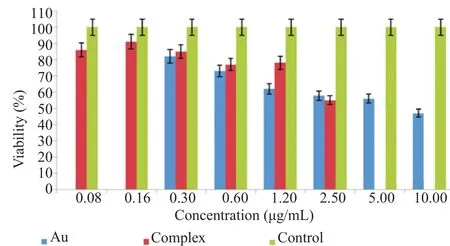
Figure 4. MTT assay evaluations on viability of MCF-7 cell line.
3.4. MTT assay of IFN-γ on BSR and MCF-7
Asseeninthe Figure5,theeffectof IFN-γonnormalcellswas similar to cancer. It means that IFN-γ in higher concentration had a low toxicity effect on cells and at lower concentrations it didn't show any toxicity.
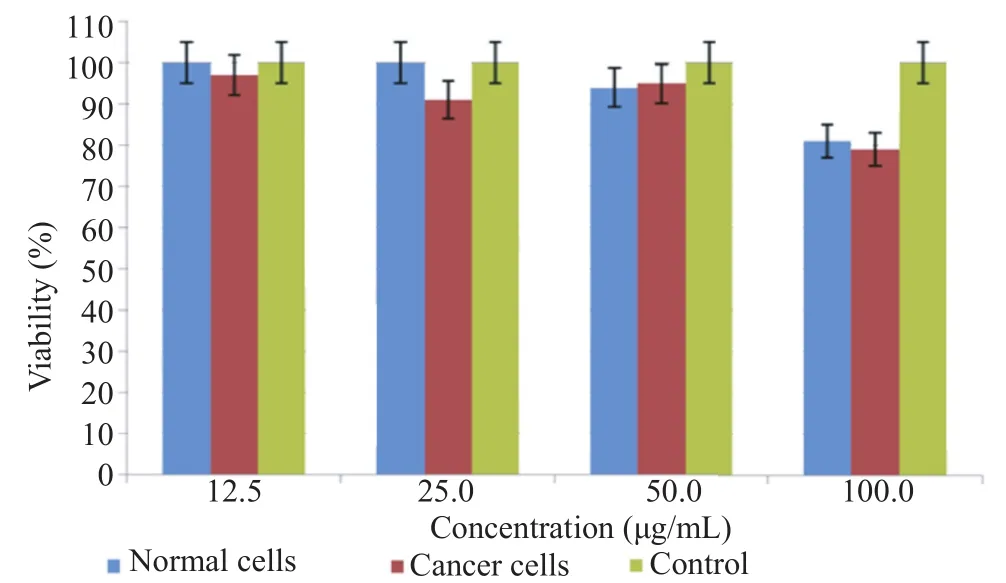
Figure 5. MTT assay of IFN-γ on BSR and MCF-7.
3.5. Hyperthermia tested on MCF-7 cell line
Single cells irradiated with laser beams for a period of 1–6 min in the absence of any material survived significantly (Figure 6).
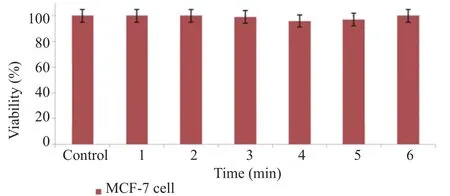
Figure 6. Effects of laser irradiation on MCF-7 cancer cells.
3.6. Laser irradiation on cells in the presence of gold nanorods at different time periods
Exposure of cells to laser irradiation in the presence of toxic and nontoxic concentration of gold nanorods alone had no effect on cell viability on cancer cells, indicating that the particles remained unattached to the cells because the gold nanorods cannot bind to cells specifically and singly (Figures 7 and 8). When the concentration was 2.5, 1.2, 0.6 and 0.3 μg/ mL, the viability was 54%, 78%, 76% and 85%, respectively (Figure 7). When the concentration was 2.5, 1.2, 0.6 and 0.3 μg/mL, the viability was 72%, 79%, 68% and 77%, respectively (Figure 8).

Figure 7. Cancer cell at different concentrations of complex before laser irradiation.

Figure 8. Cancer cell at different concentration of complex after 6 min laser irradiation.
3.7. Laser irradiation on cells in the presence of the complex at different time periods
Irradiation of MCF-7 cells was in the presence of the complex at 0.3 μg/mL concentration and higher, however, the death of cell increased after 6 min of exposure. This phenomenon could be due to the presence of methionine in the complex thatwas in favor of MCF-7 cells to use and caused the attachment of the complex to the cells. Uptake of methionine by cancerous cells was more than normal cells therefore the cells absorbed more complex and the laser beams were more effective on them. As a result, the heat generated by gold nanorods in the course of laser irradiation could kill the cells (Figure 9).
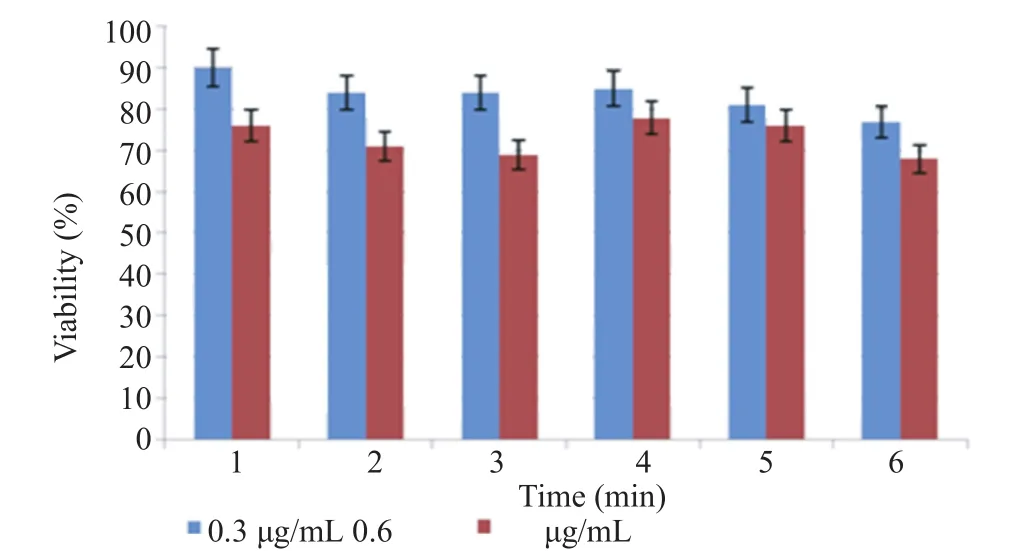
Figure 9. Laser irradiation on cells in the presence of the complex at different time periods.
4. Discussion
Overall, this study can be a main consideration in order to discuss the results including toxic or nontoxic effects of gold nanorods on MCF-7 cell line. Effects of nanoparticles to induce lethal hyperthermia after laser radiation are synergistic to the effect of gold nanorods conjugated with methionin and IFN-γ. Over 80% of MCF-7 cells in the presence of gold nanorods complex have maintained their survival and the lack of toxicity of gold nanorods was confirmed by measuring the size and concentrations (10 nm in size and concentration 0.3 μg/mL). In other words, the size and concentration of nanoparticles didn't induce cytotoxicity in MCF-7 cell line. The reports by Gomaa and colleagues [15] and Cho et al. [16] showed that if the appropriate size and concentration of gold nanoparticles to be used, it would have no toxic effect on the cells. Cho et al. [16] showed that the use of gold nanorods with a size of 10 nm could have no toxic effect on cells, whereas, due to their specific shape they absorb more laser light and induce heat to destroy cancer cells. It has also been reported that the deformation of the spheres to rod-shaped gold nanoparticles can be replaced by the visible light spectrum that would be used for gold nanosphere using near-infrared spectroscopy [7]. The cell death induced by laser irradiation in the presence of nanoparticles exacerbates hyperthermia and increases cellular death. In the mean time, the chemicals released by the immune system play an important role in eliminating and destroying cancer cells [17]. It has been known that IFN-γ shows anti-proliferative effects on large numbers of tumor cells with IFN-γ receptor and induces apoptosis in these cells [18]. The results of this study showed that IFN-γ used in the concentrations was not toxic to cells. The presence of nanorods during irradiation by near-infrared laser increased the number of dead cells during this process. Gold nanorods were not able to attach to the cancer cells alone. The presence of methionin in the complex induced the adsorption of the complex to the cancer cell and due to the presence of gold nanorods, it could absorb laser photons from the laser beam which would be converted to heat and thus raise the temperature of the cells resulting in destruction of viable cells. These results indicated a new promising approach for treatment of cancer cells.
The laser used in this study was in the form of IR and at the wavelength of 980 nm with a power of 37 W/cm2and exposure time of 1–5 min did not kill any cells. Whereas, exposing cells to the laser for 6 min at a concentration of 0.3 μg/mL of the complex destroyed them. Because of the annexing methionine to the nanorods, it facilitates the attachment of the complex to the cells which in turn produces heat after exposure to laser beams and brings about a marked cell death. Perhaps, by increasing the duration of laser beam exposure a larger population of tumor cells will be destroyed. Researchers in other studies have demonstrated the cell death after using laser beam at a wavelength of 820 nm.
Rod-shaped gold nanoparticles allow us to use optical spectra with lower frequency than visible light spectrum. In other words, instead of using light spectrum that is normally used for gold nanospheres, we can use infrared rays. Moreover, the elongated shape of gold nanorods can possibly absorb more energy and convert it to heat to destroy malignant cells in deeper tissues. The results of this study can be promising for the development of novel anti-cancer treatment in future studies.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was financially supported by Pasteur Institute of Iran, Tehran, Iran. This work has been supported by a grant from Iran National Science Foundation under the grant number 91002993.
References
[1] Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5): E359-86.
[2] Tan BK, Alitheen NB, Yeap SK, Ali AM, Mawardi R. Cytotoxic effect of 2ʹ, 3ʹ-epoxy isocapnolactone and 8-hydroxyisocapnolactone-2ʹ3ʹ-diol isolated from Micromelum minutum (G.Forst.) Wight and Arn. in human T-lymphocyte leukemia CEM-SS cells. Afr J Biotechnol 2009; 8: 4632-41.
[3] Tao W, Zhang J, Zeng X, Liu D, Liu G, Zhu X, et al. Pharmaceutical nanotechnology: blended nanoparticle system based on miscible structurally similar polymers: a safe, simple, targeted, and surprisingly high efficiency vehicle for cancer therapy (Adv. Healthcare Mater. 8/2015). Adv Healthc Mater 2015; 4(8): 1260.
[4] Telang C, Otanicar T, Dai L, Phelan P, Swaminathan R, Zhang M. Controllable optical properties of polystyrene/PNIPAM-gold composite nanoparticles. Plasmonics 2015; 10(1): 17-25.
[5] Reznikov AG, Salivonyk OA, Sotkis AG, Shuba YM. Assessment of gold nanoparticle effect on prostate cancer LNCaP cells. Exp Oncol 2015; 37(2): 100-4.
[6] Castro CM, Im H, Lee H, Weissleder R. Nanotechnology approaches for intraprocedural molecular diagnostics. In: Fong Y, Giulianotti PC, Lewis J, Koerkamp BG, Reiner T, editors. Imaging and visualization in the modern operating room. New York: Springer; 2015, p. 157-66.
[7] Li W, Rong P, Yang K, Huang P, Sun K, Chen X. Semimetal nanomaterials of antimony as highly efficient agent for photoacoustic imaging and photothermal therapy. Biomaterials 2015; 45: 18-26.
[8] Sharma H, Mishra PK, Talegaonkar S, Vaidya B. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov Today 2015; 20: 1143-51.
[9] Su R, Guo G, Zhang Q, Li C, Gan Y. Design and simulation of coil system in thermotherapy. In: Magnetic Particle Imaging (IWMPI), 2015 5th International Workshop on; 2015 Mar 26–28; Istanbul. New York: IEEE; 2015.
[10] Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperth 2015; 31: 609-14.
[11] Man J, Shoemake JD, Ma T, Rizzo AE, Godley AR, Wu Q, et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res 2015; 75(8): 1760-9.
[12] Leggio L, de Varona O, Dadrasnia E. A comparison between different schemes of microwave cancer hyperthermia treatment by means of left-handed metamaterial lenses. Prog Electromagn Res 2015; 150: 73-87.
[13] Wang YJ, Zhou SM, Xu G, Gao YQ. Interference of phenylethanoid glycosides from Cistanche tubulosa with the MTT assay. Molecules 2015; 20(5): 8060-71.
[14] Begum Z, Kumar N. Evaluation of cytotoxic effect of Punica granatum L. var spinosa extracts on malignant cell line MCF-7 in vitro. Mol Enzymol Drug Targets 2015; 1(1): 4.
[15] Gomaa IE, Gaber SAA, Bhatt S, Liehr T, Glei M, El-Tayeb TA, et al. In vitro cytotoxicity and genotoxicity studies of gold nanoparticles-mediated photo-thermal therapy versus 5-fluorouracil. J Nanopart Res 2015; 17: 102.
[16] Cho WK, Kang S, Choi H, Rho CR. Topically administered gold nanoparticles inhibit experimental corneal neovascularization in mice. Cornea 2015; 34(4): 456-9.
[17] Hao K, Yan Z, Shuang Y, Sun J, Tao S, Fu W, et al. Association between interferon gamma 13-CA-repeats polymorphism and metastasis of nasopharyngeal carcinoma in a population of Northern China. Int J Clin Exp Pathol 2015; 8(6): 7409-14.
[18] Tong R, Langer R. Nanomedicines targeting the tumor microenvironment. Cancer J 2015; 21(4): 314-21.
*Corresponding author:Masoud Ghorbani, Research and Development Department, Pasteur Institute of Iran, Tehran, Iran.
 Asian Pacific Journal of Tropical Biomedicine2016年2期
Asian Pacific Journal of Tropical Biomedicine2016年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Prevalence of refractive errors among primary school children in a tropical area, Southeastern Iran
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
- Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus)
