Sulforaphene and sulforaphane in commonly consumed cruciferous plants contributed to antiproliferation in HCT116 colon cancer cells
Piman Pocasap, Natthida Weerapreeyakul*Graduate School, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, 4000, ThailandFaculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, 4000, Thailand
Sulforaphene and sulforaphane in commonly consumed cruciferous plants contributed to antiproliferation in HCT116 colon cancer cells
Piman Pocasap1, Natthida Weerapreeyakul2*1Graduate School, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, 40002, Thailand
2Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, 40002, Thailand
Original article http://dx.doi.org/10.1016/j.apjtb.2015.11.003
Tel: +66 43 202 378
Fax: +66 43 202 379
E-mail: natthida@kku.ac.th
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
Foundation Project: Supported by Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster and Research and Development of Herbal Nutraceutics Subcluster of Khon Kaen University (F-2553-M-11 and NRU541051).
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 21 Sep 2015
Received in revised form 16 Oct, 2nd revisedform21 Oct,3rdrevisedform
26 Oct, 4th revised form 2 Nov 2015
Accepted 15 Nov 2015
Available online 19 Dec 2015
Keywords:
Cruciferous
Cancer
Isothiocyanates
Sulforaphane
Sulforaphene
Chemoprevention
ABSTRACT
Objective: To analyze two isothiocyanates (sulforaphene and sulforaphane) and their antiproliferative effect of 11 indigenous cruciferous vegetables.
Methods: Phytoconstituents identification was conducted by high performance liquid chromatography and gas chromatography-mass spectrometer techniques. The antiproliferation was evaluated in colon cancer cell line HCT116 by MTT assay.
Results: Isothiocyanate identification by high performance liquid chromatography showed that broccoli, cabbage,“Khi-Hood”(Raphanus sativus L. var. caudatus Alef) and Chinese radish contained isothiocyanates sulforaphane. Sulforaphene and sulforaphane in broccoli, cabbage and“Khi-Hood”were characterized by the gas chromatography-mass spectrometer analysis. Antiproliferation screening by MTT assay found that the potent plants which possessed IC50below 50 μg/mL were cabbage and“Khi-Hood”, while the others had low antiproliferation with IC50higher than 50 μg/mL. Difference in antiproliferation was probably due to difference existed phytochemical constituents in each plant.“Khi-Hood”possessed the highest antiproliferation against HCT116 with the lowest IC50at (9.42±0.46)μg/mL. The IC50of chemotherapeutic drug (mitomycin C) was (19.12±1.00)μg/mL, while both melphalan and 5-fluorouracil possessed the IC50value higher than 50 μg/mL.
Conclusions: Commonly consumed cruciferous vegetables exerted varied antiproliferation and isothiocyanate contents. High isothiocyanate content in“Khi-Hood”was contributed to high antiproliferation. Among 11 plants studied,“Khi-Hood”could be an alternative chemopreventive diet.
1. Introduction
In the present, many epidemiological studies have shown the associationbetweencruciferousvegetablesconsumptionandlower riskofseveralcancerincidencessuchaslungandcolorectalcancer [1]. Therefore, many researchers have focused on finding an active compound in cruciferous crops, plant of the family Brassicaceae (also called Cruciferae), as a potential chemopreventive source. The bioactive compound in cruciferous plants associated with chemopreventive property is isothiocyanates[2].
Isothiocyanates compounds have been studied intensively for its anticancer property. The compounds have not only shown chemopreventive property but also chemotherapeutic property in both in vitro and in vivo studies [3]. These properties make isothiocyanates to be a good anticancer candidate. Many studies have been performed to identify and quantify isothiocyanates in cruciferous plants. High amount of isothiocyanates, such as sulforaphane, phenethyl isothiocyanates and allyl isothiocyanate, were found in broccoli, cabbage andcauliflower [4]. Hence, these plants have been recognized as a source of isothiocyanates as well as a source of anticancer agent.
Due to the limited information of the isothiocyanates in indigenous Thai cruciferous vegetables, this study aims to identify sulforaphane, as the representative of isothiocyanates, in these plants as well as corresponding antiproliferation indicating anticancer potential of the extracts from the indigenous Thai cruciferous vegetables in colon cancer cell line HCT116. Consequently, this study provides the useful evidence for supporting the use of Thai indigenous cruciferous vegetables as a source of sulforaphane and a source of anticancer agent.
2. Materials and methods
2.1. Materials
D,L-Sulforaphane standard was purchased from Calbiochem (EMD, Darmstadt, Germany). L-Sulforaphene standard was purchased from Enzo Life Science (New York, USA). Dichloromethane (Fisher Scientific, UK) was analytical grade and acetonitrile was high performance liquid chromatography (HPLC) grade (RCI Labscan, Thailand).
2.2. Plant extraction
Cruciferous plants were collected from local market in Khon Kaen and Phayao Province, Thailand, during 2010–2011. The crude extracts were prepared according to the method previously described with slight modification [5]. Each plant was homogenized (50 g fresh weight: 50 mL water) for 30 min and placed at room temperature for 2 h to allow autolysis. The homogenates were extracted with dichloromethane. The dichloromethane layer was collected,filtered and dried by using rotary evaporator. Stock solutions of each plant extracts were prepared in acetonitrile.
2.3. HPLC fingerprints analysis
The cruciferous plant extracts were analyzed by the reversedphase HPLC (Agilent, 1100 Series G1310A, Germany) to determine sulforaphane content following the methods of Campas-Baypoli et al. [6] and Liang et al. [5] with minor modifications. The analysis was carried out with acetonitrile: ultrapure water (30:70, v/v) as a mobile phase with isocratic elution on C18 (25 cm×4.6 mm, 5 μm) column (HiQsil, Japan) at a flow rate of 0.6 mL/min. Detector (Agilent, 1100 Series G1314A, Japan) wavelength was set at 254 nm.
2.4. Gas chromatography-mass spectrometer (GC-MS) operating condition
GC-MS analysis (GC: Agilent, 6890N, China; MS: Agilent, 5973 inert, USA) was performed as previously described to characterize sulforaphane and sulforaphene in cruciferous vegetables[7]. Ultra-high purity helium was used as a carrier gas and the flow rate was set at 40 cm/s. The oven temperature was programmed at 50°C for 5 min, then increasing to 250°C in increment of 10°C/min and remaining steady at 250°C for 10 min, while injector temperature was steady at 250°C. Ion source temperature was set at 180°C and a mass spectrum was obtained by electron ionization at 70 eV.
2.5. Cell culture and antiproliferation test
The human colon cancer (HCT116) cell line was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 IU/mL penicillin and 100 μg/mL streptomycin. Cells were incubated at 37°C with 95% air and 5% CO2. Briefly, cells at a density of 5×103cells/well were plated in 96-well microplates and in 100 μL culture medium. Thereafter, 100 μL of the crude extract in acetonitrile with concentration ranging from 1 to 250 μg/mL was added into each well. The final concentration of acetonitrile in culture medium was maintained to be lesser than 0.2% to avoid solvent toxicity. MTT (Ambresco, USA) solution (in phosphate buffer saline) was added into each wells (final concentration of 0.5 mg/mL) in 22 h and incubated for another 2 h in CO2incubator. Then, the medium was discarded and 150 μL of dimethyl sulfoxide was added to solubilize formazan. Absorbance of formazan product was measured at 570 nm wavelength and percentage of antiproliferation was calculated [8]. The chemotherapeutic drugs which were mitomycin C, melphalan and 5-fluorouracil were used as positive control.
3. Results
A total of 11 local cruciferous plants used in this study were “Chun Chai Hang Hong”[Brassica juncea var. sareptana Sinskaja (B. juncea var. sareptana)],“San”[Brassica juncea var. gracilis (B. juncea var. gracilis)],“Chao Mom”[Brassica juncea (B. juncea)],“Hin”[Brassica juncea L. Czern & Coss (B. juncea L. Czern & Coss)], broccoli [Brassica oleracea var. italica (B. oleracea var. italica)], cabbage [Brassica oleracea var. capitata (B. oleracea var. capitata)],“Khi-Hood”[Raphanus sativus L. var. caudatus Alef (R. sativus L. var. caudatus Alef)], Chinese radish [Raphanus sativus var. longipinnatus (R. sativus var. longipinnatus)], Chinese cabbage [Brassica rapa var. pekinensis (B. rapa var. pekinensis)] and two different varieties of choy sum [Brassica chinensis Jusl. var. parachinensis (Bailey) Tsen and Lee (B. chinensis Jusl. var. parachinensis (Bailey) Tsen and Lee)]. The edible parts of all cruciferous vegetables were extracted and analyzed by HPLC-UV. The percentages of yields of the extracts compared to the fresh weight of each vegetable were shown in Table 1.“Khi-Hood”had the highest yield of 0.029% per fresh weight. Since the presence of isothiocyanate compounds such as sulforaphane and sulforaphene were previously reported in various cruciferous plants, sulforaphane and sulforaphene were determined in the plant extracts[9].
The HPLC chromatograms of each vegetable extracts as well as standard sulforaphane and sulforaphene were shown in Figures 1–14. The HPLC peaks of isothiocyanates, sulforaphane and sulforaphene, were presented at the retention time of 11.4 min and 11.1 min, respectively (Figures 1 and 2). When standard sulforaphane and sulforaphene were coinjected, the peaks were merged and detected at retention time 11.1 min (Figure 3). Thus, this merged peak was primarily used to indicate the presence of the combined sulforaphane and sulforaphene. It was interesting that although the extracts of each vegetable had been extracted using the same method and from the same cruciferous family, their HPLC chromatograms were clearly different indicating the existence of different phytochemical compositions in each of the plant extracts (Figures 4–14). The distinct anticancer isothiocyanates (viz., sulforaphane and sulforaphene) in the edible part of“Khi-Hood”were approximated. Only 4 of the 11 cruciferous vegetables tested contained the isothiocyanates (sulforaphane and sulforaphene). The high to low rank of extracted isothiocyanates by comparing peak areas was“Khi-Hood”(pod and flower)>cabbage (leaves)>Chinese radish (root)>broccoli (leaves).

Table 1IC50values of antiproliferation of cruciferous plants in 24 h exposure time against HCT116 colon cancer cell line.

Figure 1. HPLC chromatogram of standard sulforaphane (100 μg/mL) with the retention time at 11.4 min.

Figure 2. HPLC chromatogram of standard sulforaphene (100 μg/mL) with the retention time at 11.1 min.

Figure 3. HPLC chromatogram of the co-injection of sulforaphane and sulforaphene (50 μg/mL each).*: Retention time of sulforaphane and sulforaphene in the extract.

Figure 4. HPLC chromatogram of B. juncea var. sareptana. (4.2 mg/mL).
These four plants that showed positive HPLC peak for isothiocyanates were further identified by using GC-MS analysis. It was found that some cruciferous plants contained not only sulforaphane but also sulforaphene. GC chromatograms showed the peaks of sulforaphane and sulforaphene at retention time of 19.6 and 19.4 min, respectively (Table 2). The GC-MS profiles illustrated the presence of sulforaphane peaks (m/z = 72, 114 and 160) in three plant extracts including broccoli, cabbage and“Khi-Hood”. The latter also contained high amount of sulforaphene (m/z = 72, 112, and 175) (Table 2). Moreover, GC chromatogram of the extracts from broccoli,“Khi-Hood”and cabbage contained the peaks at 8.2 min indicating the presence of 3-butenyl isothiocyanate (m/z = 55, 72, 85 and 113), the degraded product of heat-labile sulforaphane from GC-MS analysis. Whilst, Chinese radish did not contain 3-butenyl isothiocyanate indicating no or low sulforaphane content according to GC-MS analysis. Table 3 demonstrates sulforaphane and sulforaphene contents found in these four cruciferous plants.

Figure 6. HPLC chromatogram of B. juncea (3.9 mg/mL).

Figure 7. HPLC chromatogram of B. juncea L. Czern & Coss (2.3 mg/mL).

Figure 8. HPLC chromatogram of B. oleracea var. italica (2.5 mg/mL).*: Retention times of sulforaphane and sulforaphene in the extract.
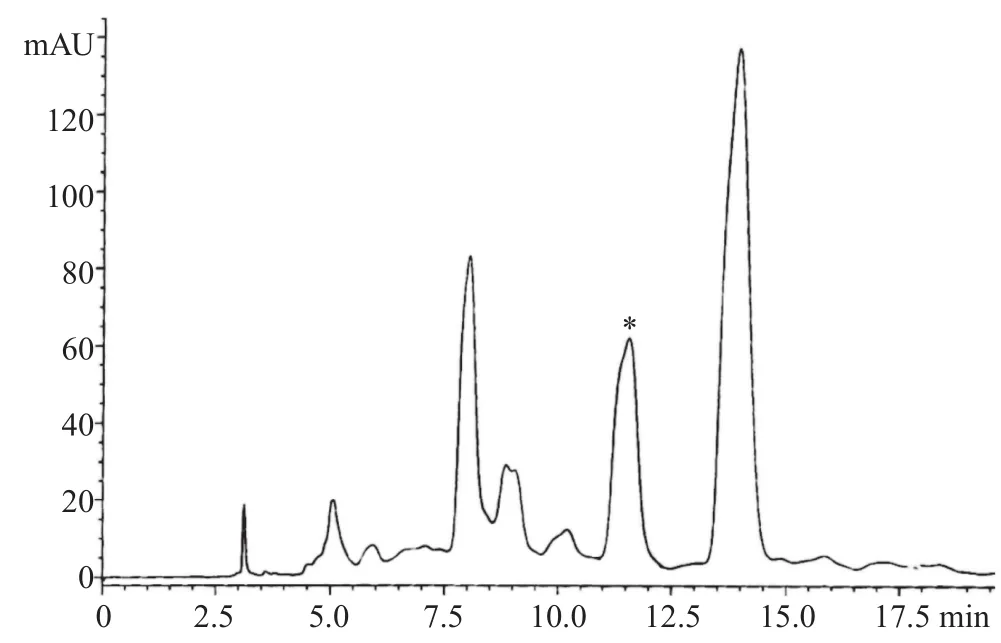
Figure 9. HPLC chromatogram of B. oleracea var. capitata (1.5 mg/mL).*: Retention times of sulforaphane and sulforaphene in the extract.

Figure 10. HPLC chromatogram of R. sativus L. var. caudatus Alef (0.27 mg/mL).*: Retention times of sulforaphane and sulforaphene in the extract.

Figure 11. HPLC chromatogram of R. sativus var. longipinnatus (2.5 mg/ mL).*: Retention times of sulforaphane and sulforaphene in the extract.

Figure 12. HPLC chromatogram of B. rapa var. pekinensis (2.3 mg/mL).

Figure 13. HPLC chromatogram of B. chinensis Jusl. var. parachinensis (Bailey) Tsen and Lee with bigger leave (8.0 mg/mL).
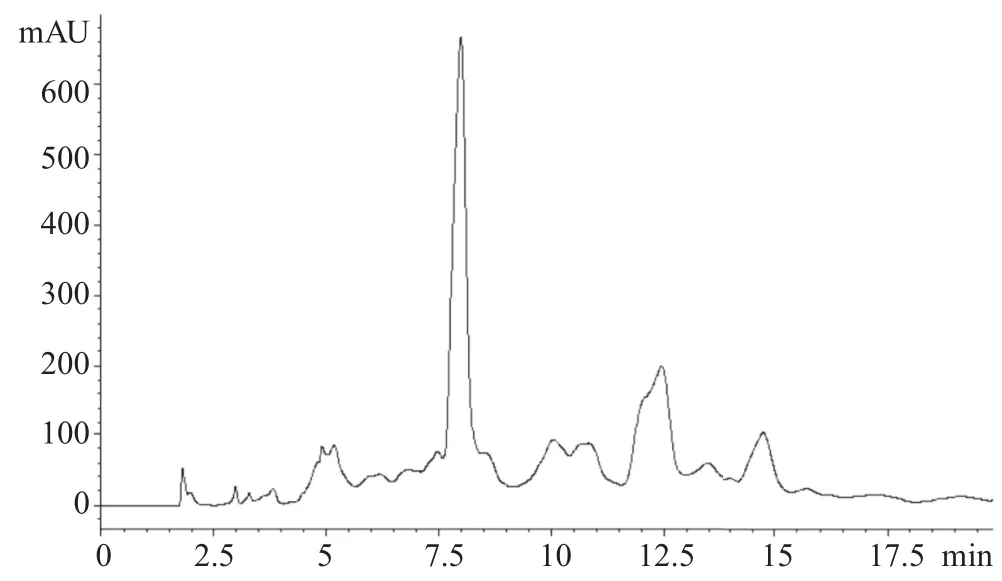
Figure 14. HPLC chromatograms of B. chinensis Jusl. var. parachinensis (Bailey) Tsen and Lee with smaller leave (2.0 mg/mL).
Due to the diversity of chemical constituents and the variable amounts of sulforaphane and sulforaphene in the cruciferous extracts, the chemopreventive activity was tested. The first chemopreventive test was antiproliferation using the MTT assay. The antiproliferation results of the test compounds against HCT116 colon cancer cell line were presented as IC50(Table 1). Of the 11 cruciferous vegetables, only“Khi-Hood”and cabbage induced HCT116 cell death. The extract from“Khi-Hood”possessed the highest antiproliferation against the HCT116 cell line [IC50= (9.42±0.46)μg/mL] and was classified as an active extract according to National Cancer Research Institute criteria because the IC50value was<20 μg/mL[9]. The cabbage extract exhibited antiproliferation against the HCT116 with an IC50value of (46.03±0.89)μg/mL, while the IC50value for the other extracts was>50 μg/mL. In order to avoid the solvent (acetonitrile) cytotoxicity, the highest concentration of the extracts tested was only 50 μg/mL.
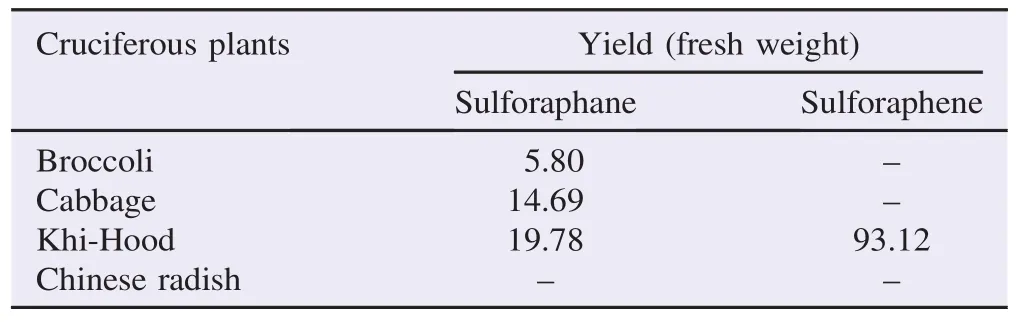
Table 3Isothiocyanates contents in cruciferous plants by GC-MS analysis.μg/g.
The chemotherapeutic drugs (mitomycin C), melphalan and 5-fluorouracil were used as the positive control to compare the sensitivity of the cancer cell line with the clinical and conventional anticancer drugs as well as with the cruciferous plant extracts. Among the chemotherapeutic drugs, only mitomycin C possessed antiproliferation against the HCT116 cells [IC50= (19.12±1.00)μg/mL], whereas the other chemotherapeutic drugs exerted less antiproliferation with IC50>50 μg/ mL. In the current study, sulforaphane and sulforaphene demonstrated a stronger antiproliferation than the chemotherapeutic drugs [IC50= (6.67±0.07)μg/mL and (10.67±2.27) μg/mL, respectively].“Khi-Hood”extract possessed the lowest IC50among cruciferous plants and chemotherapeutic drugs indicating its greater antiproliferation and suggesting a greater potential chemopreventive activity than the chemotherapeutic drugs.
4. Discussion

Table 2GC-MS of identified compounds found in cruciferous plants.
The sulforaphane content in edible part of broccoli and cabbage was previously reported [5]. Sulforaphane content in broccoli ranged from 1.4 to 32.9 μg/g fresh weight, while in cabbage it ranged from 0.7 to 4.7 μg/g fresh weight [5]. The present study on sulforaphane content in broccoli and cabbage is in accordance with the previous reported. Sulforaphene was previously reported to exist in the dichloromethane extractedfrom seed of“Khi-Hood”[10]. However, the reported amount of sulforaphene was different from that of the present study which might be due to the difference in extracted parts, extraction methods, analytical methods and plant cultivars [10].
Sulforaphane was considered to be the chemopreventive compound in various cruciferous vegetables including broccoli and cabbage. The preventive mechanism of sulforaphane activates mainly via the induction of Phase II metabolism enzymes and induction of apoptosis in cancer cell [3]. Although our study found no sulforaphane and sulforaphene in Chinese radish, another isothiocyanate [4-(methylthio)-3-butenyl isothiocyanate (m/z = 53, 72, 87 and 159)] was detected in this plant at 16.60 min by GC and structure was predicted by MS analysis. Previous studies reported the presence of isothiocyanates in the Chinese radish genus such as phenethyl isothiocyanate, benzyl isothiocyanate, 4-(methylthio) butyl isothiocyanate and 4-(methylthio)-3-butenyl isothiocyanate [11,12]. It was reported that 4-(methylthio)-3-butenyl isothiocyanate was the most abundant isothiocyanates in root of Chinese radish and varied between 70 and 400 μmol/L per 100 g fresh weight depending on plant strains [13]. In addition, this compound was a major contributor for in vivo chemopreventive activity of Chinese radish extract[13–15].
The presence of sulforaphane and sulforaphene has already been reported in cruciferous plants as well as their antiproliferative activity[7,10,16–18]. In this study, although the other cruciferous vegetables contained no sulforaphane and sulforaphene or even other isothiocyanates, it did not mean that these vegetables contained no isothiocyanates or other active compounds. It should be noted that the difference in solvents used as well as extraction methods and analytical parts may lead to different amount of isothiocyanates or active compound found in each reports. Thus, the suitable extraction and analytical method is still crucial to track the possible chemopreventive agent presented in these plants.
The different HPLC fingerprints of cruciferous plant extracts indicate the existence of different chemical constituents, which are consequently responsible for their varying antiproliferative activity against the HCT116 cell line. Our HPLC chromatogram displayed the isothiocyanates, as a merged peak from sulforaphane and sulforaphene, from four cruciferous plants including broccoli, cabbage,“Khi-Hood”and Chinese radish. However, the GC-MS analysis on these four plants showed that only broccoli, cabbage, and“Khi-Hood”contained sulforaphane, with the additional sulforaphene in“Khi-Hood”. Antiproliferative effect of these 11 cruciferous was also tested using MTT assay. Only“Khi-Hood”and cabbage extract possessed antiproliferative activity against colon cancer cell line HCT116 with IC50lower than 50 μg/mL“Khi-Hood”extract exhibited the highest antiproliferative effect among cruciferous plants, and chemotherapeutic drugs act possibly due to the contribution of both sulforaphane and sulforaphene. Our study showed that some of these cruciferous plants contained isothiocyanates. And the isothiocyanates possibly exhibit their effects via antiproliferative activity observed by MTT assay. This study substantiates the efficacy of cruciferous plant against the colon cancer cell line and suggests the possibility of its use as a potential source of anticancer agent. However, further studies are still required.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster and Research and Development of Herbal Nutraceutics Subcluster of Khon Kaen University (F-2553-M-11 and NRU541051).
References
[1] Bosetti C, Filomeno M, Riso P, Polesel J, Levi F, Talamini R, et al. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol 2012; 23: 2198-203.
[2] TangL,ZirpoliGR,GuruK,MoysichKB,ZhangYS,AmbrosoneCB, etal.Intakeofcruciferousvegetablesmodifiesbladdercancersurvival. Cancer Epidermiol Biomarkers Prev 2010; 19(7): 1806-11.
[3] Herr I, B¨uchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev 2010; 36: 377-83.
[4] Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol 2012; 19: 134-41.
[5] Liang H, Yuan QP, Dong HR, Liu YM. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J Food Compost Anal 2006; 19: 473-6.
[6] Campas-Baypoli ON, S´anchez-Machado DI, Bueno-Solano C, Ramírez-Wong B, L´opez-Cervantes J. HPLC method validation for measurement of sulforaphane level in broccoli by-products. Biomed Chromatogr 2010; 24: 387-92.
[7] Pocasap P, Weerapreeyakul N, Barusrux S. Cancer preventive effect of Thai rat-tailed radish (Raphanus sativus L. var. caudatus Alef). J Funct Foods 2013; 5: 1372-81.
[8] Boncler M, R´o.zalski M, Krajewska U, Podsędek A, Watala C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J Pharmacol Toxicol Methods 2014; 69: 9-16.
[9] Colegate SM, Molyneux RJ. Bioactive natural products: detection, isolation, and structural determination. 2nd ed. Boca Raton: CRC Press; 2007.
[10] Songsak T, Lockwood GB. Glucosinolates of seven medicinal plants from Thailand. Fitoterapia 2002; 73: 209-16.
[11] Blaˇzevi'c I, Masteli'c J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem 2009; 113: 96-102.
[12] Beevi SS, Mangamoori LN, Subathra M, Edula JR. Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Foods Hum Nutr 2010; 65: 200-9.
[13] Nakamura Y, Iwahashi T, Tanaka A, Koutani J, Matsuo T, Okamoto S, et al. 4-(Methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus; Japanese white radish). J Agric Food Chem 2001; 49: 5755-60.
[14] Salah-Abb`es JB, Abb`es S, Abdel-Wahhab MA, Oueslati R. In-vitro free radical scavenging, antiproliferative and anti-zearalenone cytotoxic effects of 4-(methylthio)-3-butenyl isothiocyanate from Tunisian Raphanus sativus. J Pharm Pharmacol 2010; 62: 231-9. [15] Okamura T, Umemura T, Inoue T, Tasaki M, Ishii Y, Nakamura Y, et al. Chemopreventive effects of 4-methylthio-3-butenyl isothiocyanate (Raphasatin) but not curcumin against pancreatic carcinogenesis in hamsters. J Agric Food Chem 2013; 61(9): 2103-8.
[16] Kuang P, Song D, Yuan Q, Lv X, Zhao D, Liang H. Preparative separation and purification of sulforaphene from radish seeds by highspeedcountercurrentchromatography.Food Chem2013;136:309-15.
[17] Kim MJ, Kim SH, Lim SJ. Comparison of the apoptosis-inducing capability of sulforaphane analogues in human colon cancer cells. Anticancer Res 2010; 30: 3611-9.
[18] Zeng H, Trujillo ON, Moyer MP, Botnen JH. Prolonged sulforaphane treatment activates survival signaling in nontumorigenic NCM460 colon cells but apoptotic signaling in tumorigenic HCT116 colon cells. Nutr Cancer 2011; 63: 248-55.
*Corresponding author:Natthida Weerapreeyakul, Ph.D., Faculty of Pharmaceutical Sciences, Khon Kaen University, 123 Mittrapap Road, Muang District, Khon Kaen, 40002, Thailand.
 Asian Pacific Journal of Tropical Biomedicine2016年2期
Asian Pacific Journal of Tropical Biomedicine2016年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Prevalence of refractive errors among primary school children in a tropical area, Southeastern Iran
- Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
