Effects of a combined nerve block on intraoperative stress and postoperative immune function in elderly patients subjected to total hip replacement: study protocol for a randomized controlled trial
Liang-de A, Guang-yuan Zhang, Hong-xiu Yan, Yan-hong Guo Yong-jin Yuan Zhen Jia
1 Department of Anesthesiology, Qinghai University Affiliated Hospital, Xining, Qinghai Province, China
2 Department of Joint, Qinghai University Affiliated Hospital, Xining, Qinghai Province, China
3 Department of Pain, Qinghai University Affiliated Hospital, Xining, Qinghai Province, China
INTRODUCTION
Elderly patients have poor tolerance of anesthesia and total hip replacement operations because of severe surgical trauma and blood loss. General anesthesia is the primary anesthesia method used for total hip replacement in the elderly, but it has many limitations. For example, it can interfere with physiological function (Li and Wang, 2012;Peng et al., 2012). Organic function and compensative ability of the elderly are often weakened to varying degrees because of cardiovascular disease, pulmonary disease, or diabetes mellitus. Thus nociceptive stimuli resulting from anesthesia and surgery can greatly in fluence stress in elderly patients (Marek et al., 2016; Lee et al., 2016; Fabio et al.,2016; Zhang et al., 2016).
Stress refers to an organism’s non-speci fic reactions to various nociceptive stimuli, i.e., stressors, in which many factors are involved. Stressors can stimulate sympathetic nerves, strengthen the function of the hypothalamic-pituitary-adrenal axis, and cause changes in various metabolic reactions, thereby playing an important role in maintaining intraoperative vital signs, and recovering postoperative immune function (Li-Tempel et al., 2016).Different anesthesia methods produce different effects on an organism’s immune function. The effects of anesthesia on immune function are closely related to complications,such as postoperative infection (Stromboni et al., 2012).In addition, immunity in the elderly is reduced compared with the non-elderly healthy population. Therefore, clinically, an optimal anesthesia method for safer surgery and postoperative recovery in the elderly is required. Speci fically, a combined nerve block has been reported to be more suitable for total hip replacement in elderly patients because of its safety and reliability (Li and Wang, 2012;Li et al., 2013).
This study has been designed to validate the hypothesis that a combined nerve block produces better outcomes including intraoperative stress, hemorheological indices, postoperative immune function, and incidence of postoperative complications compared with general anesthesia. Previous studies focused primarily on onset time of anesthesia and postoperative complications (Li et al., 2013). To the best of our knowledge, no studies have explored the effect of a combined nerve block on intraoperative stress and postoperative immune function in elderly patients undergoing total hip replacement.
METHODS/DESIGN
Study design
A prospective, single-center, randomized controlled, openlabel trial.
Study setting
Qinghai University Af filiated Hospital, China.
Study procedures
A total of 120 elderly patients will be randomly divided into an experimental group (n = 60, a combined nerve block)and a control group (n = 60, general anesthesia) to undergo total hip replacement. All patients will be followed up for 3 months (Figure 1).
Inclusion criteria
Patients scheduled to undergo total hip replacement and presenting all of the following criteria will be considered for admission to this trial:
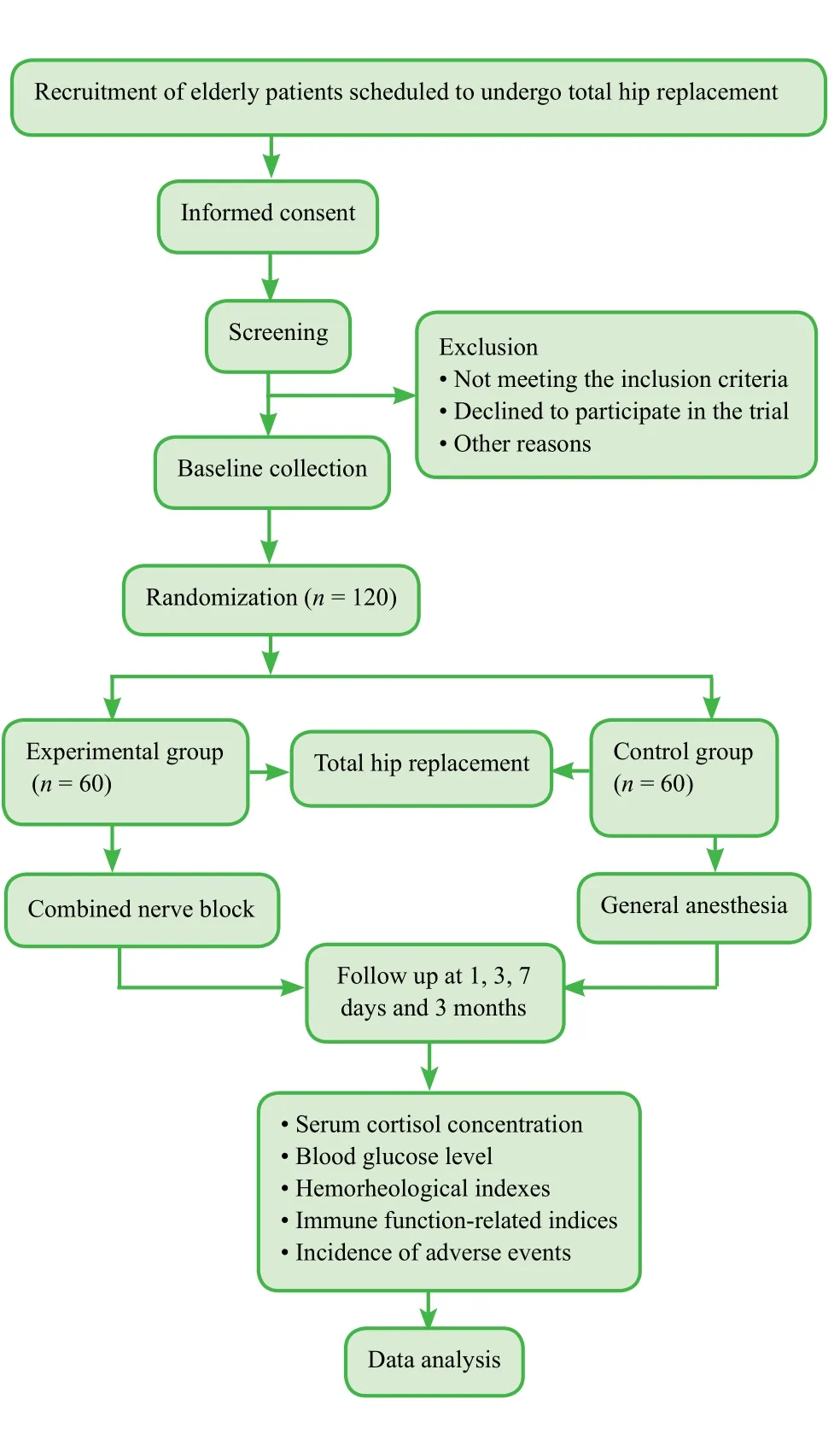
Figure 1: Flow chart of the trial protocol.
· Blood pressure below 160/90 mmHg (1 mmHg =0.133 kPa)
· Hemoglobin > 90 g/L
· Fasting blood glucose level < 10 mM
· Preoperative examinations (blood, urine, stool, hepatic and renal function, blood coagulation, electrolyte level,and electrocardiogram): normal
· Types I-II in American Society of Anesthesiology (ASA)classi fication (Rao et al., 2015)
· Age > 65 years
· Of either sex
· Provision of signed informed consent to participate in the trial
Exclusion criteria
Patients presenting with any one or more of the following will be excluded from this trial:
· Severe heart, liver, lung, kidney or hematological system diseases, severe infection or malignant tumor
· Allergy to anesthetic agents
· Taking immunosuppressive agents and/or glucocorticoid
· Viral infections
· Mental disorder, dysnoesia, hearing disorder, or poor compliance during anesthesia
· Alcohol or drug abuse
Baseline evaluation
Prior to randomization, patients’ baseline information,including demographic data and general history of disease will be collected (Table 1).

Table 1: Patient’s baseline information
Sample size
In accordance with a previous study (Li and Wang, 2012)and our previous experience, we hypothesized that compared with prior to anesthesia, serum cortisol concentration in the experimental group increases by 10% during surgery,whereas it increases by 35% in the control group. Taking β ≤ 0.1 and power = 90% with a signi ficance level of α =0.05 (two-sided), the final effective sample size of n = 55 per group is required. If a 20% rate of lost patients is considered, we will require 66 patients per group. According to the screening criteria, we aim to include 60 patients in each group.
Recruitment
Recruiting of participants will be performed through hospital bulletin board advertisements. Potential patients in the ward will be asked to contact the project manager. After providing written informed consent, those interested in participating in the trial will be screened according to the above inclusion and exclusion criteria.
Randomization
One day prior to randomization, we will number the 120 included patients using a random table generated by SPSS 17.0 software (SPSS, Chicago, IL, USA). In the random number table, the initial starting point and the order of sampling will be arbitrarily speci fied. Patients assigned even numbers will be included in the experimental group,and those with odd numbers in the control group.
Blinding
Physicians, patients, and assessors will not be blinded to group information and therapeutic regimen.
Interventions
Prosthesis material
Large diameter head metal-on-metal total hip prostheses(ASRTMXL, DePuy Orthopaedics, Inc., Warsaw, IN, USA)will be used in the experimental and control groups. Each large diameter (range 39-63 mm) head metal-on-metal total hip prosthesis has a hemisphere-shaped acetabulum with microporous hydroxyapatite coating, which facilitates early fixation and bone in-growth. The Corail femoral stem(Depuy Orthopaedics) is coated with hydroxyapatite and has a biological titanium alloy taper shank. The Summit femoral stem (Depuy Orthopaedics) is coated with microporous hydroxyapatite and has a biological titanium alloy taper shank. These meet the requirement of YY0117.1-1993 and GB13810-1997.
Total hip replacement
After successful anesthesia, a 12-cm-long incision will be made on the extension line passing through the posterior superior iliac spine and greater trochanter, and on the lateral side of the femur. The femoral neck will be cut off at 2 cm above the lesser trochanter. After separation of the surrounding ligament and articular capsule, the femoral head will be taken. The stumps of the femoral head will be trimmed repeatedly, and then the acetabular prosthesis will be implanted.
Anesthesia
· Experimental group: a combined nerve block involving the lower lumbar plexus, sciatic nerve, and paraspinal nerve L1-2will be performed.
Anesthesia at the lower lumbar plexus: The puncture point of the lower lumbar plexus will be located on the affected side and at the site 4.0-5.0 cm lateral from the point of intersection of the line between the highest iliac crests and the midline of the spinous process line.
Anesthesia at the sciatic nerve: The puncture point of the sciatic nerve will be located at the site 5 cm below the perpendicular bisector of the midpoint of the line between the posterior superior iliac spine and greater trochanter of the femur, and near the exterior margin of the gluteus maximus muscle. The initial current of the nerve stimulator will be set at 1 mA and 2 Hz to initiate contraction of quadriceps femoris and gastrocnemius muscles, and cause dorsi flexion and plantar flexion of the ankle. Then,the current of the needle tip of the nerve stimulator will be adjusted to 0.3-0.5 mA to elicit muscular contraction.Muscular contraction will cease at 0.15 mA, suggesting that the needle tip has approached the lower lumbar plexus or sciatic nerve, but without injury to the nerve. At this time,if no blood or cerebrospinal fluid is pumped back, 5 mL of 0.5% ropivacaine will be injected. One minute later, if no toxic reaction caused by local anesthetic occurs, needle tip current will be increased to 1 mA, and if no muscular contraction occurs, nerve block positioning will be correct.A total of 15 mL of 0.5% ropivacaine will be injected into the lower lumbar plexus and 10 mL of 0.5% ropivacaine into the sciatic nerve several times.
Anesthesia at the paraspinal nerve L1-2: The puncture point of the paraspinal nerve L1-2will be located on the affected side, and 2.5 cm lateral from the L1-2spinous process. If necessary, low-dose midazolam will be administered several times during surgery.
· Control group: General anesthesia will be used for total hip replacement. After induced anesthesia by intravenous injection of midazolam 0.06 mg/kg, sufentanil 0.2 µg/kg,etomidate and cisatracurium 0.2 mg/kg each, tracheal intubation will be followed by mechanical ventilation with tidal volume of 8-10 mL/kg, respiratory frequency of 12-14/min,and partial pressure end-tidal carbon dioxide (PETCO2)35-40 mmHg. Propofol 80 µg/kg/min and remifentanil 0.1 µg/kg/min will be pumped for maintenance of general anesthesia, and cisatracurium administration will be intermittently performed.
Outcome measures
Primary outcome measure
· Intraoperative serum cortisol concentration to evaluate intraoperative stress. Normal serum cortisol concentration is 138-635 nM measured at 8:00 a.m.-10:00 a.m. and 83-359 nM at 4:00 p.m.-6:00 p.m. Greater intraoperative serum cortisol concentration indicates greater stress.
Secondary outcome measures
· Serum cortisol concentration prior to anesthesia and prior to and immediately after surgery will be measured to evaluate a patient’s stress level.
· Fasting blood glucose level prior to anesthesia and prior to, during, and immediately after surgery will be used to evaluate a patient’s stress at each time. Fasting blood glucose in the normal population is 3.61-6.11 mM. Higher fasting blood glucose level indicates greater stress.
· Immune function-related indices prior to anesthesia,immediately after surgery, 1, 3, 7 days, and 3 months after surgery: these indices will include absolute leukocyte count,absolute neutrophil count, interleukin-1, interleukin-6,tumor necrosis factor-α, and T-lymphocyte subset levels,and be measured to evaluate postoperative patient immune function. Greater values indicate poorer immune function.
· Hemorheological indices prior to anesthesia, and during and immediately after surgery: these indices will include electrocardiogram, pulse, systolic pressure, diastolic pressure, heart rate, blood gas analysis, and be used to investigate changes in blood-related indices. The electrocardiogram will be used to detect arrhythmia. Pulse and heart rate measurements will be used to investigate changes in patient vital signs. Systolic pressure and diastolic pressure will be used to monitor blood pressure. Blood gas analysis will measure the pH value of blood, partial pressure of carbon dioxide (PaCO2), and partial pressure of oxygen (PaO2), and be used to monitor acid-base imbalances and respiratory failure.
· Incidence of adverse events 1, 3, 7 days, and 3 months after surgery: these adverse events include upper respiratory tract infection, pulmonary infection, acute atelectasis,and acute pulmonary embolism. The percentage of patients suffering adverse events in each group will be calculated.The schedule of outcome measurement assessment is shown in Table 2.
Adverse events
If severe adverse events including any expected or unexpected symptoms occur, information including the data of occurrence, type of adverse events, and measures takenrelated to the treatment of the adverse events will be reported to the project manager and the institutional review board within 24 hours.
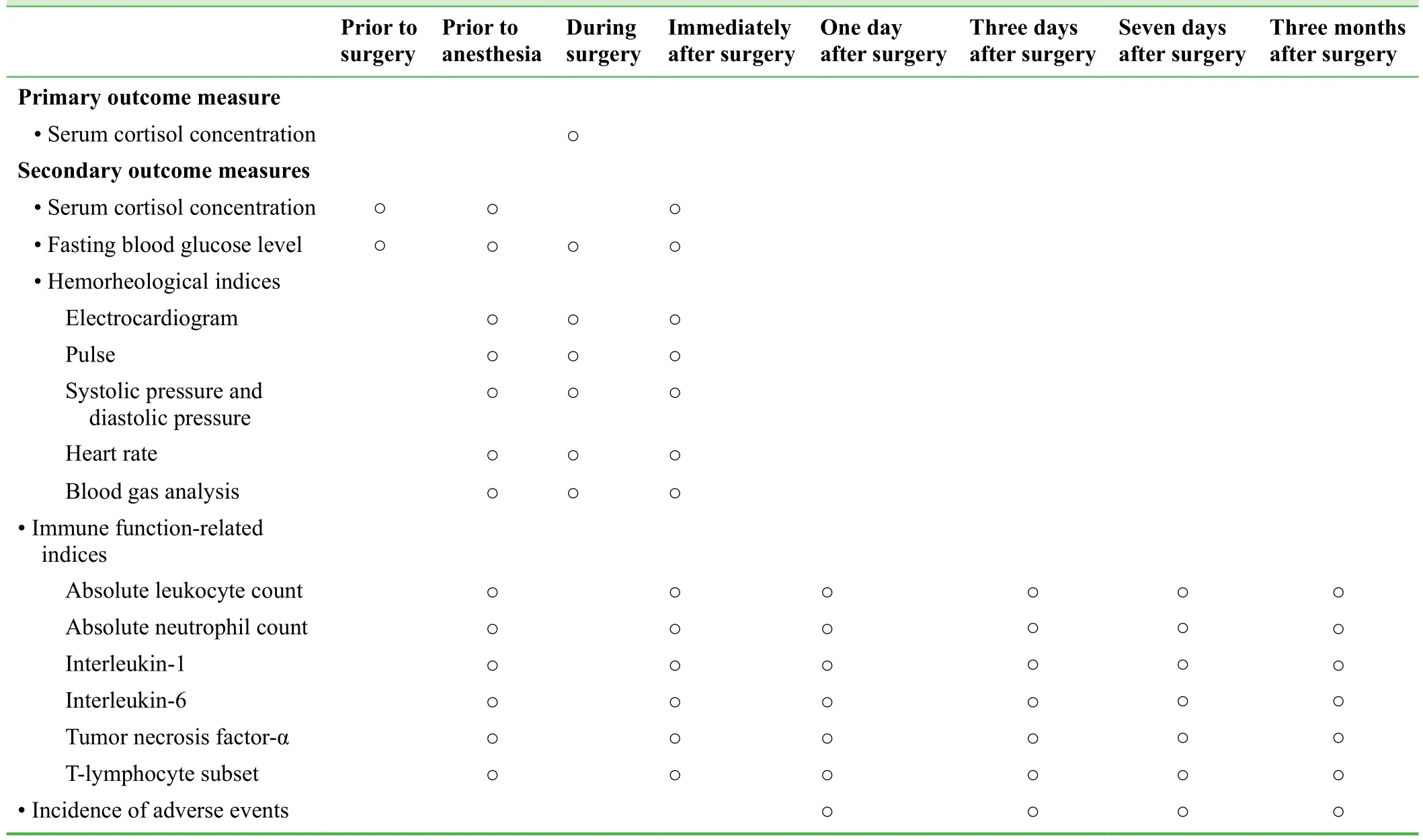
Table 2: Timing of outcome assessment
Data collection, management, analysis and open access
According to trial design type and requirement, a table will be developed to record trial data. The recorded data will be input into an electronic database using a double-data entry strategy by trained professional staff. Information accuracy will be checked when all recruited patients are followed up.The database will be locked by the researcher in charge and will not be altered. All information relating to this trial will be preserved by Qinghai University Af filiated Hospital,China. The electronic database will be made available to a professional statistician for statistical analysis. An outcome analysis report will be made by the statistician and submitted to the lead researchers. An independent data monitoring committee will supervise and manage the trial data with the goal of ensuring a scienti fic and stringent trial process,resulting in accurate and complete data. Anonymized trial data will be published at http://www. figshare.com.
Statistical analysis
Statistical analysis will be performed by a statistician using SPSS 17.0 software, and will follow the intention-to-treat principle. Normally distributed measurement data will be expressed as mean, standard deviation, min, and max. Nonnormally distributed measurement data will be expressed as lower quartile (q1), median, and upper quartile (q3). The Mann-Whitney U test will be used to compare fasting blood glucose level, serum cortisol concentration, hemorheological indices, and immune function-related indices between experimental and control groups. The chi-square test will be used to compare the incidence of adverse events between experimental and control groups, with an accepted signi ficance level of α = 0.05.
Auditing
Trial progression will be reported to the ethics committee of Qinghai University Af filiated Hospital, China every 6-12 months, and the trial’s status will be updated in the registration database.
Confidentiality
Trial data will be maintained in paper and electronic forms.Electronic data will be preserved in a dedicated passwordprotected computer and managed by a data management professional. Data reported on paper will be preserved by Qinghai University Af filiated Hospital, China.
Trial status
Recruiting is ongoing at the time of submission.
DISCUSSION
This study was designed to investigate an optimized anesthesia protocol for total hip replacement in the elderly, with the aim of reducing adverse events resulting from surgery.This prospective, single-center, randomized controlled, open label trial will investigate the effects of a combined nerve block on intraoperative and postoperative stress and immune function in elderly patients. Compared with previous reports(Li and Wang, 2012; Peng et al., 2012), this trial design is normalized and provides a suf ficiently large sample size for the effect size hypothesized. Nevertheless, although powered to investigate the effects of different anesthesia methods on stress and immune function in elderly patients undergoing total hip replacement, this study is not powered to evaluate curative effects on hip joint function and has a short follow up time. Follow up over 1 year is required to investigate long-term curative effects of total hip replacement on hip joint function. In addition, age over 65 years is too generic for the outcomes given the risk of complications in any surgery for the very elderly patient, so age could be a confounder for the outcomes in this study.
Findings from this trial will help to validate the hypothesis that a combined nerve block is superior to general anesthesia for total hip replacement in elderly patients, speci fically,from the perspectives of intraoperative stress, hemorheological indices, and postoperative immune function. We hope that a combined nerve block can alleviate stress, promote the recovery of immune function, and reduce complications in elderly patients undergoing total hip replacement.
Conflicts of interest
None declared.
Author contributions
LDA conceived and designed the trial protocol, wrote the manuscript and approved the final version of this manuscription for publication. GYZ, HXY, YHG, YJY and ZJ were responsible for experiment conduction.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Fabio S, Rossella G, Mariella de R, Nicola C, Maria S (2016) Minimizing stress with remifentanil in a patient with Takotsubo undergoing ophthalmic surgery. J Opioid Manag 12:86-88.
Lee TW, Kowalski S, Falk K, Maguire D, Freed DH, Hay Glass KT (2016) High spinal anesthesia enhances anti-in flammatory responses in patients undergoing coronary artery bypass graft surgery and aortic valve replacement: randomized pilot study. PLoS One 11:e0149942.
Li JK, Liu X, Zhu YZ (2013) Application of combined nerve block anesthesia in hip arthroplasty. Jujie Shoushuxue Zazhi 22:101-102.
Li-Tempel T, Larra MF, Sandt E, Mériaux SB, Schote AB, Schächinger H, Muller CP, Turner JD (2016) The cardiovascular and hypothalamus-pituitary-adrenal axis response to stress is controlled by glucocorticoid receptor sequence variants and promoter methylation. Clin Epigenetics 8:12.
Li ZL, Wang GL (2012) Effects of different anesthesia methods on blood gas parameters and stress response during hip replacement in elderly patients. Qiqihaer Yixueyuan Xuebao 33:1710-1713.
Marek M, Małgorzata K, Małgorzata K (2016) Effect of pre-surgical stress on recovery of patients undergoing hip replacement procedures. Przegl Lek 73:25-28.
Peng MM, Li M, Li Y, Peng Q, Huang HG (2012) The research on stress response of unilateral lumbar anesthesia in hip replacement operation in senile patients. Chongqing Yixue 41:2258-2262.
Rao AK, Gurajala I, Gopinath R (2015) Comparison of electroencephalogram entropy versus loss of verbal response to determine the requirement of propofol for induction of general anaesthesia.Indian J Anaesth 59:348-352.
Stromboni M, Menguy F, Hardy P, Leparc JM, Lortat-Jacob A, Benoit J (2002) Total hip arthroplasty and femoral head osteonecrosis in renal transplant recipients. Rev Chir Orthop Reparatrice Appar Mot 88:467-474.
Zhang C, Li C, Xu Z, Zhao S, Li P, Cao J, Mi W (2016) The effect of surgical and psychological stress on learning and memory function in aged C57BL/6 mice. Neuroscience 320:210-220.
 Clinical Trials in Orthopedic Disorder2016年3期
Clinical Trials in Orthopedic Disorder2016年3期
- Clinical Trials in Orthopedic Disorder的其它文章
- Chondromyxoid fibroma of the distal one-third of the fibula: a rare tumour at rare site
- Traction apophysitis of the medial malleolus
- Digital navigation enhances cervical pedicle screw placement accuracy and safety: study protocol of a randomized controlled trial
- Porous tantalum rods improve the hip joint function of patients with avascular necrosis of the femoral head after femoral neck fracture surgery: study protocol of a randomized controlled trial
- Safety and effectiveness of proximal femoral nail antirotation for the treatment of intertrochanteric femoral fracture: study protocol for a prospective case series
- Commentary on "clinical efficacy of tranexamic acid administration via different routes during total hip arthroplasty:study protocol for a prospective randomized controlled trial”
